The results of research form the body of knowledge of any scientific discipline, either to describe areas that are seldom described or to strengthen already known theories. Research is based on the use of the scientific method, formed by a systematic process for obtaining data that allows us to answer a relevant and specific research question. However, this first part is one of the most relevant for the construction of clinical research, being of varying complexity, depending on the skills and knowledge of the researchers. The approach to the research question will be adapted according to its methodological characteristics, whether qualitative or quantitative. Quantitative research enables a response to the study of the association or relationship between quantified variables, determining the strength of this relationship, its inference to a population, and even making causal inferences to explain why a phenomenon does or does not occur in a particular way.
The research project (RP) is a document that includes the proposal of the research team, expressing in writing all the core elements of the planning and design of the research study to be undertaken.1 The purpose of drafting a RP is1:
- •
To guide and orient all the members of the research team in executing the study.
- •
To standardise the tasks, activities and actions undertaken by members of the research team.
- •
To request funding from agencies or institutions.
- •
To request permission and authorisation to conduct the study from the clinical research ethics committees or institutions where the study is to be, carried out.
- •
To guide the preparation of reports and other dissemination documents (articles, communications to congress, theses, etc.).
In short, the RP, in addition to guiding the researchers in executing the study, constitutes the letter of presentation of our research proposal to agencies and institutions. Therefore, its drafting must be agreed by all members of the research team under quality standards. Institutions and agencies usually have standard forms for writing an RP. Below we propose an RP structure (Table 1) and what each section should include, as a guide to drafting the project.
Structure of a quantitative research project.
| 1. Title |
| 2. Authors |
| 3. Abstract |
| 4. Keywords |
| 5. Background and current status of the subject |
| 6. Justification for the study |
| 7. References |
| 8. Hypothesis |
| 9. Objectives |
| 10. Methodology |
| -Study design |
| -Reference and study population |
| -Inclusion and exclusion criteria |
| -Sample size and sampling procedure |
| -Variables (dependent and independent) |
| -Data collection and information sources |
| -Data analysis |
| 11. Ethical considerations |
| 12. Difficulties and limitations of the study |
| 13. Work plan |
| 14. Experience of the research team on the subject |
| 15. Applicability and practical utility of the results |
| 16. Means available to undertake the project |
| 17. Justification for grant requested |
| 18. Budget requested |
| 19. Annexes |
- 1
Title
The title should be explanatory, short (no more than 15 words), clear (avoid using acronyms) and precise. It should include: the type of study to be conducted, the population, and if possible, the scope.2–4
- 2
Authors
The author of the RP is considered the person who has made substantial intellectual contributions to the project and is therefore responsible for all aspects of the project, ensuring reliability and integrity in its execution.4 A distinction will be made between the principal investigator (as the project leader) and the other members of the research team (in order of intellectual contribution to the project), including the academic degree and affiliation of each team member.2 The composition of the research team is defined by the needs of the study itself. Funding agencies and clinical research ethics committees usually require the curriculum vitae of each member, usually in the standard format of the Spanish Foundation for Science and Technology (FECYT) (https://cvn.fecyt.es/), or provide a form to that effect.
- 3
Abstract
This is a structured outline, between 200 and 400 words, which reflects the essential aspects of the research to be carried out.1–3 It is advisable to include:
- •
Objectives: what it is intended should be known after the study has been completed.
- •
Methodology: including the design, scope, subjects to be studied, techniques, procedures, methods and tests that will be used to determine the main variables to be studied.
- •
Interest for practice and strategic impact.
It is advisable to write the summary once the final version of the RP has been drafted, to ensure that it is as reliable as possible.1 Assessment agencies usually indicate that an English version of the abstract should be included.3
- 4
Keywords
These are terms that represent key concepts of the RP. It is advisable to use health science descriptors; DeCS (Descriptores en Ciencias de la Salud http://decs.bvs.br/E/homepagee.htm) and MeSH (Medical Subject Headings of the U.S. National Library of Medicine https://www.ncbi.nlm.nih.gov/mesh).1,3 It is advisable to include the terms used for the formulation of the problem under study.
