To evaluate the rates and nature of the complications related to the Central Peripheral Access Catheter (CVCAP or PICC) from its insertion to its withdrawal.
MethodsProspective observational study. All patients older than 14 years of age with a PICC inserted in the polyvalent intensive care unit (ICU) during the period between May 1, 2015 and April 30, 2016 were included. Data collected included: demographic data, insertion details, reason for insertion and removal, maintenance unit, total dwell time, incidence of complications and related factors and infection rate.
Results144 PICCs were inserted, of which 94 corresponded to the ICU group (65.28%) and 50 (34.72%) to the non-ICU group. The most important complication was the suspicion of infection: 17.36% (rate of 15.2 per 1000 days of PICC). The total number of confirmed infections was 6.25% (5.5 per 1000 days of PICC), 1.39% (1.2 per 1000 days) being in the ICU group and 4.86% (4.2 per 1000 days) in the non-ICU group. There were 5 bacteraemias, all in the non-ICU group (3 per 1000 days). The most frequent germ was Staphylococcus epidermidis (6 cases). Phlebitis had an incidence of 9.03% (7.9 per 1000 days of PICC).
ConclusionsPICC, effective device for central venous access due to the minimal incidence of risks in implantation and to its advantages with regard to the classic central venous catheters, is a further nursing technique.
Evaluar las tasas y la naturaleza de las complicaciones relacionadas con el catéter venoso central de acceso periférico (CVCAP o PICC) desde su inserción hasta su retirada.
MétodoEstudio observacional prospectivo. Se han incluido a todos los pacientes con edad superior a 14 años a los que se les insertó un CVCAP en una unidad de cuidados intensivos (UCI) polivalente durante el año comprendido entre el 1 de mayo de 2015 al 30 de abril de 2016. Variables estudiadas: datos demográficos, vena de inserción, indicación, unidad de cuidado, duración, complicaciones y factores relacionados, tasa de infección y motivo de retirada.
ResultadosSe insertaron 144 CVCAP, de los cuales 94 corresponden al grupo UCI (65,28%) y 50 (34,72%) al no-UCI. La complicación más importante fue la sospecha de infección: 17,36% (tasa de incidencia de 15,2 por 1.000 días de CVCAP). El total de infecciones confirmadas fue del 6,25% (5,5 por 1.000 días de CVCAP), siendo en el grupo UCI del 1,39% (1,2 por 1.000 días) y en el grupo no-UCI del 4,86% (4,2 por 1.000 días). Hubo 5 bacteriemias, todas en el grupo no-UCI (3 por 1.000 días). El germen más frecuente fue el Staphylococcus epidermidis (6 casos). La flebitis tuvo una incidencia del 9,03% (7,9 por 1.000 días de CVCAP).
ConclusionesEl CVCAP, eficaz dispositivo de acceso venoso central debido a la mínima incidencia de riesgos en la implantación y a sus ventajas respecto a los catéteres venosos centrales clásicos, es una técnica enfermera más.
Peripherally inserted central catheters are increasingly used, and their insertion has become common practice among nurses working in intensive care units due to their many uses and indications.
What is the contribution of this?This article describes results of these catheters inserted by nurses, and shows that the complication rate, although low, is not without risk for the patient, especially when they are maintained in the hospital ward.
Implications of the studyThis study detected the need to raise awareness of infections associated with these catheters and could serve to encourage hospitals to provide clear indications on their use, and thus improve patient safety.
The peripherally inserted central catheter (PICC), is a long, thin and flexible tube, usually between 4 and 7F depending on the number of lumens, which is implanted through the basilic or cephalic veins, above the flexure avoiding the ante-cubital fossa. It can be inserted short term (grade I polyurethane) or medium term >6 months (grade III polyurethane). The catheter is inserted to the lower 1/3 of the superior vena cava (next to the venoatrial junction), and is used to administer liquids, blood products or drugs, and for haemodynamic monitoring or for drawing blood samples. According to the national data of the Study on the Prevalence of Nosocomial Infection in Spain (EEPINE-EPPS STUDY 2017), 83.24% of patients carry an intravascular device, of which 10.41% are central venous catheters (CVC).1
Many clinical practice guidelines and protocols on intravenous therapy via percutaneous catheters,2–5 recommend the use of central venous access catheters when intravenous therapy is to be administered for more than 6 days, a pH <5 or >9, increased osmolarity >600mOsm/l, total parenteral nutrition (TPN), for vesicant drugs or treatment through multiple lumens. The opinions of nurses could be added to these indications, since they are accustomed to assessing patients, observing their vascular tree and updating their knowledge through consensus documents.3,6
The nursing field of action allows central intravenous devices to be implanted via the veins of the forearm. The PICCs perform the same functions as a central line. These devices, of guaranteed durability, can be inserted by trained nurses and, in addition to preserving the patient's vascular system and enabling blood to be taken without the need for needles,7 provide many advantages over other central venous catheters, such as a very small risk of arterial puncture and easy compression should it occur, and without the thoracic complications typical of central access routes through the subclavian or jugular veins.
