Type 2 diabetes (DM2) is one of the diseases that cause the highest premature mortality and disability worldwide. Sleep disturbances have been associated with the onset of metabolic disorders and increased levels of glycated haemoglobin in diabetics.
ObjectiveTo evaluate the quality of sleep of people with type 2 diabetes and its association with sociodemographic, clinical, and metabolic characteristics.
MethodsObservational, cross-sectional, analytical study of 223 patients with DM2 between 20 and 64 years old, controlled in a primary health care centre in Chile.
ResultsMost were women (66.4%), average age 54.7 years. Only 33% slept the required number of hours (7–9 h) to maintain health. Fifty-seven point seven percent were metabolically unbalanced (Hb1Ac > 7%). Seventy-five point two percent presented sleep problems of varying severity, which were associated with being a woman, night pain, nocturia, time of diagnosis of diabetes, and depressive symptoms. Persons with poor sleep quality were 73% more likely to be metabolically decompensated, however, this result was not statistically significant: OR = 1.73 (CI: 78–3.87).
ConclusionsThe high frequency of poor sleep quality and metabolic decompensation in the sample stands out, which could complicate DM2. The association between poor sleep quality and DM2 decompensation is inconclusive. Future research will contribute to elucidating the role of sleep in metabolic compensation and in the prevention of metabolic disorders.
La diabetes tipo 2 (DM2) es una de las enfermedades que causa mayor mortalidad prematura y discapacidad a nivel mundial. Las alteraciones del sueño se han asociado a la aparición de trastornos metabólicos y a mayores niveles de hemoglobina glicosilada en personas diabéticas.
ObjetivoEvaluar la calidad del sueño de personas con DM2 y su asociación con características sociodemográficas, clínicas y metabólicas.
MétodosEstudio observacional, transversal, analítico en 223 personas con DM2 entre 20 y 64 años controladas en un centro de atención primaria de salud.
ResultadosLa mayoría eran mujeres (66,4%), edad promedio 54,7 años y sólo el 33% duerme las horas necesarias (7 a 9 horas). Un 57,7% se encontraba metabólicamente descompensado (Hb1Ac > 7%). Un 75,2% de los participantes presentó problemas de sueño de diversa gravedad, que se asoció a ser mujer, dolor nocturno, nicturia, tiempo de diagnóstico de la diabetes, y síntomas depresivos. Las personas con mala calidad de sueño tuvieron 73% más probabilidad de estar descompensados metabólicamente, sin embargo, este resultado no fue estadísticamente significativo OR = 1,73 (IC: 0,78–3,87).
ConclusionesDestaca la alta frecuencia de mala calidad de sueño y descompensación metabólica en la muestra, lo que podría complicar la DM2. No es concluyente la asociación entre mala calidad de sueño y descompensación de la DM2. Futuras investigaciones aportarán a dilucidar el papel del sueño en la compensación metabólica y en la prevención de trastornos metabólicos.
Sleep quality is associated with metabolic disorders. There is an association between problems with sleep and low glycaemic control in patients with diabetes mellitus type 2. The prevalence of sleep alterations is above 50% in the Chilean population.
What does this paper contribute?This study reflects that a high percentage of people with diabetes mellitus type 2 present with multimorbidity and sleep alterations but there is no concordance between the perception of bad quality sleep with the objective outcomes obtained through the Pittsburg Sleep Quality Index (PSQI) in this population. The above makes it necessary to consider sleep as a habit that should be assessed in the check-ups of people with multimorbidity, given the impact on their health and compensation for their pathologies.
