This study arose from the need to improve all tasks related to monitoring pain in post cardiac surgery patients.
ObjectivesChecking and quantifying the pain suffered by patients undergoing cardiac surgery in the first 24 h of their stay in the intensive care unit (ICU), treated with Dexmedetomidine (Dex) as analgesic adjuvant, as well as their degree of sedation and the need for opiates such as rescue analgesia.
Material and MethodsUnicentric study, observational, descriptive, from April 2016 to September 2017. Both genders, all adult, undergoing cardiac surgery, operating theatre-extubated and Dex continuous infusion carriers. Pain degree level was evaluated by the Visual analogue Scale of Pain (VAS), from immediately post-surgery until 24 h from ICU entry and sedation degree, by the Richmond Sedation -Agitation Scale (RASS), only while the Dex infusion lasted.
Results109 patients were included. The results obtained showed that the average pain suffered by patients during the first 24 h, was 0.47 with standard deviation (SD) of 1.25; the average maximum pain experienced was 3.58 with a range of 0–8 and the RASS average was -0.68 (SD: 080). In addition, 44.04% of the patients needed rescue opiates, although only 7.32% showed severe pain greater than 6.
ConclusionsThe Dex infusion patients showed mild levels of pain, however, a small percentage, who must be taken into account, suffered severe pain.
El estudio surgió de la necesidad de mejorar el control del dolor en los pacientes postoperados de cirugía cardiaca.
ObjetivosConocer el grado de dolor que experimentan los pacientes sometidos a cirugía cardiaca en las primeras 24 horas de estancia en la unidad de cuidados intensivos (UCI) con perfusión continua de dexmedetomidina (Dex) como coadyuvante analgésico, así como el grado de sedación y la necesidad de opiáceos como analgesia de rescate.
Material y métodosEstudio unicéntrico, observacional, descriptivo, de Abril de 2016 a Septiembre de 2017. Pacientes mayores de 18 años sometidos a cirugía cardiaca, extubados en sala quirúrgica y portadores de perfusión continua de Dex. Se evaluó el grado de dolor mediante la escala visual analógica (EVA), durante las primeras 24 horas del postoperatorio inmediato desde el ingreso en la UCI y la sedación, mediante la escala de sedación y agitación de Richmond (RASS), tan solo mientras durara la perfusión de Dex.
ResultadosSe incluyeron 109 pacientes. Los resultados obtenidos fueron que la media de dolor experimentado durante las primeras 24 horas fue de 0,47 con una desviación típica (DT) de 1,25 la media del dolor máximo experimentado fue de 3,58 con un rango de 0 a 8 y el RASS medio fue de -0,68 (DT:0,80). Un 44,04% de los pacientes precisaron opiáceos de rescate, aunque solo un 7,32% de ellos presentaba un dolor severo mayor de 6.
ConclusionesLos pacientes con perfusión de Dex presentan un grado de dolor leve aunque un pequeño porcentaje, no por ello despreciable, tienen un dolor severo.
Cardiovascular surgery is a medical speciality dealing with the disorders and diseases of the cardiocirculatory system which require surgical treatment. This surgical intervention is an aggressive procedure for the patient, which involves pain. Postoperative pain is acute in its nature and the aim of the nurse is to reduce or eliminate the pain and the patient’s sensation of malaise.
What does this paper contribute?This is the first study conducted in Spain which observes and describes pain management in the immediate postoperative period following heart surgery in extubated patients and with a perfusion of dexmedetomidine. Results show that dexmedetomidine provides good sedoanalgesia for pain management, with pain being minimal.
Study implicationsThe study describes and shows that postoperative extubated heart surgery patients who receive a perfusion of dexmedetomidine perceive of a mild level of pain. Studies like this one may lead to an improvement in the use of opiates with a reduction in their administration.