The use of vascular catheters occasionally causes local or systemic infections such as uncomplicated or complicated bacteraemia, manifested by persistent bacteraemia, septic thrombophlebitis, endocarditis, and other metastatic complications such as lung or brain abscesses, osteomyelitis and endophthalmitis. These types of complications have important morbidity as well as not inconsiderable mortality, and are the most frequent reason for the removal of devices of all types.8–10 Therefore, and to prevent complications, it is important to implement evidence-based indications.11,12 Recently, in 2015, the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) was published. It provides a summary of how to choose the intravenous device with the lowest risk that most efficiently supports the patient's treatment plan.13
Vascular catheter-related bacteraemia is one of the most frequent hospital-acquired infections. In the EPINE-EPPS study of 2017,1 central venous catheter-related bacteraemia (CVCRB) accounted for 34.36% of all bacteraemia and dropped to 26.41% when the bacteraemia was confirmed by microbiological culture of the catheter tip. In the national study on Nosocomial Infections Surveillance in ICU (ENVIN-HELICS 2017)14 CVCRB accounted for 11.82% of all infections acquired in intensive care units (ICU).
Surveillance programmes for the prevention of CVCRB, principally intended to apply a simple set of preventive measures of proven effectiveness, combined with educational campaigns for staff and implemented in collaboration with the institutions’ management structures, such as the Zero Bacteraemia project,15 have had significant impact in reducing the rates of CVCRB in ICU as evidenced by the results of the 24 years of ENVIN-HELICS studies.14 In 2001, the rate of bacteraemia of unknown origin, associated with a CVC was 3.7 episodes per 1000 catheter days16 and in 2017 had dropped to 2.93 episodes per 1000 catheter days (in that year CVC-related bacteraemia was studied specifically, and an incidence of 1.58 episodes per 1000 days with a CVC was found).14
With venous catheters, as with other devices, setting up surveillance programmes implies being aware of the pathogenesis of catheter-related infections (CRI). Microorganisms can access these catheters intraluminally or extraluminally. The adherence and incorporation of these microorganisms to form biofilms causes colonisation of the catheters, with the possibility of haematogenous spread.17
There are 3 important ways that microorganisms can gain access via a PICC or any other intravascular device: contamination of the infusion product, contamination of the connection and of the intraluminal space, and contamination of the skin adjacent to the insertion site and the extraluminal surface.18
Contamination of the product administered is very unusual due to the rigorous controls that these products undergo. Only TPN solutions with prepared lipids have a higher risk if they have not been prepared using appropriate safety measures.8,9 Contamination of the connection and intraluminal space is the second most common cause, especially if inserted more than 2 weeks ago.18,19 Access of microorganisms from the skin around the catheter insertion site is the most important pathogenic mechanism for colonisation of the extraluminal surface, and is possibly the only entry route in catheters that have been in place for less than 8 days. Colonisation by haematogenous spread of a microorganism originating from a distant site is very rare and is essentially observed in critical patients with long-term catheters or patients with chronic intestinal diseases who are carriers of intravascular devices for parenteral nutrition. This possibility should be considered when the infection recurs despite removal of the catheter.8,9,20
The setting up of surveillance programmes, such as Zero Bacteraemia,15 has had a significant impact in reducing infectious complications, encouraging increased use of PICCs due to the following major advantages:6,21,22
A rapid and less costly nursing product than central venous catheters that are not peripheral access.
The vascular tree is preserved since it spares patients from repeated needle sticks.
It is less invasive than a conventional CVC and, being peripherally inserted, eliminates the risk of pneumo- and/or haemothorax.
It is a good alternative if there are coagulation problems.
It has the same benefits of any central line: the possibility of multi-lumen usage and administration of drugs such as chemotherapy, TPN, vesicant drugs and large volumes. It also enables haemodynamic monitoring and blood sample extraction.
The general aim of this paper was to assess the rates and nature of PICC-related complications from insertion to removal.
MethodologyDesignProspective observational study.
ScopeThe scope of the study was a secondary level hospital with one polyvalent ICU. A general population of 105,600 people is attended according to the individual health card data of 2015. Nurses insert PICCs, nurses of the unit and those that need to do so on the wards. The study period was from 1 May 2015 to 30 April 2016.
SubjectsAll patients over the age of 14, who had a PICC inserted during their hospital stay were included in the study.
Patients under the age of 14 were not included because critically or semi-critically ill paediatric patients are transferred from our hospital to the paediatric referral hospital. There were no exclusions on the grounds of sex, department or reason for inserting the catheter.
Depending on the care after inserting the catheter, the patients were divided according to care in the unit (ICU group) and care in the ward (non-ICU group).
The sample size was calculated according to an expected 10% incidence of CRI. A minimum sample of 130 patients would result for a 95% confidence and 5% precision level.
