The latest advances in the vaginal microbiome and molecular diagnosis of bacterial vaginosis have allowed for a better knowledge of this entity, characterising aspects of its pathogenesis and the establishment of the vaginal biolayer, the models and new theories of its aetiology, how it is transmitted, with it being considered nowadays as a probable sexually transmitted infection, the separation of other entities such as aerobic vaginosis, its molecular diagnosis and treatment with new molecules to prevent frequent relapses. This entity and the study of the vaginal microbiome have made it possible to consider these infections as a polymicrobial syndrome, putting an end to the dogma: one microorganism, one disease. In addition, a lesser-known entity such as aerobic vaginosis and the methods for its detection are updated.
Los últimos avances en el microbioma vaginal y el diagnóstico molecular de la vaginosis bacteriana han permitido un mayor conocimiento de esta entidad caracterizando aspectos de su patogenia y el establecimiento de la biocapa vaginal, los modelos y nuevas teorías de la etiología de la misma, cómo se transmite al considerarse hoy como una probable infección de transmisión sexual, la separación de otras entidades como la vaginitis aerobia, el diagnóstico molecular de la misma y el tratamiento y nuevas moléculas que eviten las recaídas frecuentes. Esta entidad y el estudio del microbioma vaginal han permitido considerar estas infecciones como un síndrome polimicrobiano acabando con el dogma: un microorganismo, una enfermedad. Además, se actualiza una entidad menos conocida como es la vaginitis aerobia y los métodos para su detección.
The human vagina is a balanced and dynamic ecosystem, with a complex population of aerobic and anaerobic bacteria, which reach up to 109CFU/ml of vaginal fluid.1–3 Typically, the presence of Lactobacillus spp. is considered to have a protective effect in this ecosystem due to three complementary mechanisms: (a) exclusion by means of the formation of a biofilm, which covers the epithelial cell receptors and blocks the binding of pathogenic microorganisms; (b) growth inhibition due to generation of different antimicrobial compounds: (b1) lactic acid from the fermentative catabolism of sugars, especially glucose, which excretes glycogen and hydrolysis that makes the pH of the vagina 3.5–4.5, (b2) production of hydrogen peroxide (H2O2), fundamentally from Lactobacillus crispatus and Lactobacillus jensenii, (b3) bacteriocins whose effect has only been demonstrated in vitro; and (c) co-aggregation with pathogens. These effects increase the barrier function in the epithelial cells and stimulate innate immunity.
The onset of genital discomfort (exudate, pruritus, dyspareunia and unpleasant odour) is common in women and is of varied aetiology: vulvodynia, contact dermatitis, atrophic vaginitis or lichen sclerosus. Within the infections, there are those caused by yeasts, trichomonas and bacterial vaginosis (BV), which represent 19% of diagnoses in these women.4 These infections produce dysbiosis, in which another more recently recognised clinical entity also intervenes: aerobic vaginitis (AV), which is sometimes confused with BV.5
The focus of this review was to update aspects of the clinical entity known as BV, and the characteristics which enable the diagnosis and treatment of AV in relation to the role of vaginal microbiota are included.
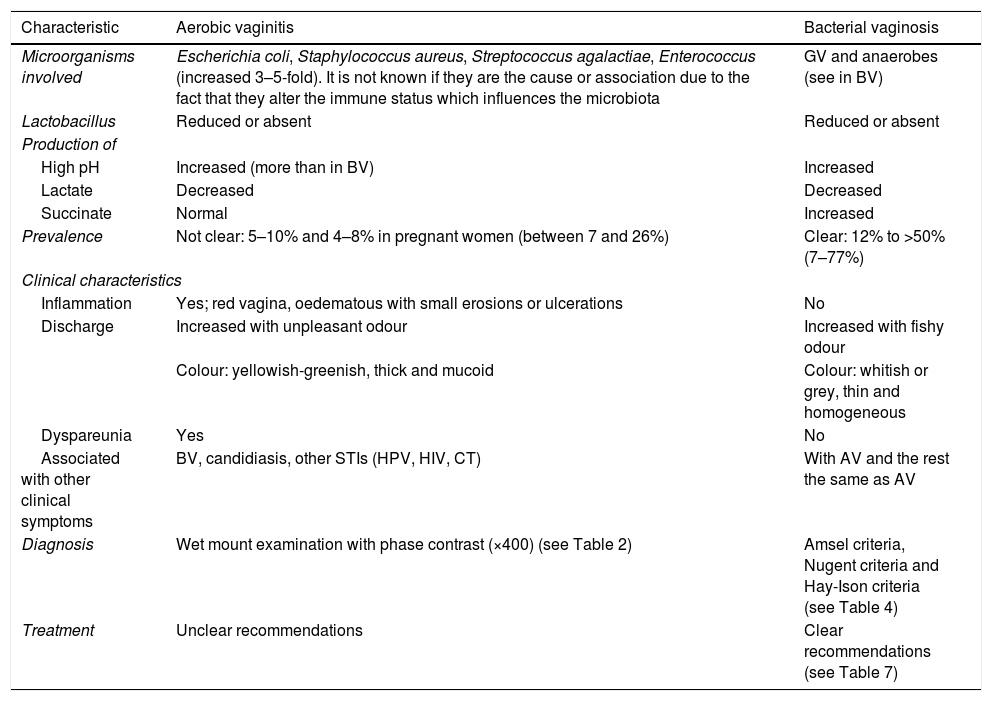