Heart surgery has experienced a spectacular development over the last 10 years, and therefore offers better outcomes, thanks to several factors such as: improvements and consolidation of diagnostic techniques (heart catheterisation); perfectioning of surgical technique (training of surgical teams); design and manufacture of prosthetic valves; improvements in extracorporeal circulation (ECC) and lastly, the quality of postoperative care in both resources and personnel. The medical and the nursing bedside care teams spend the most time with the patient after surgery.1
According to the International Association for the Study of Pain (IASP), pain is defined as “an unpleasant sensory and emotional experience, associated with actual or potential tissue damage, or described in terms of such damage.” 2
Postoperative pain is an acute pain which presents as a result of surgical aggression. It has its own characteristics which differentiate it from chronic pain, because it is predictable and self-limiting over time. The aim of postoperative pain treatment is to reduce or eliminate pain and the patient’s sensation of malaise, with minimum secondary or adverse effects, with the most economical methods posible.3
Defective or inadequate pain control during the postoperative period may lead to complications in breathing function, with impaired ventilator mechanics, often the cause of collapsed lung, which is a highly common complication in postoperative heart surgery patients. 4–6
Circulatory complications caused by pain, together with respiratory complications are one of the most important causes of postoperative morbimortality. We also find there are complications at metabolic, endocrine, inflammatory levels and on haemostasis.4–6
Up until 2015, heart surgery patients from the Centro Médico Teknon Quirón-Salud (CMT) were admitted to the intensive care unit (ICU) intubated, and extubation was performed from 10−16 hours following admittance to the unit. Sedation was normally given with a perfusion of protpofol, almost always combined with a perfusion of fentanyl or morphine derivatives. The patient also required conventional analgesia to combat post-extubation pain. This led to late initiation of tolerance and mobilisation which could result in complications such as venous thrombosis and pulmonary thromboembolisms, characteristics of immobilization, and an increase in morbidity derived from the complications of prolonged intubation, such as infections, anxiety and delirium and the use of opioids. The most severe of these is respiratory depression.4,7–12
Since 2015, in the CMT, the fastrack technique has been used on heart surgery patients, which essentially guarantees a short postoperative recovery time and with it a reduction in hospital stay and costs. It is centred upon rapid surgery extubation (from 1 to 6 h after surgery), even directly in the operating theatre with no remarkable morbidity presenting.13 In our case almost all patients are extubated in the operating theatre. In the majority of cases the patients are given a continuous intravenous perfusion of dexmedetomidine (Dex), in addition to the prescribed standard analgesia and an elastomeric pump of ropivacaine at local sternotomy level. Rescue analgesia is also prescribed from admission to the ICU—it is of note that in the CMT there is no analgesia protocol for this type of surgery—,and restricted liquid management to thereby ensure appropriate mechanical ventilatory support during the postoperative period and early mobilisation of the patient. This leads to a shorter ICU and hospital stay.5,13 This advance may be measured by the fact that the patient who has been operated on for heart surgery is no longer than 2 or 3 days in the ICU, and is then transferred to the hospital ward.
Up until the use of Dex and consequently the arrival of extubated patients, an essential tool was lost for pain quantification – i.e. that of the patient. Their ability to communicate was withdrawn and we were forced to rely on indirect tools ( behaviour scales). In our case, the Campbell scale. It should be noted that the lack of specificity of the signs in this type of scale may result in them being misinterpreted and usually they are underestimated by the staff involved in their care. Bearing in mind that pain causes major adverse effects, which alters the course of patient evolution, their identification and control the outcome is an increase in the quality of care of the critically ill patient.14–16
Dex is a selective agonist of alpha-2 receptors which has been proven to provide sedation and analgesia, with a minimum of respiratory depression, indicated for patients who require a level of sedation no deeper than waking up in response to verbal stimulation, which corresponds to the level between 0 and −3 on the Richmond agitation and sedation scale (RASS).4,17,18
The use of Dex allows us to obtain continual sedation with respiratory stability, leading to effective sedoanalgesia.4,7,9,13,19–25
In our unit patients arrive from the operating theatre with a continuous perfusion of Dex at a concentration of 4 µg/mL. This perfusion starts with an initial dose of 0.7 µg/kg/h which increases and decreases according to the patient’s haemodynamic status and sedation status (RASS). The duration of the Dex perfusion is prescribed by the clinician with consideration always of when oral tolerance will be initiated, since this is considered to be mild sedation.
The nurse plays a highly important role in heart surgery. He or she is another piece in the huge puzzle, from the time of hospital admission until discharge home. The patient in a heart surgery postoperative situation must be extremely closely monitored, with control of medical symptoms, control of pain and assessment of sedation.
This study arose from the nurses’ need to improve pain control in this type of patient. We made an assessment of the pain experienced by patients who had undergone heart surgery during the first 24 h in the ICU, with continuous perfusion of Dex as analgesic coadjuvant. The primary objective was to determine the degree of pain suffered postoperatively by heart surgery patients. Secondary objectives were to determine the degree of sedation of the patients and the administration of opiates as rescue analgesia.
Our hypothesis was that patients with intravenous Dex perfusion have a mild degree of pain and therefore do not require opiates.
Material and methodsDesign: Unicentric, observational, descriptive, quantitative study.
Scope: Conducted from April 2016 until September 2017, in the multi-purpose ICU of the CMT.
Inclusion criteria: Patients over 18 years of age operated on for heart bypass surgery with venous and/or arterial graft, replacement and/or repair of valve, combination of both (coronary artery and valvular), ascending aortic surgery, septal defects and atrial myxoma resection, with median sternotomy and ECC support who were admitted extubated and with Dex perfusion.
Exclusion criteria: All patients who were admitted to the ICU, intubated and all those who arrived extubated and without Dex perfusion.
Calculation of sample size and selection: According to the Spanish Society for Thorasic and Cardiovascular Surgery (SECTCV for its initials in Spanish) 19,772 major heart operations with ECC support were performed in 2016 in Spain.26 In consideration of this data and the sample size we wished to study, with a 95% confidence level, and a 5% error margin, the sample size to study was 109 patients.