Diabetes type 2 (DM2) is one of the main diseases which causes premature mortality and disability on a worldwide level. In Chile, DM2 has increased in prevalence from 9.4%–12.3% between years 2010–2017 and 67.6% of controlled patients are metabolically unbalanced.1
Population and clinical research studies link quality and quantity of sleep, considering both too little sleep (less than 7 h) and too much (more than 9 h), with systemic level effects,2 among them: the increased risk of suffering from diseases such as DM2, high blood pressure, obesity and other cardiovascular diseases.3–6 Sleep disorders also lead to changes such as intolerance to glucose, insulin resistance and alteration, and finally the appearance of DM2.7 Along these lines, researchers have shown that there is an association between sleep problems and glycaemic control in patients with DM2.8–11
It has also been observed that there is a relationship between sleep disorders and metabolic syndrome in obese people, snorers who are or are not obese, and with sleep-disordered breathing patterns (hypoventilation syndrome), particularly in people with obstructive sleep apnoea (common in patients with diabetes mellitus), a pathology which generates minor and major awakenings, leading to repeated activation of the sympathetic nervous system and loss of glucose and blood pressure control.12
One mechanism which explains these relationships could be the fact that the changes in sleep patterns lead to high levels of cortisol, TNF-alpha, interleukin- 6 and C-reactive protein, which in turn impact the increase of insulin resistance. It is of note that insulin levels are regulated in deep-sleep phases, which is when there is less use of glucose and lower secretion of corticosteroids. Metabolic requirements are therefore minimal in this phase, which is more difficult to reach when sleep problems arise.13
There is also a relationship between sleep disorders and DM2 which appears in the neuroendocrine deregulation of appetite through the interaction between leptin (satiety hormone) and ghrelin (antagonist), both produced during sleep. When sleep quality and quantity is poor leptin falls whilst ghelin rises, and this leads to an increase in appetite (a preference for food rich in carbohydrates) and probably to an increased ingestion of foodstuffs. This is the contrary to what happens during exercise, since this boosts sleeping well and therefore leads to a neuroendocrine regulation of appetite.12
It should be noted that good quality sleep helps the pancreas to regulate blood sugar levels. In the case of people with DM2, they usually present with continuous awakenings due to the underlying conditions of this pathology, such as diabetic leg neuropathy, polydipsia, polyuria and fatigue, to the detriment of insulin regulation by the pancreas.13
The prevalence of sleep disorders in the Chilean population is between 63.2% and 67.2% measured with different instruments.1,14
There are no publications in Chile regarding sleep quality among people with chronic conditions such as DM2 nor is it assessed within the Cardiovascular Health Programme (CVHP) or mentioned in the ministerial clinical guidelines for management of this pathology.15,16
This study seeks to assess sleep quality in people with DM2 and its association with sociodemographic, clinical and metabolic characteristics. Also to predict the effect of sleep quality on metabolic compensation, to help make this habit visible as part of a healthy lifestyle for these patients.
MethodObservational, cross-sectional and analytical study of people with DM2 who subscribed to the CVHP of the Family Health Centre (CESFAM for its initials in Spanish) Dr. Osvaldo Ruz from the Machalí commune, from the Libertador Bernardo O’Higgins region in January 2017, corresponding to the summer season. Machalí is a commune in central Chile, with approximately 52,000 inhabitants, 95% urban population and 5% rural. It has a temperate Mediterranean climate with a dry season in summer and a wet season in winter. Economic activity mainly comprises industrial and mining sectors. It has 4 primary health level centres, one of which the participants of this study were attached to.
A simple random sample was collected, in a population of 631 people with DM2 aged between 20 and 65 years, who had subscribed to the CVHP of the CESFAM, with a 95% confidence level, a 5% precision level and prevalence of sleep disorders in 67.2% of the adult population attending the CESFAM, based on the study by Castillo et al.,14 amounting to a sample size of 222 patients.
From the list of patients subscribing to the CVHP provided by the CESFAM, people were chosen using a list of random numbers. These people were contacted by phone or in the actual family health centre if they were there for other reasons, by the research team and staff of the CESFAM. Inclusion and exclusion criteria were corroborated: confirmed diagnosis of DM2; being in treatment for at least one year and having had a serum glycosylated haemoglobin (HbA1c) measurement in the 3 months prior to the start of the study. Exclusion criteria were: the presence of surgical intervention which required general anaesthesia in the last 3 months; life expectancy under 3 months; addiction to illegal drugs or alcohol; shift work; conditions of life which impeded sleeping the whole night through, and severe mental health disorders. Most of the measurements were made in the family health centre and in some cases in the person’s home.