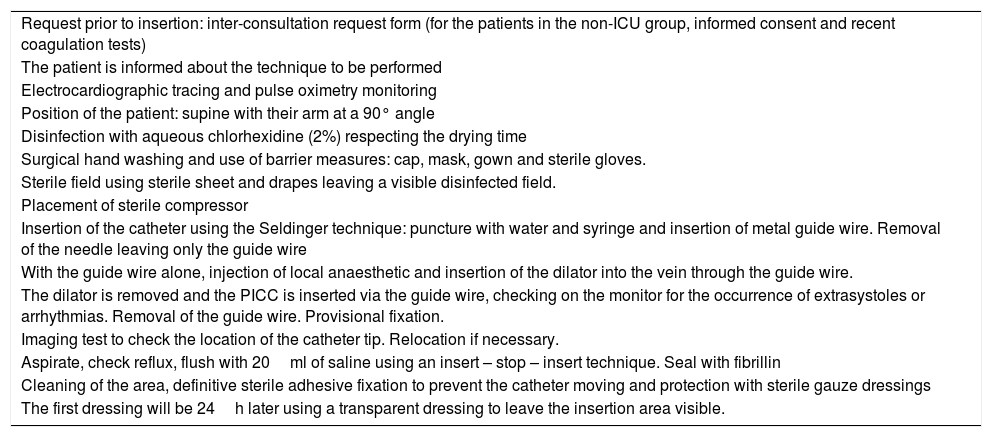
To provide the study with safety and reduce the infectious complications as much as possible, the insertions were carried out following the recommendations of the Zero Bacteraemia project,15 in line with the protocol set out in Table 1.
Protocol for PICC insertion in the unit.
| Request prior to insertion: inter-consultation request form (for the patients in the non-ICU group, informed consent and recent coagulation tests) |
| The patient is informed about the technique to be performed |
| Electrocardiographic tracing and pulse oximetry monitoring |
| Position of the patient: supine with their arm at a 90° angle |
| Disinfection with aqueous chlorhexidine (2%) respecting the drying time |
| Surgical hand washing and use of barrier measures: cap, mask, gown and sterile gloves. |
| Sterile field using sterile sheet and drapes leaving a visible disinfected field. |
| Placement of sterile compressor |
| Insertion of the catheter using the Seldinger technique: puncture with water and syringe and insertion of metal guide wire. Removal of the needle leaving only the guide wire |
| With the guide wire alone, injection of local anaesthetic and insertion of the dilator into the vein through the guide wire. |
| The dilator is removed and the PICC is inserted via the guide wire, checking on the monitor for the occurrence of extrasystoles or arrhythmias. Removal of the guide wire. Provisional fixation. |
| Imaging test to check the location of the catheter tip. Relocation if necessary. |
| Aspirate, check reflux, flush with 20ml of saline using an insert – stop – insert technique. Seal with fibrillin |
| Cleaning of the area, definitive sterile adhesive fixation to prevent the catheter moving and protection with sterile gauze dressings |
| The first dressing will be 24h later using a transparent dressing to leave the insertion area visible. |
PICC: peripherally inserted central catheter; ICU: intensive care unit.
A structured data sheet for the collection of the patients’ data and variables was prepared, compiling the following:
Patient affiliation: age, sex, speciality from which the request came (ICU or not ICU).
Risk factors relating to the patient: coagulopathy, evidenced by prothrombin activity below 50%, activated partial thromboplastin time (APTT) more than 1.5 times the control, and platelet count below 100×109/l, neutropenia, expressed by a neutrophil count below 0.5×109/l, and the need for isolation measures.
Catheter: site (cephalic or basilic), number of needle sticks, relocation after X-ray screening, longevity in days.
Data on the insertion: indication for PICC (attempt at another site, anatomical problems or multi-lumen treatment), preferential use (need for a central line, need for TPN, administration of vesicant and/or vasoactive drugs and haemodynamic monitoring), and reason for removal (end of treatment and/or patient discharge, suspicion of fever, redness and/or exudate from the insertion site or the possibility of catheter-related sepsis – phlebitis, accidental removal, rupture or death).
Complications: acute (insertion-related) such as anomalous migration of the catheter tip after insertion, bleeding and arrythmia; and late (catheter care-related until its removal), phlebitis and thrombosis.
Microbiological data: culture of catheter tip, haemocultures of peripheral blood and results obtained.
Confirmation of the infection differentiating between colonisation of the catheter with the presence of one to 14 colony-forming units (CFU), catheter-related infection with a presence of 15 or more CFU, catheter-related bacteraemia, presence of 15 or more CFU + positive haemocultures with the same germ, according to the manual of definitions and terms of the (ENVIN-HELIS) study.14
Statistical analysisA description was made of the patients included in the study, of their basic epidemiological features and those relating to aspects concerning their admission to hospital.
The complication rates per 1000 catheter-days were calculated over the total number of catheters inserted, specifying the results with 95% confidence intervals.
Significance tests were used to determine the factors relating to the onset of complications, chi-squared tests specifically, and comparisons of means with the Student's t-test.
A significance level of 5% was set for these comparisons.