- 5
Background and current status of the subject
The problem to be studied must be stated in this section, indicating its magnitude (macro and micro epidemiology), the contributions of previous research, emphasising what is known and what aspects remain to be analysed in the literature, being explicit about the need to address the problem under study. It is essential in this section to indicate the theoretical or conceptual framework in which the problem is framed, and the theoretical approaches used by other researchers to solve it, guiding the relationships that already exist after previous research. This section should guide the justification for undertaking the RP, and therefore the objectives of the project.1,2,4,5
- 6
Justification for the study
After the research question status, a description will be given as to why it is necessary to undertake the RP: what contribution will be made to existing knowledge and its relevance, indicating the inference of its results to the general population, possible applications and consequences of the knowledge it will generate, its contribution to theory and its potential to improve practice.3,5,6
- 7
References
The background and current status of the subject, as well as the methodology should be supported by a synthesis of relevant, current references (preferably from the last 5 years, unless there is a fundamental reference on this subject that is older) after a critical analysis. The use of literature reference managers is recommended for drafting and it should be adapted to the style required by the agency or institution to which it is to be presented.
- 8
Hypothesis
The hypothesis will guide the analysis of the data. It must be based on a well-established theoretical framework.3 It is a provisional statement expressing the probable relationship between 2 or more variables, usually a cause and an assumed effect, which can be verified empirically.7 The hypothesis statement should include the research variables, and the relationship between the variables and the population under study. After contrasting the explanatory hypothesis, there are 2 possibilities: the hypothesis is confirmed, or the hypothesis must be refuted.7 In descriptive studies, no hypotheses of causality are formulated.3
- 9
Objectives
An objective has to be3,7:
- •
Measurable, observable, achievable: this means that they must be expressed so as to allow the qualities or characteristics of the object of investigation to be measured.
- •
Specific: expressed briefly and in simple language (without ambiguity).
- •
Logical and pertinent: they must be attainable and consistent with the study hypothesis, providing an answer to it.
A single general objective is usually formulated, headed by a verb in the infinitive that specifically indicates measurement (e.g.: determine, compare, analyse, etc.). Like the hypothesis, it must include the main variable under study and the population. These elements will determine the design of the study.
Specific objectives should only be included if they cover detailed actions that are not included in the general objective. Between 4 and 6 should be drafted and put in chronological order or in ascending order of complexity.3
- 10
Methodology
The procedure to be followed to achieve the proposed objectives and thus be able to confirm or otherwise the proposed hypothesis6 is included in this section. It includes different aspects:
- •
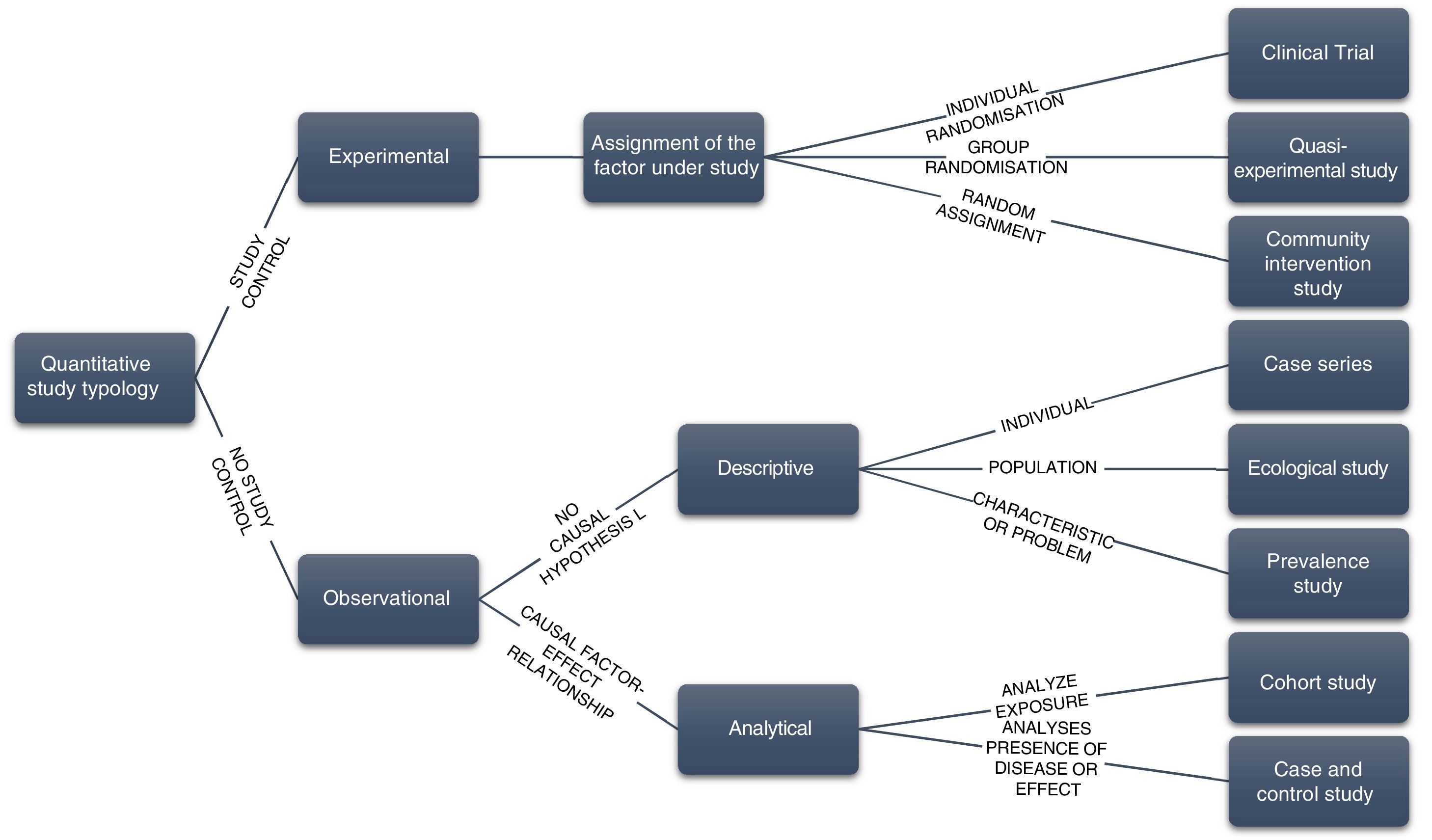
Study design. This will be adapted to the problem or research question and must be described clearly and concisely (Table 2 and Fig. 1). Depending on the object of study, a mixed design may be proposed although in general it is not advisable to include different designs in the same RP.