A questionnaire was supplied in an interview and this included sociodemographic data, sex, age, education, civil status and occupation (especially designed for this study), Pittsburg Quality of Sleep Index (PQSI) and the Patient Health Questionnaire (PHQ-9) scale. Primary variables were quality of sleep (outcome variable) and metabolic compensation assed by HbA1c (predictive variable). Secondary variables were sociodemographic data; anthropometric data; the presence of nocturia; time from diagnosis of diabetes; presence of night pain and the presence of depressive symptoms. For the age variable the cut-off point was taken as being over or under 45 years of age, since most of the DM population with poor sleep quality are those aged between 42.2 and 66 years according to the meta-analysis by Azharuddin et al.17
Updated anthropometric data (body mass index), comorbidities and HbA1c levels were obtained from the clinical record and those found to be out of date were re-measured. The “glycaemic control” variable was created from the HbA1c level, and defined as “decompensated” if the HbA1c level was equal to or above 7% and “compensated” if it was under 7%, based on HbA1c control health objectives of the Chilean Ministry of Health Cardiovascular Programme Clinical Guideline.18 Regarding the years since DM2 diagnosis, these were classed as under 6 years and over 6 years, which is the same figure used in similar studies.19
Sleep quality and quantity was assessed with the PQSI, scores of which vary from 0 to 21 and consist of 7 components: subjective sleep quality; latency; duration; normal effectiveness; changes; use of medication, and daytime dysfunction. This tool was validated into Spanish for use in the Latin American population with an average reliability ratio of .78.20 In this study we considered a PQSI ≤ 5 points as good sleep quality and above 5 points as poor sleep quality, so that scores above 5 indicated poor sleep quality and serious difficulties in at least 2 areas of the 7 components, using similar study values to those found in the literature.21
Depressive symptoms were assessed using the PHQ-9 scale, validated in Chile, with 92% sensitivity and 89% specificity.22
Analysis was made using descriptive statistics of central estimation and dispersion for the continuous variables and frequency distributions for the categorical variables. Logistic regression models were made to calculate the effect on the quality and quantity of sleep from the sociodemographic and clinical variables, adjusted by the covariables of interest, using the stepwise method. The Stata 14.0 programme was used.
This study was approved by the Scientific Ethics Committee of the Pontificia Universidad Católica de Chile Faculty of Medicine. People showed their willingness to participate through the process of informed consent.
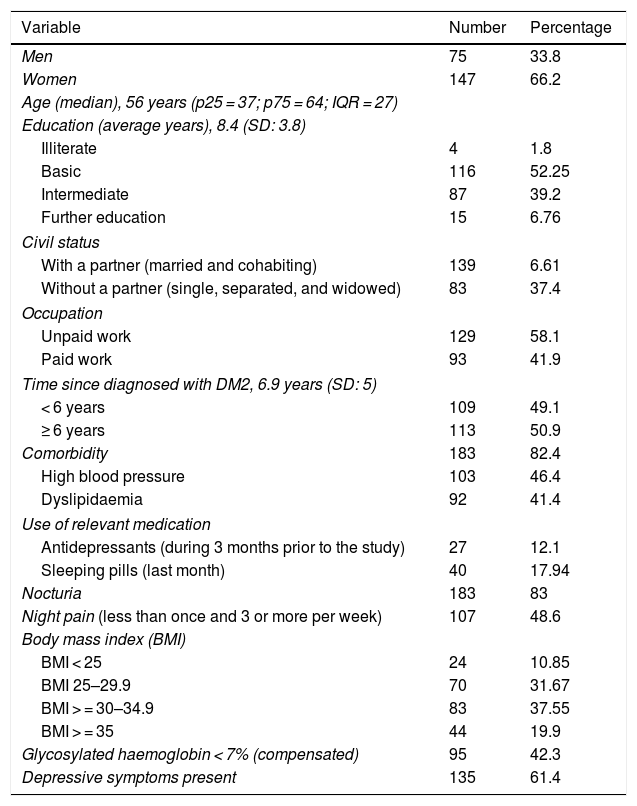
ResultsSociodemographic and health profileTwo hundred and twenty two people with DM2 were studied, with sociodemographic and health data being described in Table 1.
Sociodemographic and health profile.