SPSS® 11.0 was used for the data analysis.
Ethical considerationsThis research study complies with the current legislation on personal data protection and the benchmark regulatory framework for undertaking research projects in Spain and the European Union. The standards of good clinical practice and the ethical principles for research on human beings established in the Declaration of Helsinki and its subsequent revisions were respected at all times.
According to ethical principles, before the data was collected the patients/family members were informed of the objectives of the study, they were invited to participate and they signed their informed consent, where it was explained that the data would be used in aggregated form.
The study protocol was approved by the Hospital's Ethics Committee and Management.
ResultsData was collected on 144 patients who had undergone PICC insertion. The mean age was 65.15 (SD: ±14.58) years, 61.80% (n=89) were male, and 38.20% (n=55) female.
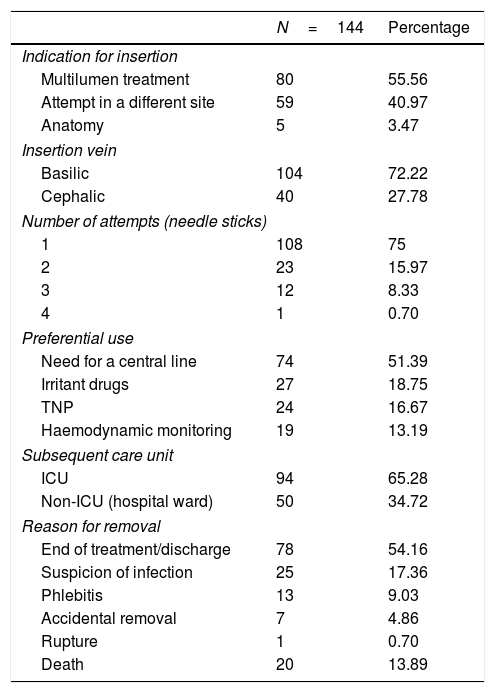
The data on the insertion of the catheter, subsequent care unit and reasons for removal are shown in Table 2.
Data referring to catheter insertion, subsequent care unit and reason for removal.
| N=144 | Percentage | |
|---|---|---|
| Indication for insertion | ||
| Multilumen treatment | 80 | 55.56 |
| Attempt in a different site | 59 | 40.97 |
| Anatomy | 5 | 3.47 |
| Insertion vein | ||
| Basilic | 104 | 72.22 |
| Cephalic | 40 | 27.78 |
| Number of attempts (needle sticks) | ||
| 1 | 108 | 75 |
| 2 | 23 | 15.97 |
| 3 | 12 | 8.33 |
| 4 | 1 | 0.70 |
| Preferential use | ||
| Need for a central line | 74 | 51.39 |
| Irritant drugs | 27 | 18.75 |
| TNP | 24 | 16.67 |
| Haemodynamic monitoring | 19 | 13.19 |
| Subsequent care unit | ||
| ICU | 94 | 65.28 |
| Non-ICU (hospital ward) | 50 | 34.72 |
| Reason for removal | ||
| End of treatment/discharge | 78 | 54.16 |
| Suspicion of infection | 25 | 17.36 |
| Phlebitis | 13 | 9.03 |
| Accidental removal | 7 | 4.86 |
| Rupture | 1 | 0.70 |
| Death | 20 | 13.89 |
TPN: total parenteral nutrition; ICU: intensive care unit.
Taking the results from Table 2 into account that refer to the number of needle sticks, the mean number was 1.35 (SD: ±0.66), and in 75% (n=108) of the cases one attempt was enough.
We were also able to confirm a relationship between the presence of complications and the number of needle sticks required. In the patients who had a complication of any type, the results indicate that they underwent more needle sticks, mean 1.49 (SD: ±0.77), compared to those with no complications, where the mean number of needle sticks was 1.19 (SD: ±0.46). And this difference is statistically significant (p=0.005).
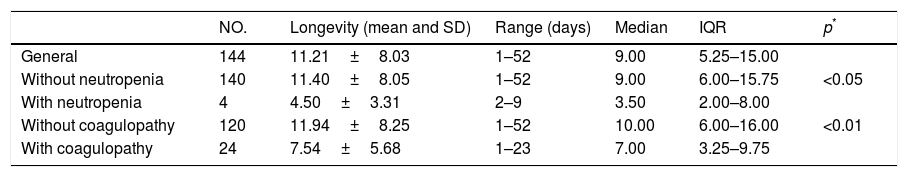
The PICCs were inserted for a total of 1647 days. The median insertion time of the catheter was 9 (CRI: 7–17) days.
Of the patients’ diseases prior to insertion, 4 (2.77%) had neutropenia and 24 (16.67%) impaired coagulation. Table 3 shows the bivariate analysis of the catheter days in the patients with these characteristics.