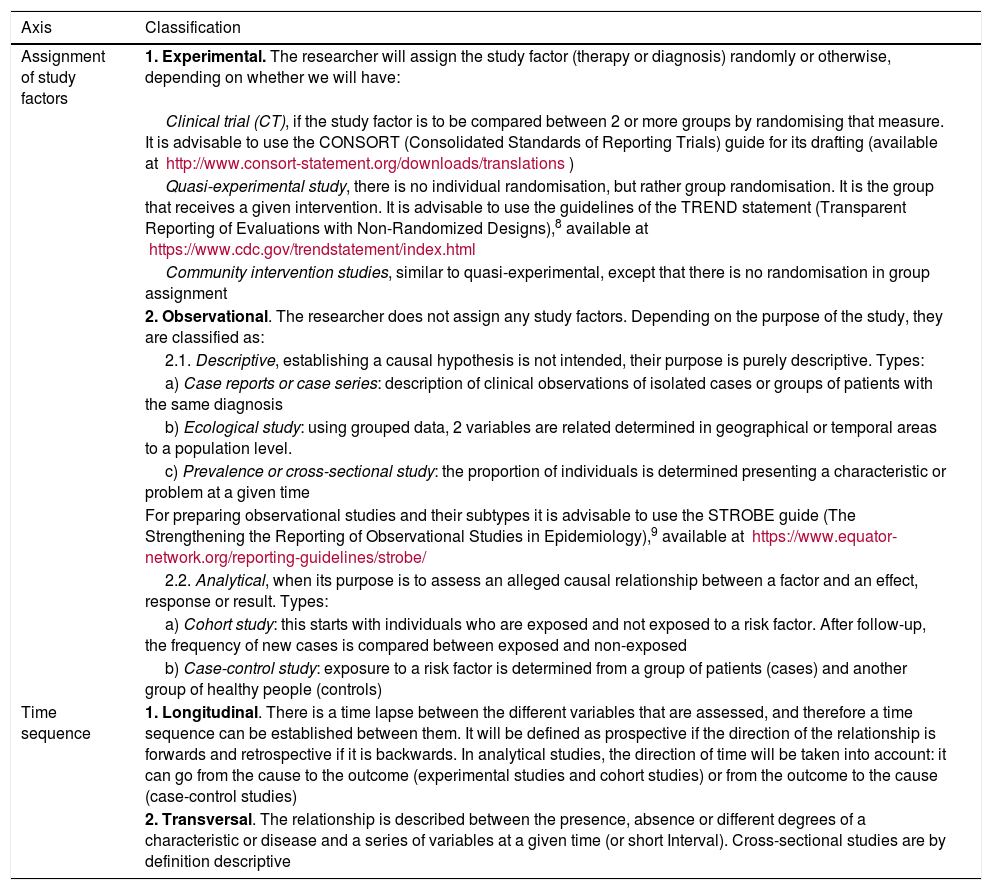
Table 2.Classification of types of quantitative epidemiological studies.
Axis Classification Assignment of study factors 1. Experimental. The researcher will assign the study factor (therapy or diagnosis) randomly or otherwise, depending on whether we will have: Clinical trial (CT), if the study factor is to be compared between 2 or more groups by randomising that measure. It is advisable to use the CONSORT (Consolidated Standards of Reporting Trials) guide for its drafting (available at http://www.consort-statement.org/downloads/translations) Quasi-experimental study, there is no individual randomisation, but rather group randomisation. It is the group that receives a given intervention. It is advisable to use the guidelines of the TREND statement (Transparent Reporting of Evaluations with Non-Randomized Designs),8 available at https://www.cdc.gov/trendstatement/index.html Community intervention studies, similar to quasi-experimental, except that there is no randomisation in group assignment 2. Observational. The researcher does not assign any study factors. Depending on the purpose of the study, they are classified as: 2.1. Descriptive, establishing a causal hypothesis is not intended, their purpose is purely descriptive. Types: a) Case reports or case series: description of clinical observations of isolated cases or groups of patients with the same diagnosis b) Ecological study: using grouped data, 2 variables are related determined in geographical or temporal areas to a population level. c) Prevalence or cross-sectional study: the proportion of individuals is determined presenting a characteristic or problem at a given time For preparing observational studies and their subtypes it is advisable to use the STROBE guide (The Strengthening the Reporting of Observational Studies in Epidemiology),9 available at https://www.equator-network.org/reporting-guidelines/strobe/ 2.2. Analytical, when its purpose is to assess an alleged causal relationship between a factor and an effect, response or result. Types: a) Cohort study: this starts with individuals who are exposed and not exposed to a risk factor. After follow-up, the frequency of new cases is compared between exposed and non-exposed b) Case-control study: exposure to a risk factor is determined from a group of patients (cases) and another group of healthy people (controls) Time sequence 1. Longitudinal. There is a time lapse between the different variables that are assessed, and therefore a time sequence can be established between them. It will be defined as prospective if the direction of the relationship is forwards and retrospective if it is backwards. In analytical studies, the direction of time will be taken into account: it can go from the cause to the outcome (experimental studies and cohort studies) or from the outcome to the cause (case-control studies) 2. Transversal. The relationship is described between the presence, absence or different degrees of a characteristic or disease and a series of variables at a given time (or short Interval). Cross-sectional studies are by definition descriptive Figure 1.Diagram of the different types of quantitative epidemiological studies according to the investigator’s control of the factor under study.3,4,7.