| Variable | Number | Percentage |
|---|---|---|
| Men | 75 | 33.8 |
| Women | 147 | 66.2 |
| Age (median), 56 years (p25 = 37; p75 = 64; IQR = 27) | ||
| Education (average years), 8.4 (SD: 3.8) | ||
| Illiterate | 4 | 1.8 |
| Basic | 116 | 52.25 |
| Intermediate | 87 | 39.2 |
| Further education | 15 | 6.76 |
| Civil status | ||
| With a partner (married and cohabiting) | 139 | 6.61 |
| Without a partner (single, separated, and widowed) | 83 | 37.4 |
| Occupation | ||
| Unpaid work | 129 | 58.1 |
| Paid work | 93 | 41.9 |
| Time since diagnosed with DM2, 6.9 years (SD: 5) | ||
| < 6 years | 109 | 49.1 |
| ≥ 6 years | 113 | 50.9 |
| Comorbidity | 183 | 82.4 |
| High blood pressure | 103 | 46.4 |
| Dyslipidaemia | 92 | 41.4 |
| Use of relevant medication | ||
| Antidepressants (during 3 months prior to the study) | 27 | 12.1 |
| Sleeping pills (last month) | 40 | 17.94 |
| Nocturia | 183 | 83 |
| Night pain (less than once and 3 or more per week) | 107 | 48.6 |
| Body mass index (BMI) | ||
| BMI < 25 | 24 | 10.85 |
| BMI 25–29.9 | 70 | 31.67 |
| BMI > = 30–34.9 | 83 | 37.55 |
| BMI > = 35 | 44 | 19.9 |
| Glycosylated haemoglobin < 7% (compensated) | 95 | 42.3 |
| Depressive symptoms present | 135 | 61.4 |
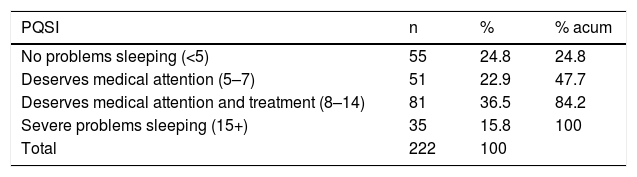
Regarding sleep quality, the average score on the PQSI was 8.88 (SD: 4.99) range 1−20. 75.2% of the participants presented with sleep problems of varying severity (Table 2).
The average hours of sleep reported by the patients was 6.2 h (SD: 1.9). Only 33% said they slept between 7 and 9 h. Regarding the subjective perception of sleep quality, 62.2% considered that they slept well and 37.8% that they slept badly.
Regarding the period of time it took to get to sleep, 54.7% took up to 30 min to get to sleep and 45.3% longer, with a notable 33.2% taking longer than one hour.
During the last month 17.9% of patients took sedatives/sleeping pills, and of these 12.5% took them between one and 3 times per week.
Metabolic compensationHbA1c of the sample reached a median of 7.2% (IQR = 3.2), with no significant differences between the sexes (Wilcoxon rank sum of the two sexes = −.459; p = .646).
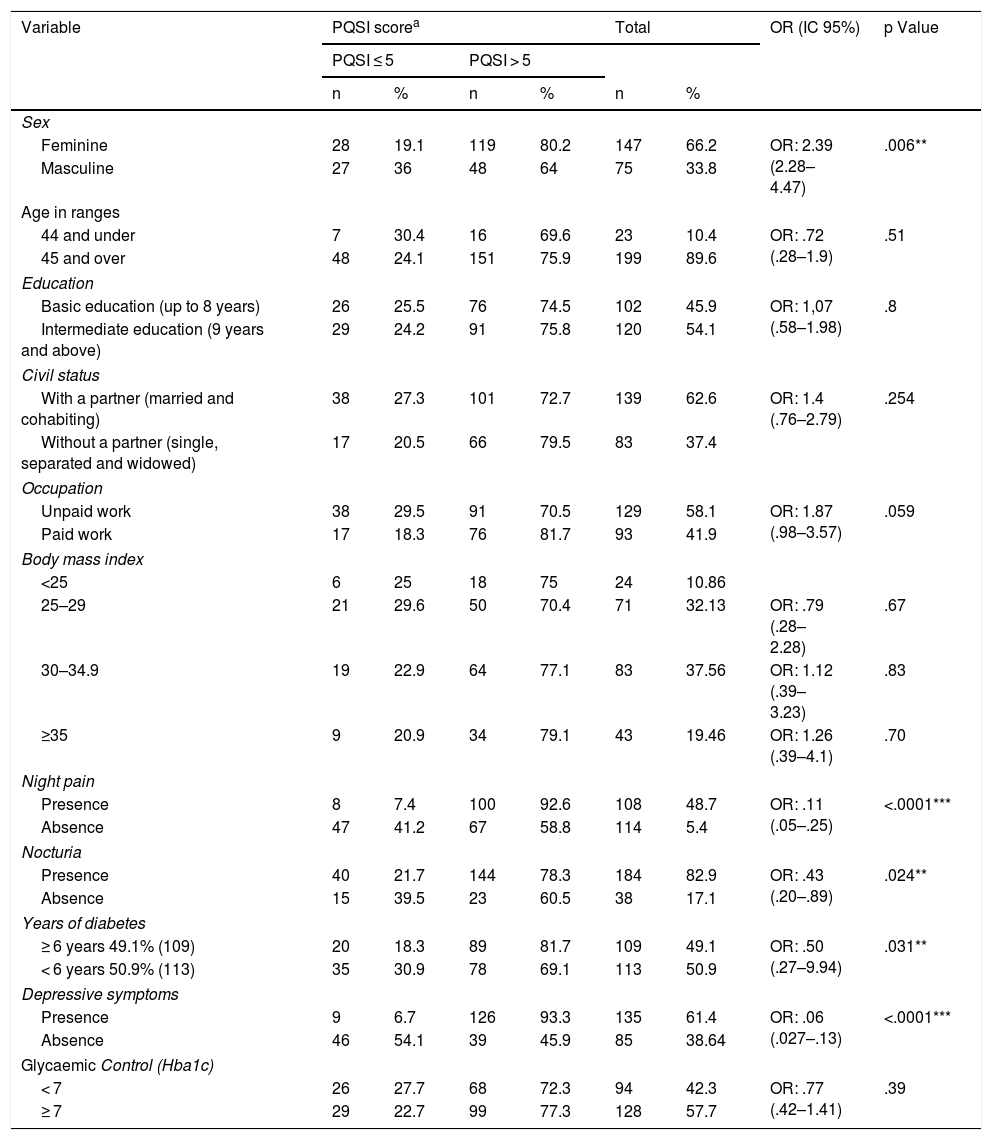
Sleep quality and its association with sociodemographic and clinical characteristicsThe association between sleep quality and sociodemographic and clinical characteristics is shown in Table 3. The variables which showed statistically significant association with the PQSI were: being a woman, night pain, nocturnia, time since diagnosis of diabetes and depressive symptoms. Not having paid work marked a tendency for poor sleep.
