Many healthcare improvements are the result of the latest progresses made. The rapid development of scientific knowledge also means that in just a few years the contents of the information received during undergraduate nursing training on different interventions, procedures and care becomes obsolete. If we also accept that it is impossible to know everything, we will find that there are often situations in our clinical practice where we have to take decisions without having of all possible information at our fingertips. These situations may lead to anxiety or feelings of guilt, where the need for information is ignored or decisions are delayed until all information is available. However, the effort required to formulate clinical questions likely to be answered and the search for responses to those questions is so complex that the need for immediate information is frequently not provided.1
Information from clinical research, known as scientific evidence,2 is an essential part of the knowledge necessary for appropriate decision taking and the formulation of a clinical question is the first step towards finding the answer in the literature to knowledge gaps. When the aim is to have the best scientific evidence based on several previously established eligibility criteria for taking clinical decisions, the best study is a systematic review (RS).3
The importance of systematic reviewsSocial recognition of the nursing profession partly depends on investigation since through this we can show how nursing care helps to improve peoples’ health and wellbeing. Systematic review is an information tool of synthesis where the units of analysis are the original studies and where systematic and explicit methods are used to minimize bias.3,4 In fact many of the problems of primary studies and clinical trials could be controlled by a review of the literature which has attempted to respond to a specific clinical question and has located all the studies covering this same question, critically assessing the methodology and synthesizing the knowledge. As a result, the evidence provided by a systematic review exhaustively and rigorously carried out becomes part of existing knowledge and therefore contains a global and specific outlook which makes it essential.5
By attaching value to reviews we acknowledge that the knowledge has become consolidated, accumulatively, through the contribution of different studies constructed on one subject. As nurses we need to have tools like reviews to obtain appropriate information and filter it in terms of quantity, quality and topicality.
Response to this challenge comes from the reviews carried out by the Cochrane Collaboration, a not-for-profit international organisation whose purpose is to aid decision-making by preparing, maintaining and disseminating systemic reviews.6 A Cochrane review is complex and may involve many appraisals. To avoid this, it is suggested the review protocol be published previously, reducing the risk of bias, improving the transparency of the process and permitting paired review.
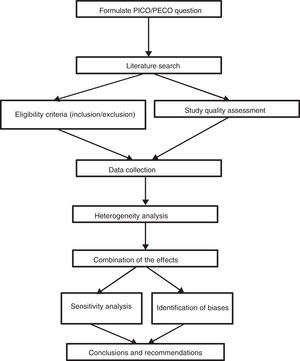
Generating questions that are likely to receive answersAny systematic review should start with a clearly formulated specific question and thus establish explicit and reproducible methods to systematically cover the successive stages in the review process: the search and identification of relevant studies, the description and analysis of their quality or risk of bias, data extraction, analysis of results and their interpretation (Fig. 1). These questions may be broad or restricted in their scope. Determining the scope of a question depends on different factors such as the relevance of the question, its impact or available resources.
Formulating a question means reducing it to clear and precise terms, identifying its main components. This process includes the determination of the four components of the question: PICO/PECO and its classification. P=population or specific health problem, I=intervention (exposure or diagnostic procedure), C=comparison, O=clinical outcome. The most appropriate types of study must subsequently be specified to respond to the question. A correct approach in question formulation makes the search for information simpler and more effective.
One of the aspects which differentiate a systematic review from a narrative one is the eligibility criteria (inclusion and exclusion). In systematic reviews, these are specified prior to the review and are a combination of the population, intervention and comparison of clinical questions. The outcomes or result variables are not included as eligibility criteria.7–9 These aspects will determine the most appropriate inclusion and exclusion criteria of the studies and the type of study we must search for.