Aerobic vaginitisDefinitionThis entity was named in 2002, and it can be defined as an alteration of the microbiota (less lactobacilli and greater quantities of enteric aerobic bacteria) with variable levels of inflammation, deficiency of epithelial maturation and of immune response with local elevation of IL-1, IL-6 and IL-8. In pregnant women, it is associated with a risk of preterm birth, chorioamnionitis and funisitis of the foetus, and also cervical dysplasia in general.5,6 It is important to differentiate AV from BV (Table 1), although sometimes it is not easy. The majority of clinicians agree with Nugent's score of 7 or more for BV and 3 or less for normal microbiota, but the meaning of intermediate microbiota with a score of 4–6 is not clear.5 In this intermediate group, AV may explain unclear aspects of BV: (a) the very concept of this intermediate microbiota; (b) the variability of the symptoms with Nugent's score; (c) so-called inflammatory BV, and (d) the failure of treatment with metronidazole to prevent preterm birth in many women with BV.5 Therefore, it is believed that this intermediate group may be a mixed group that may include women with AV associated with BV.
Differential characteristics between aerobic vaginitis and bacterial vaginosis.
| Characteristic | Aerobic vaginitis | Bacterial vaginosis |
|---|---|---|
| Microorganisms involved | Escherichia coli, Staphylococcus aureus, Streptococcus agalactiae, Enterococcus (increased 3–5-fold). It is not known if they are the cause or association due to the fact that they alter the immune status which influences the microbiota | GV and anaerobes (see in BV) |
| Lactobacillus | Reduced or absent | Reduced or absent |
| Production of | ||
| High pH | Increased (more than in BV) | Increased |
| Lactate | Decreased | Decreased |
| Succinate | Normal | Increased |
| Prevalence | Not clear: 5–10% and 4–8% in pregnant women (between 7 and 26%) | Clear: 12% to >50% (7–77%) |
| Clinical characteristics | ||
| Inflammation | Yes; red vagina, oedematous with small erosions or ulcerations | No |
| Discharge | Increased with unpleasant odour | Increased with fishy odour |
| Colour: yellowish-greenish, thick and mucoid | Colour: whitish or grey, thin and homogeneous | |
| Dyspareunia | Yes | No |
| Associated with other clinical symptoms | BV, candidiasis, other STIs (HPV, HIV, CT) | With AV and the rest the same as AV |
| Diagnosis | Wet mount examination with phase contrast (×400) (see Table 2) | Amsel criteria, Nugent criteria and Hay-Ison criteria (see Table 4) |
| Treatment | Unclear recommendations | Clear recommendations (see Table 7) |
There are no very reliable data due to the lack of studies performed, but it is estimated in between 5 and 10% of non-pregnant women and 4–8% of pregnant women,7 although it may vary between 7 and 26%.6 The risk factors are similar to those of BV.
Signs and symptomsA purulent yellowish-greenish discharge with inflammation and altered epithelial cells is produced. The signs are a reddened, inflamed vagina, with foul-smelling discharge, burning with ecchymotic haemorrhages, erosions and dyspareunia. The symptoms may be prolonged for up to 12 months or more, and it is sometimes indistinguishable from desquamative inflammatory vaginitis.5
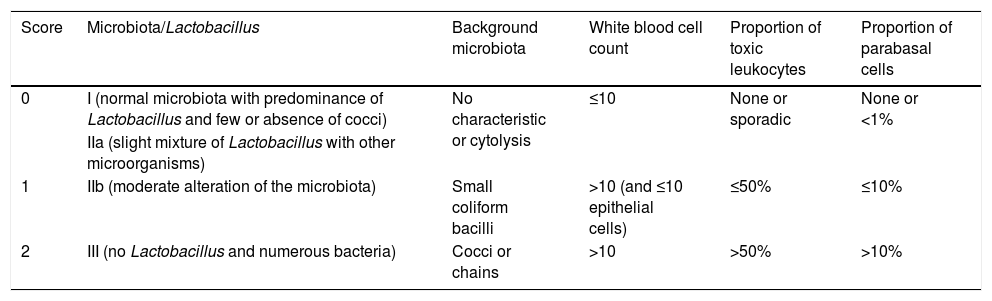
DiagnosisThe most accurate and preferred method is the wet mount examination with phase-contrast microscopy (×400) (Table 2),6 applying a score like in Nugent's criteria: from 0 to 2 means absence of AV; between 3 and 4, mild AV; between 5 and 6, moderate AV; and from 7 to 10, severe. Some studies consider a score of between 5 and 10 to be pathological.