Study variablesIndependent variables: the Dex dose and the hours of duration together with the administration of opiates.
Dependent variables: pain assessment and level of sedation.
The visual analogue scale (VAS) was used to assess pain. This is a subjective assessment tool for each individual which consists of a maximum value of 10 for maximum pain experienced and a minimum of 0 for absence of pain. Assessment is performed using a visual metric scale from 0 to 10, shown to the patient who points to the pain scores. In the VAS scale, pain is quantified as 0: absence of pain; mild: 1–3; moderate: 4–6 and severe > 6.2
For sedation assessment the RASS scale was used. This scale is based on observation and interaction with the patient, with 0 indicating an alert and peaceful patient and with a rising order of agitation, with the maximum being +4 (pugnacious and violent); and descending order, reaching up to −5 (absence of response).27
The VAS and RASS scale scores were obtained by the unit nurses who were in charge of the patient, who had been familiarised and trained to accurately perform these evaluations.
Patient-related variables: demographic (age, sex), type of heart surgery, duration of stay in the ICU.
ProcedureAn exhaustive search was made on the variables to be studied and a bibliographic review, from February 2016 until September 2017, based on Medline via Pubmed, Cuiden and Google Scholar data. Searches adapted to the different data bases used were carried out using the descriptors “cardiac surgery/cirugía cardíaca”, “pain/dolor”, “dexmedetomidine/dexmedetomidina”, “sedation/sedación”, “analgesia/analgesia”, “opiate/opiáceo”, combining between them with the Boolean operator “and”, excluding the paediatric and neonatal population from the study.
A data collection sheet was then designed where we extrapolated all the variables we needed (see additional material appendix).
Socio-demographic data were contained in the first sheet, including age, sex, body mass index (BMI), background obtained from computerised clinical histories using the Tesis® programme installed in the ICU, complemented by the researchers to facilitate data collection.
The second sheet concluded a 24 h Schedule where all variables relating to patient pain and sedation were collected hourly, such as VAS, RASS, hourly Dex dose, prescribed analgesia and rescue opioids. It should be noted that in our unit there is no analgesia protocol and this varied depending on the medical guideline. However, all patients were prescribed with paracetamol, metamizol and/or dexketoprofene to be alternated as standard analgesia and methadone or chloride as rescue analgesia.
When the patient was admitted to hospital, and their meeting with the inclusion criteria had been confirmed, the nurse on duty was in charge of recording the data of the second sheet during the first 24 h after admission.
After this, the data were stored in a file located in the unit and were transferred weekly to the database by the researchers.
Data analysisBasic descriptive statistics were performed, with expression of quantitative data as mean (standard deviation) and qualitative data as proportions.
The Microsoft Excel 2016 statistical package was used.
Ethical considerationsThe study was approved by the Clinical Research Ethics committee of the hospital of reference.
All patients signed an informed consent form prior to intervention by the anaesthesiologist, stating they had been informed of all mechanisms of action of the drug, its risks and benefits.
Professional confidentiality was maintained at all times, with data treatment during the study period and strict compliance with Law 15/1999, of 13th December governing guaranteed personal data protection.28
The Project met all currently required precepts for biomedical research.29
ResultsThe study included 109 patients, 62% of whom were men and 38% of whom were women. The mean age of the patients was 62 years (SD: 12.8). Mean stay in ICU was 4 days (SD: 1.3). Twenty four per cent of the patients underwent coronary bypass surgery, 65% heart valve replacement, 6% combined and 6% other surgery such as ascending aorta surgery, septal defects and atrial myxoma resection. One hundred per cent of patients underwent median sternotomy and required ECC support.