The criteria for selecting the population included in a review must be broad enough and fair enough, ensuring the necessary balance between the sensitivity to find all the information relating to our question and the specificity or degree of precision of the results. As a result, restrictions regarding specific characteristics of the population must be explicitly minimized or justified. The second component of the PICO question is intervention of interest and intervention with which they will be compared. The definition of the intervention or the exposure is equally important and we should know how to differentiate between whether this is a prognostic factor, a diagnostic test, an exposure to risk or protection or a pharmacological or non pharmacological intervention. The final component is the result variables which should be relevant outcomes so that they can be measured without confusion. These results may be survival, clinical events, outcomes communicated by the patients, adverse events, disease burden, and financial results.5 The authors should have available information on the type of measurement (objective or subjective) and the moment when the result variables were measured.
Formulating these questions involves the reflection and explicitness which clinically experienced nurses automatically apply every day in their work.
In addition to the four components of the PICO questions, the type of design is specified since some designs are more appropriate than others for responding to certain questions. (Table 1).
Classification of the questions and study type.
| Type of question | Type of study | Type of synthesis study |
|---|---|---|
| Diagnosis | Cross-sectional study | CPGSystematic review |
| Aetiology | Case study and controls | CPGSystematic review |
| Cohort study | ||
| Prognosis | Cohort study | CPGSystematic review |
| Treatment | Clinical trial | CPGSystematic review |
The information search and review creation process has been documented in many previous editions of “Enfermería Intensiva”8,9 (Intensive Nursing) and therefore we will only comment upon aspects of the search plan formulation.
Data collection has to be exhaustive, extensive and reproducible to avoid selection and publication bias. It cannot be limited to a single data base nor a single language. The search begins with the appropriate formulation of the key words and the research into data bases such as Medline, Embase, Lilacs or Cochrane Collaboration, either manually from journals or from data bases, clinical trial registries or consultations from experts. The reading of doctoral theses, conference minutes or technical reports may also be of use.
The search process should be meticulously documented and reported to the point where it can be reproducible. All complete search strategies for each data base must be included in the review appendices. The search strategy is not defined from the beginning but requires many attempts and often also advice from external parties.
At least two researchers should independently and blindly identify and select the titles and abstracts obtained from electronic bases. With these a set of eligible studies will be created and if there are differences between the assessors a consensus should be reached. If this is not possible a third assessor will be required. Following this, the complete articles are obtained and the compliance of inclusion criteria are independently assessed and the excluded studies are listed, together with the reasons for exclusion.10 In the chapter on protocol methods, all the relevant information on reviewers and the process must be mentioned. Non compliance of a single criteria of eligibility will exclude any review study. When the review has been made by many reviewers, on finalising the included and excluded study selection, the level of agreement between reviewers is measured with the Kappa statistic.10
On finalising the process of article selection we proceed to codify the information. At least two researchers extract the data independently using a standard format which includes the study variables and the information relating to the participants, intervention, exposure, purchasers and outcomes.10 Lack of agreement over quality, eligibility and data extracted are resolved through discussion until consensus is formed. If necessary, a third author will participate to reach consensus and if the information is unclear they will contact the authors to obtain the missing information.
Assessment of the individual quality of the studies should include pre-established criteria or scales which guide the interpretation of results and help us to control the bias. There is currently great diversity of tools for assessing the quality of the evidence, among them the Alejandro Jadad scale, the JAMA evaluation guide, the NewCastle-Ottawa scale, the Cochrane bias risk tool or GRADE to assess evidence and to generate recommendations. Each of them serves for a different type of study.11,12
Evaluation of evidence, bias risk assessment and quality assessment are the final phases to precede analysis of results of the systematic reviews and deserve detailed attention.
ConclusionsThe systematic reviews are an essential support for taking decisions and in recent years have increased exponentially because they offer us highly structured response to clinical practice problems. However, they are not exempt from problems since primary research may be low quality with major biases and inappropriate review methods.
The use of scientific evidence must be a permanent goal for sustained professional development offering excellence in care with a positive impact on results and patient safety. To do so it is essential that as healthcare professionals we change our research priorities, prioritising values such as honesty, transparency and a critical outlook.
Please cite this article as: Robleda G. Pregunta estructurada para generar la búsqueda de una revisión sistemática. Enferm Intensiva. 2019. https://doi.org/10.1016/j.enfi.2019.07.001