Longevity of the catheters according to the associated disease.
| NO. | Longevity (mean and SD) | Range (days) | Median | IQR | p* | |
|---|---|---|---|---|---|---|
| General | 144 | 11.21±8.03 | 1–52 | 9.00 | 5.25–15.00 | |
| Without neutropenia | 140 | 11.40±8.05 | 1–52 | 9.00 | 6.00–15.75 | <0.05 |
| With neutropenia | 4 | 4.50±3.31 | 2–9 | 3.50 | 2.00–8.00 | |
| Without coagulopathy | 120 | 11.94±8.25 | 1–52 | 10.00 | 6.00–16.00 | <0.01 |
| With coagulopathy | 24 | 7.54±5.68 | 1–23 | 7.00 | 3.25–9.75 |
SD: standard deviation; IQR: interquartile range.
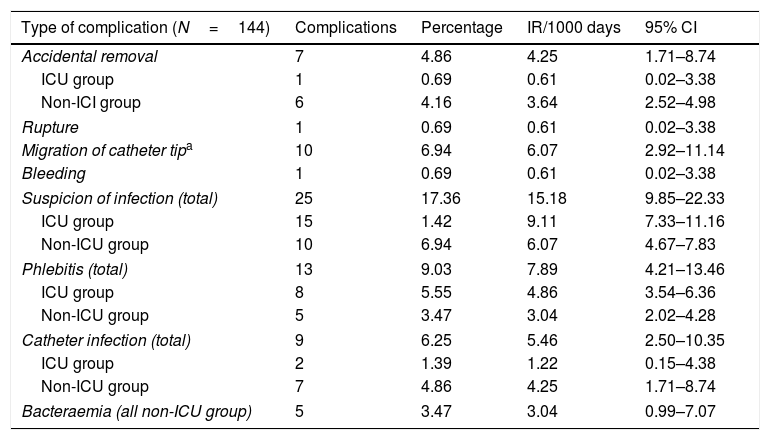
Table 4 shows the complications expressed in percentages and incidence rate per 1000 catheter-days (IR/1000) together with the CI.
Complications expressed in percentages and incidence rates.
| Type of complication (N=144) | Complications | Percentage | IR/1000 days | 95% CI |
|---|---|---|---|---|
| Accidental removal | 7 | 4.86 | 4.25 | 1.71–8.74 |
| ICU group | 1 | 0.69 | 0.61 | 0.02–3.38 |
| Non-ICI group | 6 | 4.16 | 3.64 | 2.52–4.98 |
| Rupture | 1 | 0.69 | 0.61 | 0.02–3.38 |
| Migration of catheter tipa | 10 | 6.94 | 6.07 | 2.92–11.14 |
| Bleeding | 1 | 0.69 | 0.61 | 0.02–3.38 |
| Suspicion of infection (total) | 25 | 17.36 | 15.18 | 9.85–22.33 |
| ICU group | 15 | 1.42 | 9.11 | 7.33–11.16 |
| Non-ICU group | 10 | 6.94 | 6.07 | 4.67–7.83 |
| Phlebitis (total) | 13 | 9.03 | 7.89 | 4.21–13.46 |
| ICU group | 8 | 5.55 | 4.86 | 3.54–6.36 |
| Non-ICU group | 5 | 3.47 | 3.04 | 2.02–4.28 |
| Catheter infection (total) | 9 | 6.25 | 5.46 | 2.50–10.35 |
| ICU group | 2 | 1.39 | 1.22 | 0.15–4.38 |
| Non-ICU group | 7 | 4.86 | 4.25 | 1.71–8.74 |
| Bacteraemia (all non-ICU group) | 5 | 3.47 | 3.04 | 0.99–7.07 |
The incidence rates are calculated per 1000 catheter-days (number of days of total catheter usage: 1647).
CI: 95% confidence interval; IR: incidence rate; ICU: intensive care unit.
There was a total of 9 (6.25%) infections among the inserted catheters, 2 infections were in the ICU group (1.39%), and 7 in the non-ICU group (4.86%); i.e., 22.22% of the PICC infections occurred in patients admitted to ICU, and 77.78% were in the non-ICU group.
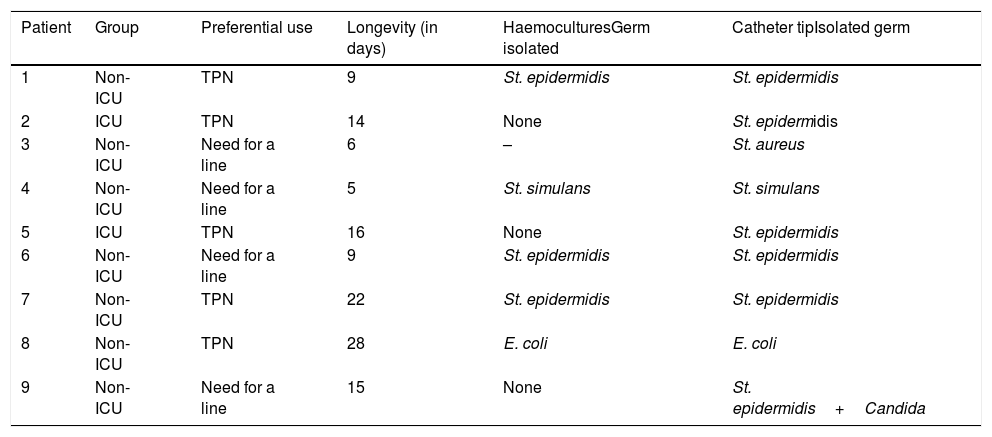
Staphylococcus epidermidis was the most frequent microorganism found in the CRI. Table 5 shows this frequency, and other data of interest relating to the CRI. It is important to highlight that TPN, as the preferential use of this line, was present in 5 (55.56%) of the CRI (n=9) and in 3 (60%) of all the CVCRB (n=5), as well as the difference of infections between the ICU group and the non-ICU group (Table 6).