Association between sleep quality and sociodemographic and clinical characteristics.
| Variable | PQSI scorea | Total | OR (IC 95%) | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| PQSI ≤ 5 | PQSI > 5 | |||||||
| n | % | n | % | n | % | |||
| Sex | ||||||||
| Feminine | 28 | 19.1 | 119 | 80.2 | 147 | 66.2 | OR: 2.39 (2.28–4.47) | .006** |
| Masculine | 27 | 36 | 48 | 64 | 75 | 33.8 | ||
| Age in ranges | ||||||||
| 44 and under | 7 | 30.4 | 16 | 69.6 | 23 | 10.4 | OR: .72 (.28–1.9) | .51 |
| 45 and over | 48 | 24.1 | 151 | 75.9 | 199 | 89.6 | ||
| Education | ||||||||
| Basic education (up to 8 years) | 26 | 25.5 | 76 | 74.5 | 102 | 45.9 | OR: 1,07 (.58–1.98) | .8 |
| Intermediate education (9 years and above) | 29 | 24.2 | 91 | 75.8 | 120 | 54.1 | ||
| Civil status | ||||||||
| With a partner (married and cohabiting) | 38 | 27.3 | 101 | 72.7 | 139 | 62.6 | OR: 1.4 (.76–2.79) | .254 |
| Without a partner (single, separated and widowed) | 17 | 20.5 | 66 | 79.5 | 83 | 37.4 | ||
| Occupation | ||||||||
| Unpaid work | 38 | 29.5 | 91 | 70.5 | 129 | 58.1 | OR: 1.87 (.98–3.57) | .059 |
| Paid work | 17 | 18.3 | 76 | 81.7 | 93 | 41.9 | ||
| Body mass index | ||||||||
| <25 | 6 | 25 | 18 | 75 | 24 | 10.86 | ||
| 25–29 | 21 | 29.6 | 50 | 70.4 | 71 | 32.13 | OR: .79 (.28–2.28) | .67 |
| 30–34.9 | 19 | 22.9 | 64 | 77.1 | 83 | 37.56 | OR: 1.12 (.39–3.23) | .83 |
| ≥35 | 9 | 20.9 | 34 | 79.1 | 43 | 19.46 | OR: 1.26 (.39–4.1) | .70 |
| Night pain | ||||||||
| Presence | 8 | 7.4 | 100 | 92.6 | 108 | 48.7 | OR: .11 (.05–.25) | <.0001*** |
| Absence | 47 | 41.2 | 67 | 58.8 | 114 | 5.4 | ||
| Nocturia | ||||||||
| Presence | 40 | 21.7 | 144 | 78.3 | 184 | 82.9 | OR: .43 (.20–.89) | .024** |
| Absence | 15 | 39.5 | 23 | 60.5 | 38 | 17.1 | ||
| Years of diabetes | ||||||||
| ≥ 6 years 49.1% (109) | 20 | 18.3 | 89 | 81.7 | 109 | 49.1 | OR: .50 (.27–9.94) | .031** |
| < 6 years 50.9% (113) | 35 | 30.9 | 78 | 69.1 | 113 | 50.9 | ||
| Depressive symptoms | ||||||||
| Presence | 9 | 6.7 | 126 | 93.3 | 135 | 61.4 | OR: .06 (.027–.13) | <.0001*** |
| Absence | 46 | 54.1 | 39 | 45.9 | 85 | 38.64 | ||
| Glycaemic Control (Hba1c) | ||||||||
| < 7 | 26 | 27.7 | 68 | 72.3 | 94 | 42.3 | OR: .77 (.42–1.41) | .39 |
| ≥ 7 | 29 | 22.7 | 99 | 77.3 | 128 | 57.7 | ||
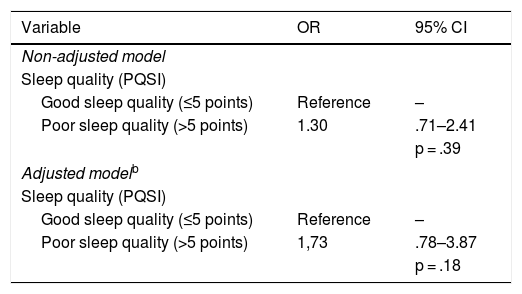