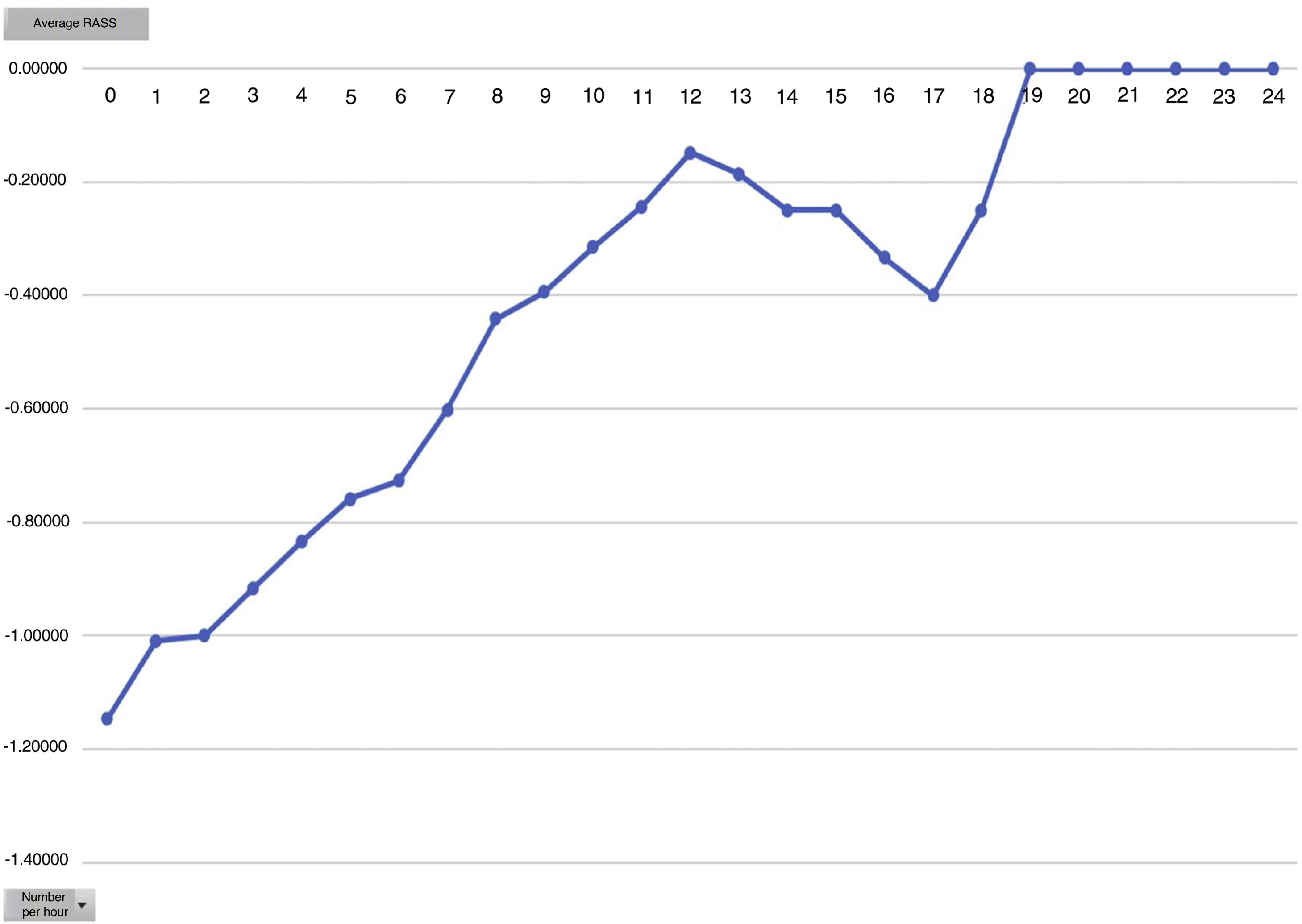
The mean hours of Dex perfusion were 12 h (SD: 2.5), with range of perfusion hours from 2.5–20 h,with a mean dose of 0.4 µg/kg/h (minimum being 0.048 and maximum 1 µg/kg/h). the mean score for the RASS scale was −.68 (SD: 0.8), with −3 being the maximum value of RASS they reached.
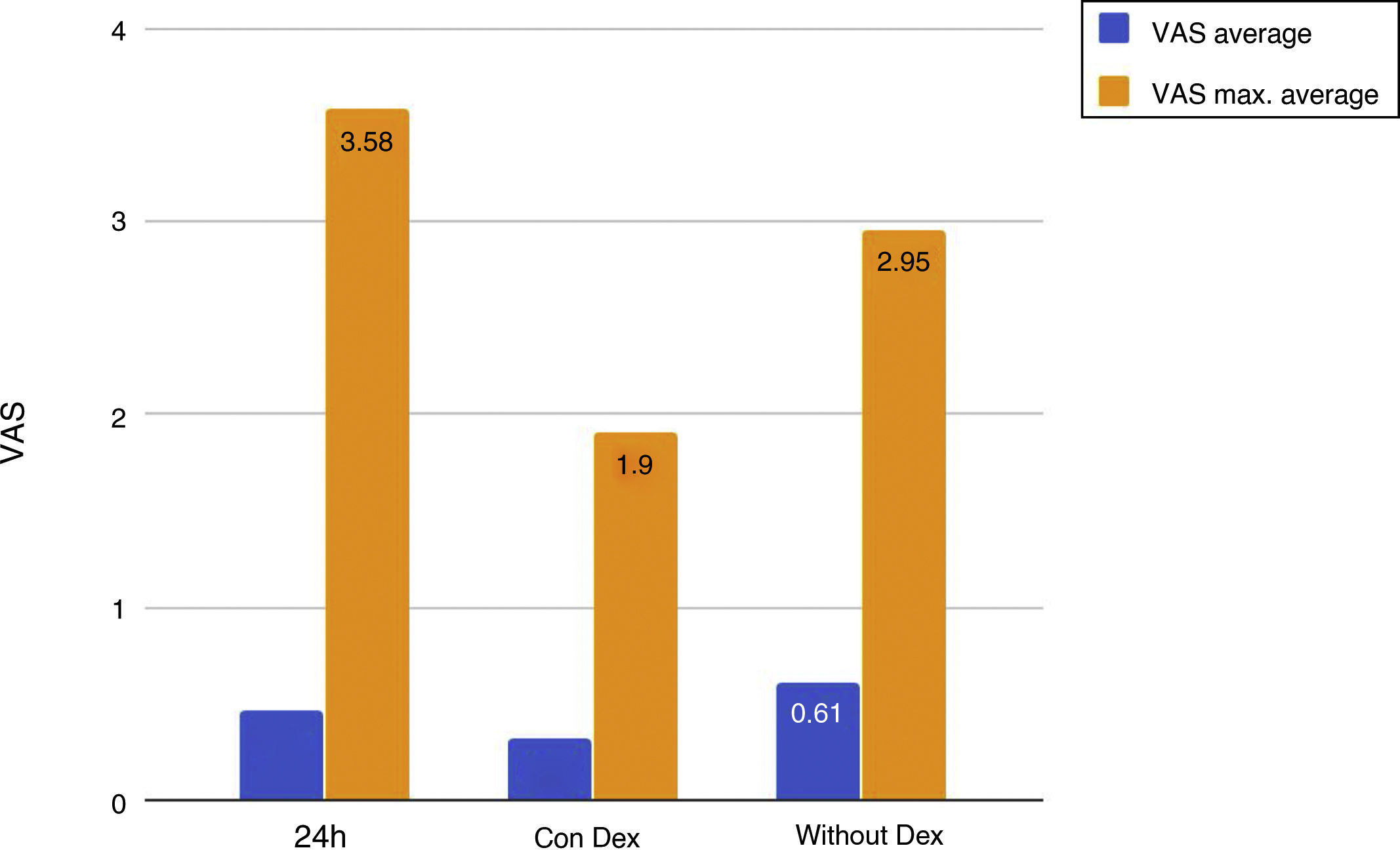
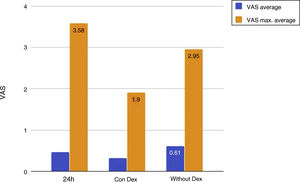
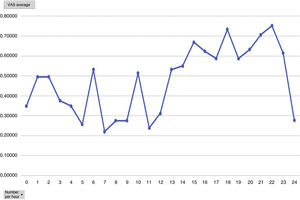
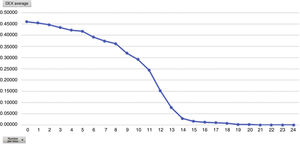
Mean pain experienced during the first 24 h of stay in the ICU was 0.47 (SD: 1.25), with mean pain suffered by the patients of 3.58 (SD: 2.18), with a range of 0–8. During the time the Dex perfusion lasted the mean values of the VAS were .32 (SD: 1.03), with mean maximum pain