- •
Reference and study population. The reference population is the one in which we want to generalise the results obtained in our study, defining its characteristics (sociodemographic, clinical, exposure), which may be individuals, groups or objects susceptible to study. The study population is the subset of the reference population that we intend to study. It is defined by previously established selection criteria and has certain geographical and temporal characteristics that make it accessible to researchers. The sample will comprise the set of individuals eventually studied.4
- •
Inclusion and exclusion criteria. These must be established in realistic terms that allow the inclusion of the desired number of subjects in the expected time. The inclusion criteria describe the characteristics that the study participants must have. The exclusion criteria describe the characteristics that imply a participant not being included in the study. They usually refer to sociodemographic characteristics of the disease or exposure, accessibility or other.3,4
- •
Sample size and sampling procedure. Sample size refers to the number of patients needed to achieve the study objectives, either to be able to estimate a specific parameter with the desired level of confidence, or to be able to perform a hypothesis contrast (detect a particular difference between the study groups, if one exists). The premises used for its calculation should be specified: prevalence values, incidence or means, standard deviations and types of errors. If this is not possible to calculate this, it can be described on the basis of the population number that can be included in the study period. If it is not possible to include a minimum number of cases, it is advisable to conduct a multicentre study. The sampling procedure should be described, indicating whether case selection will be by random, systematic, consecutive or other types of sampling. If the proposed design is a randomised clinical trial, how subjects will be assigned to the different study groups will be specified.3,4
- •
Variables (dependent and independent). In this section, the variables should be described so as to avoid ambiguities, indicating in the categorical variables all the possible measurement categories, and in the numerical variables the scales and units of measurement. A distinction should be made between independent variables (exposure factors, confounding factors, etc.) and dependent variables (response, result or outcome variables).3,4,7
- •
Data collection and information sources. Information sources, instruments and measurement procedures should be described (how measurements are to be carried out on the subjects, by whom, where and when).
- •
Data analysis. The statistical analysis strategy will be indicated in this section: descriptive (frequencies and percentages for qualitative variables, and means, standard deviations, maximum and minimum values for quantitative measurements), bivariate (techniques used to check relationships between 2 variables, an independent and a dependent variable) or multivariate (when seeking simultaneous relationships between a dependent variable and several independent variables).3,4
- 11
Ethical considerations
The following aspects should be made explicit, in accordance with current legislation7:
- 1)
The known benefits and risks for the subjects participating in the study.
- 2)
The precise description of the information to be provided to the participating subjects and when it will be communicated orally or in writing
- 3)
How the confidentiality of the participants’ information will be preserved.
- 4)
The requirement for informed consent.