A logistic regression model was adjusted to predict the effect of sleep quality on metabolic compensation, the levels of which are shown in Table 4. Adjustment variables to the model included those which were statistically significant with sleep quality, using the stepwise method: presence of depressive symptoms, sex, presence of night pain, nocturia and time with diabetes. The model yielded an OR = 1.73 (CI: .78–3.87), i.e. the people with poor quality sleep were 73% more likely to be metabolically decompensated than those with good quality sleep, adjusted by the stated variables. Despite this result not being statistically significant, given that the confidence interval surpasses the critical 1 value, it is a clinically relevant outcome.
Effect of sleep quality on glycaemic control.a
| Variable | OR | 95% CI |
|---|---|---|
| Non-adjusted model | ||
| Sleep quality (PQSI) | ||
| Good sleep quality (≤5 points) | Reference | – |
| Poor sleep quality (>5 points) | 1.30 | .71–2.41 |
| p = .39 | ||
| Adjusted modelb | ||
| Sleep quality (PQSI) | ||
| Good sleep quality (≤5 points) | Reference | – |
| Poor sleep quality (>5 points) | 1,73 | .78–3.87 |
| p = .18 | ||
This is the first study to be published that assesses sleep quality and its association with sociodemographic and metabolic variables in diabetic patients controlled by primary healthcare centres in Chile.
Most of the sample were women, who had completed basic education, and had been diagnosed with FM2 for an average of 6 years. There was a presence of high blood pressure, dyslipidaemia and similar HbA1c levels to those reported by other researchers.19,23–25 The patient profile was similar to that normally found in primary healthcare control in Chile.26 The presence of depressive symptoms in this population was 61.4%, a much higher figure than that reported in the international literature in diabetic patients (40.1% and 43.6%),10,24,27 and also in users of primary care in Chile (25%).28 It is striking that, despite the high percentage of people with depressive symptoms, only 12.1% stated they were receiving treatment (antidepressant or anxiolytic) over the 3 months prior to the interview.
The average hours of sleep reported by people in this study (6.2 h) were similar to those reported in the study by Shamshirgaran et al.19 and higher than those reported by Knutson et al.29 The amount of sleep was under that considered appropriate30 and may be affecting the health of these users, particularly considering their chronic condition.