suffered of 1.90 (SD: 2.17). the mean pain score once Dex perfusion had terminated was 0.62 (SD: 1.42), with a mean maximum perceived score of 2.95 (SD: 2.32) (Fig. 1).
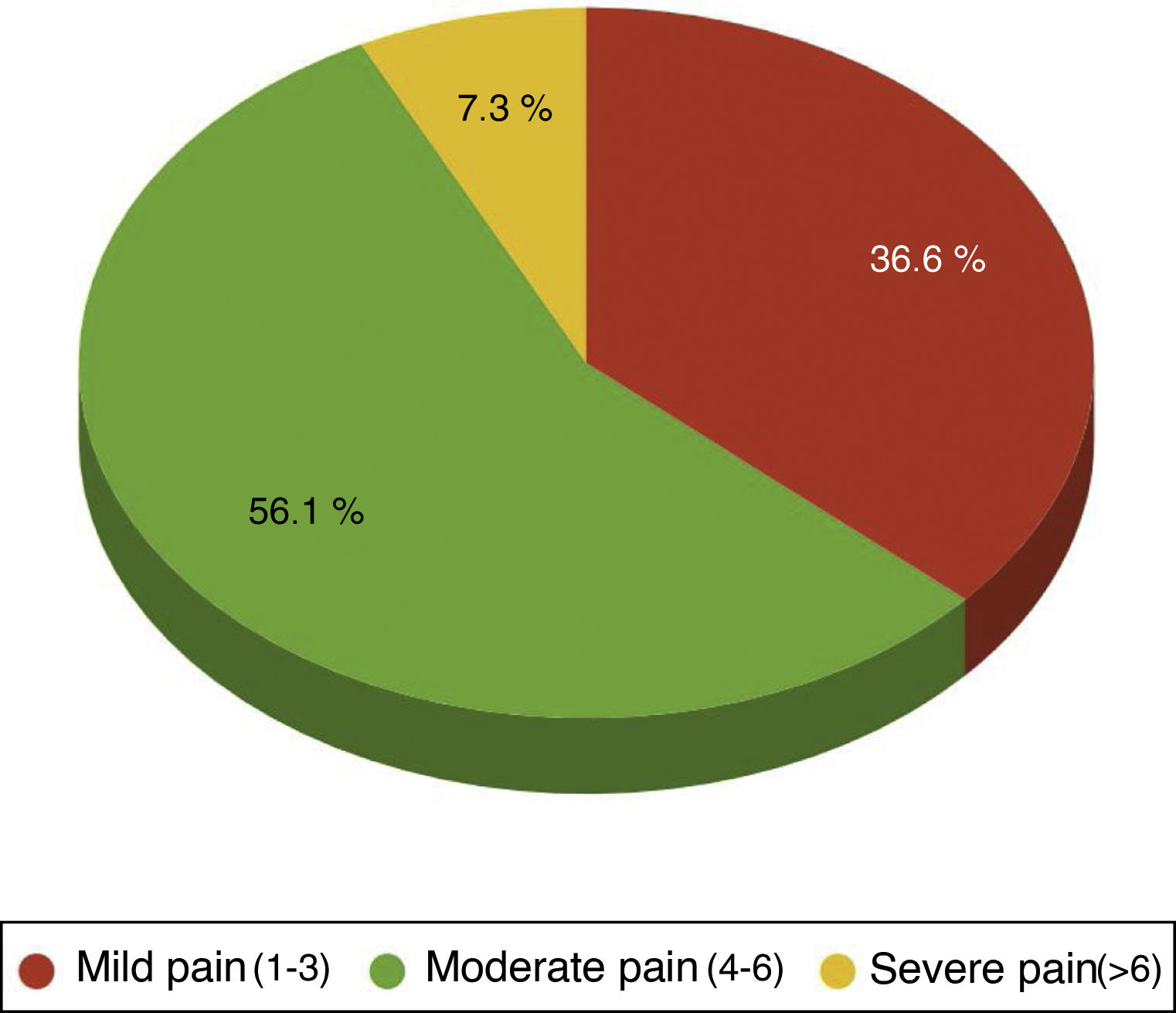
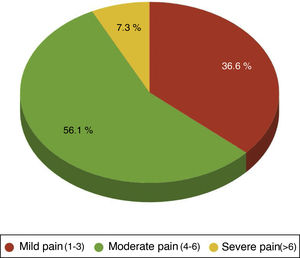
One hundred per cent of the patients were administered with the standard analgesia prescribed by the clinician and carried an elastomeric pump of ropivacaine at local sternotomy level. Rescue opiates were also administered in case the treatment administered was ineffective, methadone and /or morphic chloride as prescribed by the physician. Forty four point four per cent of patients required rescue opiates. Out of these patients the VAS variable score was analysed, observing that 36.59% of patients had been administered opiates for mild pain(VAS between1and 3), 56.10% for moderate pain (VAS between 4 and 6) and 7.32% for severe pain (VAS > 6) (Fig. 2).
Discussion and conclusionsThis research study is the first to link the use of Dex as analgesic coadjuvant in postoperative heart surgery patients in Spain by nurses.
The main objective of our research study was to assess the degree of pain of the patient operated on for heart surgery with a Dex perfusion. Results suggested that the patients had a low level of pain and good general comfort. Comparing the results of pain with and without the Dex perfusion we reached the conclusion that there is a significant difference, and were able to confirm that during the Dex perfusion the patients had better pain control.