- 12
Difficulties and limitations of the study
The problems that could arise when carrying out the research will be outlined, also explaining the mechanisms that the researchers will use to ensure the quality of the project, and/or justify how far these mechanisms do not have to modify what is to be measured or highlight the fact that it is inevitable that they occur.3,4
- 13
Work plan
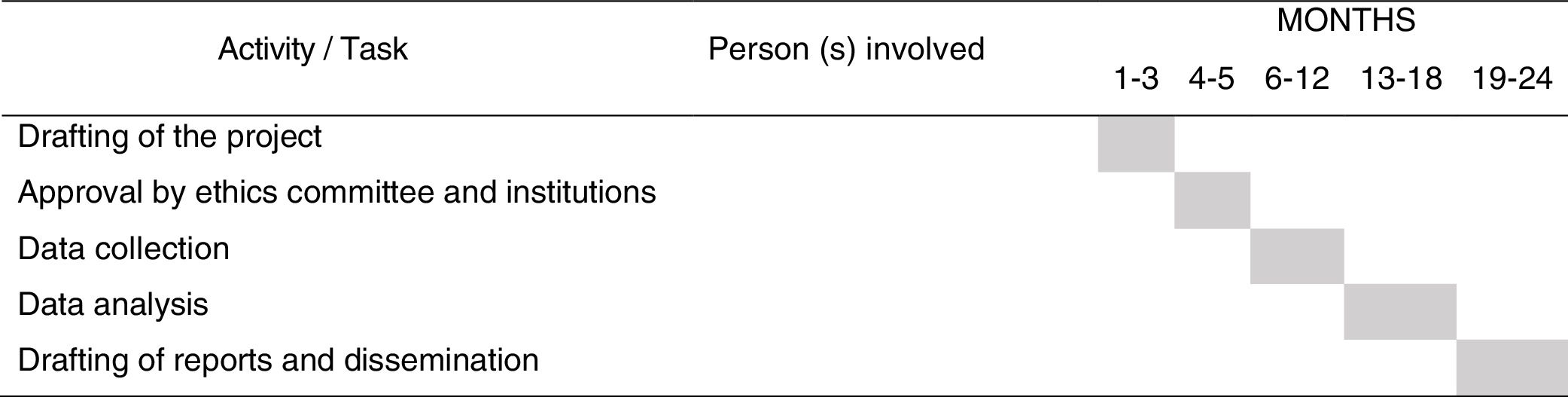
The detailed RP implementation plan will be outlined, by preparing a schedule, with the distribution of tasks for each of the participating researchers and time period (Fig. 2).
- 14
Experience of the research team in the study area
The experience of the researchers in the subject must be highlighted in this section, indicating the lines of research on which the project applicants have worked within the subject to be investigated, since it supports the feasibility of the project and is a strength when it comes to being awarded funding in competitive bids. If it is a study that is not within the line of research of the team members, other strengths can be expressed, for example: previous experience of the team members participating in other studies, clinical experience in the subject under study, previous lines of research related to the current subject, etc., and created for the project. It is not necessary to describe in detail the research curriculum since normally the complete curriculum of each member of the team that comprises it is attached to the RP.
- 15
Applicability and practical usefulness of the results
This section is of vital importance, as it specifies the relevance of the research and the extent of its applicability. This section will also include the impact of the project (at clinical, economic and social levels), its capacity for transfer and innovation, and alignment with institutional strategic lines.
- 16
Available means to undertake the project
Both the material and the human resources available for which funding will not be required will be detailed (multiparametric monitors, computers, digital library, etc.).10
- 17
Justification of the support requested
The necessary resources that are not available for implementing the RP will be indicated and their detailed justification.
- 18
Budget requested
This is usually represented using a table with the breakdown of the budget into items3,7:
- •
Personnel expenses: salary of scholarship holders, research or administrative staff.
- •
Implementation costs: inventoriable material (infrastructure and equipment), consumables (stationery, etc.).
- •
Contracting of services: statistician, translator and publications costs.
- •
Travel/expenses: travel, registration and daily allowance for meetings, conferences or scientific congresses.
- 19
Annexes
This section will include the documents that complement the RP in a relevant way: data collection notebook, measuring instruments, ethics committee approval, etc.
ConclusionsThis guide to the core elements of an RP is intended to guide scientific writing for new and emerging researchers. Thus, the complete drafting of these elements can help to improve the scientific quality of the entire content and be adapted according to the call for competitive bids to which the RP request is presented.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Cobo-Sánchez JL, Blanco-Mavillard I. Elementos nucleares para la elaboración de un proyecto de investigación con metodología cuantitativa. Enferm Intensiva. 2020;31:35–40.