Catheter-related infection: data referring to the care group, preferential use, longevity of the catheter in days, germ responsible and isolation culture (haemocultures or on the catheter tip).
| Patient | Group | Preferential use | Longevity (in days) | HaemoculturesGerm isolated | Catheter tipIsolated germ |
|---|---|---|---|---|---|
| 1 | Non-ICU | TPN | 9 | St. epidermidis | St. epidermidis |
| 2 | ICU | TPN | 14 | None | St. epidermidis |
| 3 | Non-ICU | Need for a line | 6 | – | St. aureus |
| 4 | Non-ICU | Need for a line | 5 | St. simulans | St. simulans |
| 5 | ICU | TPN | 16 | None | St. epidermidis |
| 6 | Non-ICU | Need for a line | 9 | St. epidermidis | St. epidermidis |
| 7 | Non-ICU | TPN | 22 | St. epidermidis | St. epidermidis |
| 8 | Non-ICU | TPN | 28 | E. coli | E. coli |
| 9 | Non-ICU | Need for a line | 15 | None | St. epidermidis+Candida |
E: Escherichia; TPN: total parenteral nutrition; ICU: intensive care unit; St: Staphylococcus.
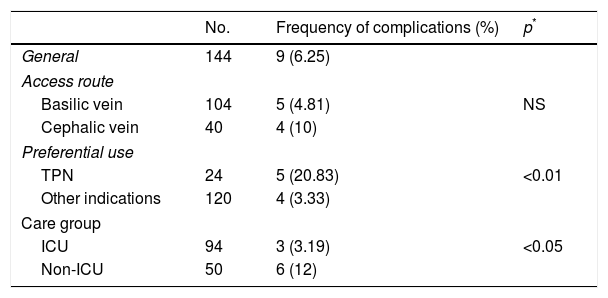
Factors related to the onset of infections.
| No. | Frequency of complications (%) | p* | |
|---|---|---|---|
| General | 144 | 9 (6.25) | |
| Access route | |||
| Basilic vein | 104 | 5 (4.81) | NS |
| Cephalic vein | 40 | 4 (10) | |
| Preferential use | |||
| TPN | 24 | 5 (20.83) | <0.01 |
| Other indications | 120 | 4 (3.33) | |
| Care group | |||
| ICU | 94 | 3 (3.19) | <0.05 |
| Non-ICU | 50 | 6 (12) | |
TPN: total parenteral nutrition; NS: not significant; ICU: intensive care unit.
Other factors were studied, relating to the onset of infections, where the subgroups created were so small in size that their comparison was not statistically significant, such as the CRI between patients with or without neutropenia or between catheters that needed to be relocated due to poor initial positioning of the catheter tip compared to those who did not require this. One of the 4 neutropenic patients had an infection (25%), whereas 8 (5.7%) (p=0.1) of the 140 who were not neutropenic became infected. Of the 10 patients who required re-siting of their catheters, one had an infection (10%), whereas of the 134 who did not require re-siting, 8 (5.9%) became infected. These differences in percentages, although obvious, did not reach the statistical significance threshold.
DiscussionNurses who care for critical patients are often faced with problems in administering parenteral treatments. The aggressiveness of some routine drugs such as vasoactive amines, antibiotics, antiarrhythmic medications such as amiodarone, etc., often necessitate the insertion of more than one non-central peripheral venous catheter, which increases the risk of phlebitis, extravasation and complications.23
Classic or conventional central venous catheterisation (CCVC) has been widely implemented in hospitals for many years and is still associated with complications that can be life-threatening for patients, prolonged hospital stays and increased health costs.24,25 The PICC is a great alternative to the CCVC. A Spanish study conducted by Gómez-Palomar and Miquel Pérez26 published in 2009 comparing the two catheterisation systems, concluded that PICCs could be recommended to trained nurses because, with a fully aseptic insertion technique and acquired skill, it is just as valid as the CCVC, with minimal risks in insertion, and with no increase in complications through its use. Other studies have shown that PICCs reduce physician workload and costs.27,28 These advantages, together with the fact that the current multilumen PICCs enable the simultaneous administration of various treatments, central venous pressure (CVP) monitoring, and laboratory tests, make them an ideal choice for patients who require a central line.