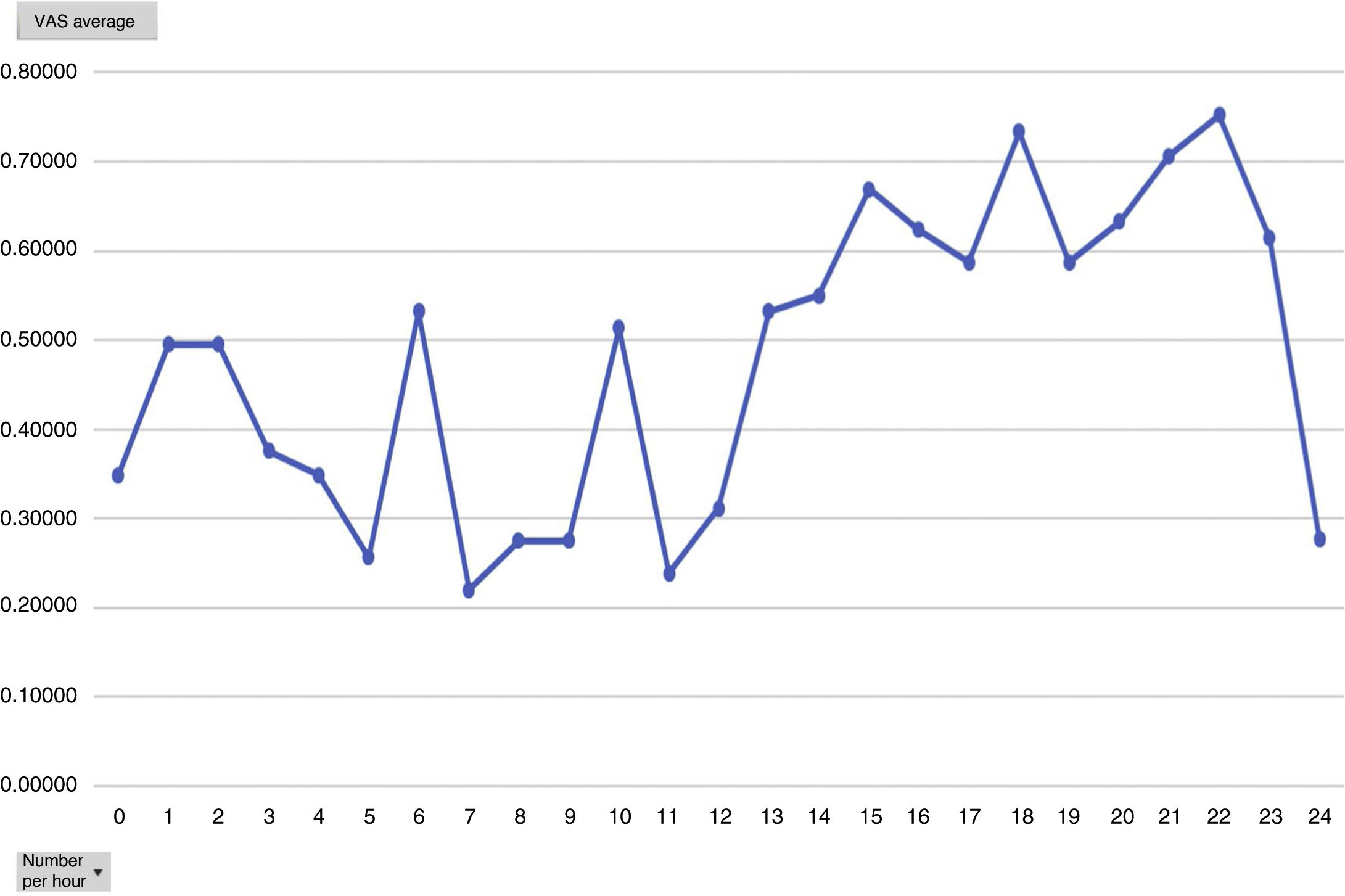
Analysing the average pain per hours, an increase in pain average was obvious from 12 h onwards, with this being the mean hours that patients had received the Dex perfusion (Fig. 3).
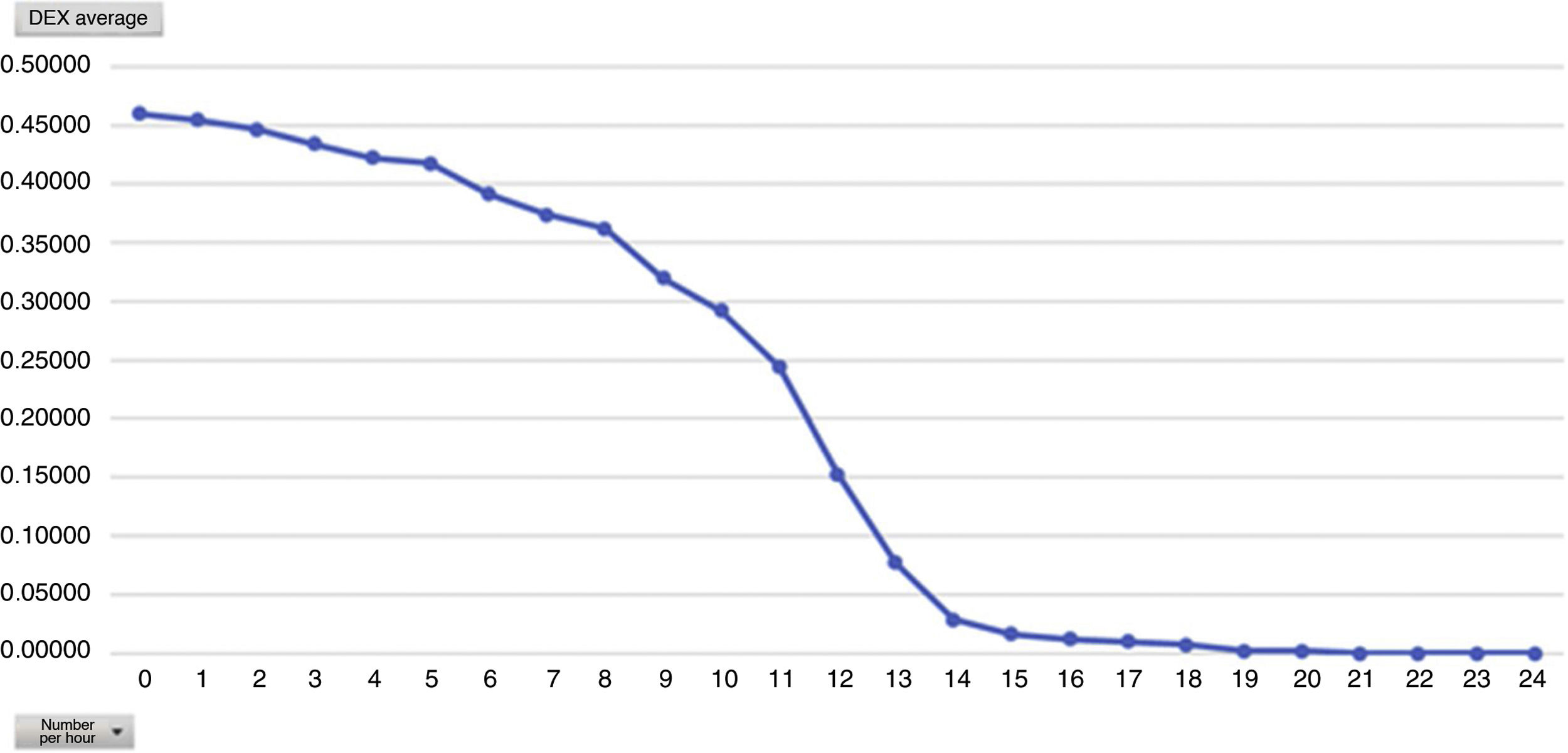
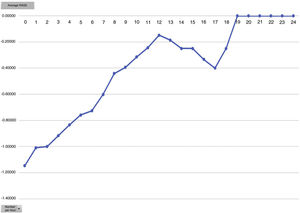
One of the secondary objectives was to assess the degree of sedation in these patients since, despite being extubated in the ICU, they were attached to the Dex perfusion, considered as sedation. We believe optimal sedation for extubated patient’s to be 0 to −2, which falls within the optimal sedation range. Analysed by average hours of Dex dose we observed that this is directly proportional to the mean level of sedation, i.e. the higher the Dex dose the more sedated the patients (Figs. 4 and 5). During outcome analysis we observed that there was a large variety in both hours of perfusion and the level of RASS of patients, and we recommend that a sedation protocol and the guidelines for desired sedation level by made by the medical team.
Our hypothesis aimed to demonstrate that patients with intravenous Dex perfusions have a mild pain level and therefore do not require opiates. Although we were able to observe that the level of pain was low, the use of opiates was high. If we perform in-depth analysis what stands out is the high level of opiates administered for mild pain. In the light of these findings we would consider that there is a lack of training with the healthcare team. Furthermore, although there is no one specific analgesic technique that is superior to any other for the management of heart surgery pain, we believe the lack of a protocol results in a highly subjective patient treatment manner since each nurse decides when is the best moment to administer a rescue opiate and this may lead to over usage.
We believe that with a broader analgesic protocol, bearing in mind the analgesic recommendations such as multimodal analgesic or the “pain ladder” would help to reduce opioids administration and treat pain more accurately and equitably.30–32