Diagnosis of aerobic vaginitis via a wet mount examination with phase-contrast microscopy (×400).
| Score | Microbiota/Lactobacillus | Background microbiota | White blood cell count | Proportion of toxic leukocytes | Proportion of parabasal cells |
|---|---|---|---|---|---|
| 0 | I (normal microbiota with predominance of Lactobacillus and few or absence of cocci) | No characteristic or cytolysis | ≤10 | None or sporadic | None or <1% |
| IIa (slight mixture of Lactobacillus with other microorganisms) | |||||
| 1 | IIb (moderate alteration of the microbiota) | Small coliform bacilli | >10 (and ≤10 epithelial cells) | ≤50% | ≤10% |
| 2 | III (no Lactobacillus and numerous bacteria) | Cocci or chains | >10 | >50% | >10% |
Parabasal epithelial cells: immature, round, small epithelial cells with a high nucleus-to-cytoplasm ratio.
Toxic leukocytes: leukocytes with abundant secretory granules.
This method is not very widespread and alternative methods are usually used, such as quantitative PCR (its sensitivity [SEN] and specificity [SPE]) are unknown, cultures to detect Streptococcus pyogenes and/or Trichomonas vaginalis (TV) or histological analysis. The observation of the coccoid microbiota under the microscope is a rapid technique, but only reflects a subgroup of patients with AV.6
There is also another test for measuring five enzymatic indicators6: (a) activity of hydrogen peroxidase (indicator of H2O2-producing lactobacilli); (b) activity of leukocyte esterase (indicates inflammation); (c) activity of sialidase (due to high production in AV by Streptococcus agalactiae and in BV by Atopobium vaginae [AV], Gardnerella vaginalis [GV] and Prevotella bivia); (d) activity of beta-glucuronidase (specific to Escherichia coli), and (e) coagulase activity (of Staphylococcus aureus and Enterococcus faecalis); by means of these five indicators obtains a SEN of 90%, but its SPE has not been studied.
Other less common methods are: measuring the oestrogen content in blood (a low oestrogen content in the vagina suggests the presence of immature epithelial or parabasal cells, but it is not relevant when measuring in serum); the pH test, by self-testing, for screening (SEN of 90% but low SPE because it also increases the pH in TV, BV, yeasts, due to sperm or due to menstrual blood) and next-generation sequencing (NGS).
TreatmentIt is not clear and there are no conclusive data with the use of antibiotics. The following can be used: (a) antiseptics such as dequalinium chloride or nifuratel 500mg intravaginal for 10 days; and (b) antibiotics such as clindamycin, kanamycin in 100-mg ovules for six days, rifaximin vaginal for six days or oral moxifloxacin 400mg for six days in one dose. Metronidazole is not appropriate as it does not cover the microorganisms involved in AV. The treatment guidelines advise: when there is atrophy, prescribe estradiol with or without probiotics; if there is inflammation, prescribe local corticosteroids; and when there is clear infection (grade IIb or III [Table 2] and/or abundant growth of E. coli, S. pyogenes, S. agalactiae or staphylococci in culture) include antibiotics. During pregnancy it seems better to use clindamycin, which is active for AV and BV, in addition to reducing preterm birth, but again this regimen is controversial.6
Bacterial vaginosisIntroductionUntil the emergence of molecular techniques, the concept of “a microorganism, a disease” was a dogma, as well as that microorganisms were in planktonic form and as a single species, when in reality they are complex polymicrobial communities forming a biofilm.8 Since 1892, the year in which Albert Döderlein discovered the bacilli that bear his name or Lactobacillus, these have been considered as normal vaginal microbiota.1 This normal microbiota consists of aerobic and anaerobic bacteria, with Lactobacillus representing >95% of all of them. They therefore maintain an acidic pH, ensuring that the H2O2 is present in the environment.9
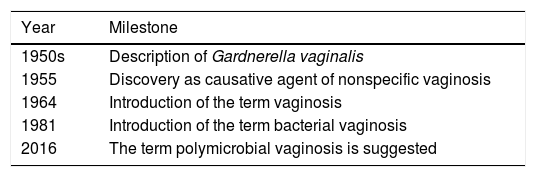
The term vaginosis emerged in the literature in 1964, but it was not until 1981 that the term BV was used, which is now questioned by that of polymicrobial vaginosis. Table 3 shows the historical milestones of BV.10,11
Historical milestones of bacterial vaginosis.