Relating to sleep quality assessed through the PQSI, the average scores in this study were similar to those reported by Bing-Qian et al.31 and higher than those published by Shamshirgaran et al.19 75.2% of the people had a sleep problem according to the PQSI, similar to that reported by Navarro,32 but this figure was higher than that found in other studies, where it was lower than 50%.24,25,30 It is striking that over half of the patients of this study had a good perception of their sleep quality, despite the high PQSI reported scores.
In our results, being a woman had a significant association with bad quality sleep, similarly to the Chao et al.33 and Song et al.34 studies. However, Narisawa et al. found no differences between the sexes.23 These differences could be associated with gender issues relating to the multiplicity of roles many women have, particularly in the domestic domain.
The presence of night pain and nocturia were significantly associated with poor quality sleep, similarly to that found by Narisawa et al.23 Both situations could be independent factors of poor quality sleep, but they could also be complications of diabetes, which appear in advanced stages of the disease (time of disease diagnosis), a condition in this study was also associated with poor sleep quality, as in the studies by Zhu et al.10 and Chao et al.33
Another variable which showed a statistically significant association with sleep quality was the presence of depressive symptoms, as shown by the Knutson et al.29 Depression would be an independent risk factor for poor quality sleep,29 a condition that is highly frequent in this study.
With regard to the relationship between sleep quality and metabolic compensation, results were controversial. This study, together with that of Shamshirgaran et al.18 and that of Narisawa et al.,23 did not find any statistically significant association between these variables, but the differences could be relevant from a clinical viewpoint. In the meta-analysis by Lee et al., which included 15 studies of patients with highly heterogeneous characteristics and with different cut-off points in the PQSI to identify people with good and poor quality sleep, it was shown that the reduction or excess in total hours of sleep was associated with an increase in HbA1c. Notwithstanding, the evidence for the association between sleep quality and metabolic compensation assessed by HbA1c was insufficient,11 similar to outcomes found in the meta-analysis by Azharuddin et al.17
It is of note that the majority of people presented with multimorbidity and poor quality sleep, which could contribute to further health impairment, given that evidence shows the effects of sleep quality on the metabolism of glucose and other basic physiological factors.
This study has several strengths and limitations. Its main strengths are that to date few published studies exist regarding sleep quality in diabetic people in Latin American and none in Chile, with this being the first. This study could provide valuable information for clinicians about the quality of sleep of people with DM2 from the CVHP.
One of its limitations is that this was a cross-sectional study with no assessment of participant follow-up, and therefore evidence of the causes of poor quality sleep in this population was not provided. Also, the study did not include patients who require insulin, since they were not controlled in these primary healthcare centres. Therefore, the results cannot be extrapolated to all DM2 patients. Another limitation may reside in the ability of participants to remember previous events (recall bias), such as for example, night pain and nocturia. The study was conducted during the summer which may have impacted sleep quality.
ConclusionResults show that a large proportion of people with DM2 have poor sleep quality (according to the PQSI), but, half of them have a good perception of their sleep quality. Sleep quality was statistically significantly associated with being a woman, having been diagnosed with diabetes for over 6 years, having night pain, nocturia, and depressive symptoms. Furthermore, an association was found between sleep quality and metabolic compensation with no statistical significance, but it could be clinically relevant.
The majority of the population studied had comorbidities (such as high blood pressure and dyslipidaemia) and over 2 thirds presented with depressive symptoms (much higher than in national and international studies), even when only 12.1% showed they had received treatment (antidepressant or anxsiolytic) during the 3 months prior to the interview.
Given the evidence which show the effects of sleep quality in the metabolism of glucose, further research is needed to highlight sleep quality in people with chronic conditions, as part of healthy lifestyle habits, to be included in clinical guidelines for managing the care for these patients.
FinancingVice-rectory of research, Pontificia Universidad Católica de Chile, Santiago-Chile.
Conflict of interestsAll the authors have no conflict of interests to declare.
Our thanks to the participants who generously provided their data. To the managers and professionals of the Family Health Centre Dr. Osvaldo Ruz Machalí Commune, VI Region, Chile.
Please cite this article as: Campos-Romero S, Barrios Araya SC, Masalan-Apip MP, Guajardo Tobar V, Arias-Ortiz NE, Bobadilla-Beiza L. Calidad del sueño en personas con diabetes tipo 2 controladas en el nivel primario y su asociación con características sociodemográficas y clínicas. Enferm Clin. 2022;32:45–53.