The huge diversity of information contained in the studies which assess Dex administration hinders comparison of outcomes. Almost all studies involve anaesthesiology, which assess both pain and haemodynamic stability with the administration of Dex during intervention, and case-control studies of intubated patients compared with sedatives such as propofol. Even so, all of them show the benefit of Dex for pain control and how the need for opiates with it falls.4,12,13,19–23 We also found strong recommendations and a moderate level of evidence in the “Clinical practice guide based on evidence for the management of sedoanalgesia in the critically ill adult patient “from 2013, where the use of Dex was recommended as the single agent or combined with analgesic opioids as short duration sedoanalgesia because it significantly reduces the incidence and duration of delirium in patients in heart surgery.33
We reached the conclusion that the implementation of Dex in pain management of the postoperative heart surgery patient is effective for pain control. Still, the use of opiates continues to be high, and in view of the results, we believe an analgesic and sedation protocol requires putting into place, together with greater healthcare team training and posterior re-assessment.
Conflict of interestsThe authors declare they have not received any type of financial aid for this study, no economic or personal conflict of interests exist which could inappropriately bias or influence their actions.
Our thanks to the multidisciplinary team of the CMT intensive care unit who collaborated in data collection, to Dr. Ruyra for her contributions to the study, and to Sara Tor, the Director of nursing. nursing.
Please cite this article as: Nacher-Fuentes L, Sanchez-Pujol A, Rodriguez-Navarro S, Duran-Ayra L. Implementación de la dexmedetomidina en el manejo del dolor en el postoperatorio inmediato de cirugía cardíaca. Enferm Intensiva. 2020;31:105–112.