Phlebitis as a cause of catheter removal, which we study independently in this paper, and not as suspected infection, to determine its real incidence, remains a common hospital-acquired disorder that is infection-related, and of multifactorial aetiology. Its incidence, irrespective of the use of protocols, ranges from 15.25% to 2%.23,29 It can present as a single entity or complicate with thrombosis.30 With the PICC, it is most usually mechanical, related with the catheter insertion site, onset tends to be in the first 10 days following placement, and is related to the obstruction caused to blood flow (most likely when the PICC exceeds 50% of the venous lumen).31 Depending on the inflammation of the vein and, according to the VIP Score,32,33 it can present with or without clinical symptoms. Ultrasound, an easy-to-use technique, serves to reduce the complications relating to insertion of the PICC, and to assess the presence of thrombosis.34–36
Adding suspected infection and phlebitis has led to, as a reason for removal, a similar result to that of Seisdedos Elcuaz et al.37 This result can be striking when compared with confirmed infections, but a series of studies have shown that a major percentage of catheters removed due to suspected infection have no association with the catheter.38,39 Bouza et al.,38 in a population of patients without neutropenia, found 204 cases that were likely to be related to CVCRB, but only 28 (14%) were really associated with the catheter, 27 (13%) had no association with the catheter, 36 (18%) had been colonised with negative haemocultures, and the remaining cases were negative. Ferrer and Almirante,18 remind us that it is necessary to document and confirm a catheter-related infection before the catheter is removed. For Rijnders et al.,40 the exclusive presence of fever in patients with a low catheter-related infection risk, such as immunocompetent individuals, with no intravascular foreign body, no evidence of infection at the insertion site, or severe sepsis or septic shock, bacteraemia or fungaemia, would not necessitate removal of the catheter, and the microbiological results could be awaited. For these same authors,40 the presence of a state of haemodynamic shock or instability should make early removal of the catheter a priority. However, in some circumstances, the risks of immediate catheter removal (for example, coagulopathy with a risk of bleeding diathesis or difficulty in replacing the catheter), doctors should assess the risk-benefit relationship. We believe that this same assessment criterion should apply in the presence of local signs of infection with fever and without accompanying bacteraemia.
Although among the causes for catheter removal there was only one case of PICC rupture, caused when contrast was injected to perform computerised axial tomography (CAT), it is important to remember that this examination requires liquids above 5ml/s and not all PICCs are prepared for this speed. The recent development of power-injectable PICCs has managed to overcome these technical limitations. Even with these catheters we recommend, before injecting contrast, that the maximum infusion speed and which of the lumens is enabled for this indication are always checked. Accidental removal found in this study was lower than that found by Grau et al. (8.9%).41 It is possible that the difference is because all the patients our study were hospitalised, and those of the abovementioned study were both inpatients and outpatients.
Safdar and Maki,42 in a similar study undertaken with the insertion of 215 PICC, found that the mean longevity of the PICC was 11.3±19.8 days, a very similar figure to the general mean found in this study. The study also shows that there was a significant difference when the duration was compared between patients with and without neutropenia and with or without coagulopathy, this result was logical as these were seriously ill patients with underlying diseases that made them more vulnerable to infections and complications.43
Although it has been theorised that PICCs have supposedly higher safety and lower risk of infection than the non-tunnelled CVC because the skin of the upper middle part of the forearm has a lower temperature and bacteria density than other venous catheter insertion sites,27,44 controlled trials that compare both types of catheter have concluded that the PICCs used in high-risk hospitalised patients are associated with a similar rate CVCRB to CCVC implanted in the subclavian or internal jugular veins (2–5 episodes per 1000 catheter days), a longer mean longevity and a lower incidence of thrombosis.22,42,45 The IR/1000 of infection and CVCRB of the results are in line with the abovementioned literature references.
The initial limitations of PICCs: low flows, difficult CVP monitoring, lack of safety for radio diagnostic procedures, single lumen, etc., prevailed over their abovementioned supposed theoretical advantages and delayed the implementation of these devices as common practice in ICU. Today nobody doubts that PICCs are easy to insert and safely implanted in the peripheral veins of the forearm, the advantages of a central location for a catheter tip suitable for all types of solutions regardless of osmolarity and pH, and that they are much more appropriate for patients with impaired coagulation since, because they enable compression and there is less insertion trauma, the risk of bleeding is reduced.21,22
Although major advances have been made in the prevention of CVCRB, these infections continue to occur with estimated rates from 1.3 per 1000 catheter-days on inpatient wards to 5.6 per 1000 catheter-days in intensive care burns units.46,47 In Spain the annual ENVIN-HELICS studies have witnessed these advances over the past 24 years.14,16
The results of this study show the difference in infections found in the ICU group and the non-ICU group, and indicate the need to improve maintenance care, especially in the non-ICU group. This is confirmed by our finding that all the CVCRB occurred in the non-ICU group.
Many publications show that the incidence rate of CVCRB is higher in ICU than on the hospital wards.34,43,45,46 There is an interesting study by Ajenjo et al.43 on a total of 163 PICC blood stream infections (BSIs) (which presented in 162 patients) with interesting results and conclusions. The use of PICCs was much higher in the ICU group than in the non-ICU group (0.109 compared to 0.059 catheter/days per patient-day). A total 3.13 BSIs per 1000 catheter-days was found, and a greater CVCRB IR/1000 was observed in the ICU group compared to the non-ICU group (4.79 vs. 2.78 days). This result is logical and in line with these incidence rates being higher in the ICU group since these are seriously ill patients, who could have underlying diseases and other risk factors such as multiple catheters, more frequent catheter access, continuous IV infusions, etc., which make them more vulnerable to infection. But analysis of these results shows another interesting result and conclusion: of the162 CVCRB, 44 (27%) were in the ICU group and the remainder (73%) in the non-ICU. In other words, the infection rates were considerably higher in the patients of the ICU group than those of the non-ICU group, a similar figure to that obtained in our study. The findings of this study in relation to the infections found and the evolution of the ENVIN-HELICS studies14,16 show that the rigorous application of the Zero Bacteraemia protocol15 for catheter insertion and maintenance have had a significant impact in reducing CVCRB rates in ICU, but highlight that PICC care on the hospital wards is inadequate. In short, multidisciplinary strategies are required to prevent and control catheter maintenance-related infections, especially when they are to be cared for in units other than ICU.
In line with the literature reviewed, the staphylococci, particularly the coagulase-negative strains such as S. epidermidis and Staphylococcus simulans, and to a lesser extent Staphylococcus aureus, are the most commonly found aetiological agents, followed by enteric gram-negative bacilli and Candida species.1,18,37,47–49S. epidermidis is a habitual skin microorganism and is particularly able to colonise vascular catheters on secreting an adherent substance that facilitates its bonding to plastic surfaces and form a biofilm that protects it from the host's defence mechanisms and helps it to multiply.50Escherichia coli grew in a patient with TPN who was subsequently diagnosed and operated for an intra-abdominal abscess. This germ is usually found in patients with intestinal diseases, under treatment with TPN and causes recurrent infections.8,9,20
Administration of TPN via the PICC, as with any other CCVC, carries an increased risk of bacteraemia and/or local infection,48,51 and the results of this study are in line with those of the previous literature.
This study has its strengths and its limitations. Its strengths are, on the one hand, that it was possible to compare maintenance care and its association with infection, once all the PICC had been inserted in the ICU, between the ICU group and the non-ICU group, and on the other, in the way the complications presented. Except for bacteraemia, the incidences tend to be expressed in proportion to the number of complications found over the total number of PICC inserted, rather than in rates per 1000 catheter-days. We believe, like other authors,34,52 that when incidences are expressed in percentages the lifespan of the catheters is not considered. It could happen that 2 studies present phlebitis in 3% of 100 PICC inserted, and yet the rates are 4.3/catheter-days if they last for 7 days, and 0.3/catheter days if they last for 90 days.
The study's major limitation is not having routinely discounted, by ultrasound or even contrast venography, the possibility of thrombosis-complicated phlebitis, although the cases of phlebitis that presented in the patients were all asymptomatic. This complication is frequently cited in the specialist literature and is associated with patients with major risk factors.12,43,53–56 Another limitation is that because the study was undertaken in a hospital where there is frequent rotation of nursing staff, it might not necessarily be possible to extrapolate the results to other settings.
The ever-present worry that problems with infections and the insertion of both peripheral venous and central venous catheters6,57 ensures that there will be future research studies to continue to reduce their incidence. The data obtained in this study will serve as a basis to identify aspects for improvement, establish strategies by creating working groups with the departments involved to draw up and disseminate a consensus protocol and then compare the results of applying the protocol, with reference fundamentally to the number of complications, especially infections, with the results that are presented.
ConclusionsThe PICC is an efficient device as central venous access due to the minimal incidence of implantation risks, CVCRB rates do not increase in high-risk inpatients when compared with the CCVC, due to its advantages over the latter, including its longer lifespan and lower thrombosis rate.
The PICC, considered the great alternative to the CCVC, has become another nursing procedure. The high likelihood of success is ensured when it is inserted by trained nurses.
According to the evidence we obtained with this study, it seems essential to raise the awareness of health professionals on the risk of infections related to these catheters, through organising multidisciplinary training throughout the hospital. This will result in improved insertion and maintenance procedures, infection prevention, patient safety, and ultimately health costs will be reduced.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the nursing team of the Intensive Care Unit of Huesca's Hospital General San Jorge, Teo Lorente for his help with the statistical analysis and Visi Ortega in helping with the references.
Please cite this article as: Lacostena-Pérez ME, Buesa-Escar AM, Gil-Alós AM. Complicaciones relacionadas con la inserción y el mantenimiento del catéter venoso central de acceso periférico. Enferm Intensiva. 2019. https://doi.org/10.1016/j.enfi.2018.05.002