| Year | Milestone |
|---|---|
| 1950s | Description of Gardnerella vaginalis |
| 1955 | Discovery as causative agent of nonspecific vaginosis |
| 1964 | Introduction of the term vaginosis |
| 1981 | Introduction of the term bacterial vaginosis |
| 2016 | The term polymicrobial vaginosis is suggested |
For some time, BV has been considered as a syndrome with different aetiologies. With current methods such as fluorescence in situ hybridisation (FISH), the characteristics of these biofilms in the case of BV have been checked.12 BV represents dysbiosis of the vaginal microbiota13 and the concentration of Lactobacillus is reduced while that of bacterial pathogens is increased in this polymicrobial syndrome.9 Despite the fact that there is not only one microorganism whose presence potentially confirms the diagnosis, GV and AV are strong indicators of BV.14
Although it is accepted that BV is caused by a conglomerate of microorganisms, there is a debate regarding the trigger which would start the alterations: if the founding microorganism of this altered microbiota is GV, if it is a consortium of microorganisms, or if it is due to other factors such as the use of a vaginal douche.
PrevalenceIt affects 12% of women in Australia, 29% in the US and >50% of women in the Sub-Saharan area,15 although there is great variation between 7 and 70% of women.16,17
EpidemiologyBV is the most common vaginal dysbiosis in women of childbearing age,1 and the most common cause of vaginitis with abundant vaginal discharge and odour in pregnant and non-pregnant women.9 Furthermore, it is more common in women who have sex with women (WSW) or a mixture of women and men, than in women who have sex with men (WSM). The mechanism could be due to sharing sexual objects, but it is not known if the vaginal fluid shared between women is more efficient than masculine penetration. In addition, the transmission of anaerobes is less efficient, meaning that it would be in favour of the hypothesis of primary transmission of GV.18
There is a debate surrounding whether it can be defined as a true sexually transmitted infection (STI). It was suggested historically in the 1950s when Gardner and Dukes saw the transmission from infected women to non-infected women, but this theory was abandoned as (a) there was no counterpart theory in men; (b) the treatment of men did not reduce BV in couples (although these studies were of low methodological quality), and (c) the presence of various pathogens went against this possibility.
Currently, the theory in favour of an STI has been taken up again due to various noteworthy and highly significant aspects15:
- (a)
There are many cross-sectional and longitudinal studies linked to sexual activity.
- (b)
It is associated with low condom use.
- (c)
Women with BV have a greater number of sexual partners than women without BV.
- (d)
It appears earlier in sexually active women.
- (e)
There is a reservoir in men, which hosts the microorganisms involved in the subpreputial space and distal urethra.
- (f)
Only tobacco (and not vaginal douches, dietary factors and stress) has been consistently associated.
- (g)
It has been linked to sexual transmission between women, to a female partner with symptoms or a history of BV and to receptive oral sex.
- (h)
There is concordance of biotypes and oligotypes between men and women who are a couple.
- (i)
Circumcision reduces the probability in women who are the partner of these men.
- (j)
Non-gonococcal urethritis (NGU) is more common in men who have female partners with BV, but only Sneathia, BV-associated bacteria (BVAB), such as BVAB2 and BVAB3, has been found in a non-statistically significant manner.
- (k)
It is linked to balanoposthitis caused by GV in men.
The concept of BV is influenced by the diagnostic method which is carried out:
- (a)
Clinical, using the Amsel criteria.17
- (b)
Research, using the Nugent criteria16 or their modification of the Hay-Ison criteria.19
The Amsel criteria have been used in clinical studies, while the Nugent criteria based on the Gram stain have been used in research studies (Table 4): 0–3 normal, 4–6 intermediate with GV/Bacteroides morphotypes and from 7 to 10 with numerous GV/Bacteroides and appearance of curved anaerobic bacilli (Mobiluncus and possibly BVAB1) in 9–10.
Amsel criteria, Nugent criteria and Hay-Ison criteria.
| Amsel criteria (clinical)a | Characteristics (%) |
|---|---|
| Vaginal discharge | Stringy, homogeneous, white, stuck to the walls and even |
| Vaginal pH | >4.5 (90%) |
| 10% KOH | Fishy smell |
| Clue cells (×40) | >20% (>90%) |
| Nugent criteria (Gram stain)b | Count per oil immersion field (value) |
|---|---|
| Lactobacillus type gram-positive bacteria | >30 (0) |
| 5–30 (1) | |
| 1–4 (2) | |
| <1 (3) | |
| 0 (4) | |
| GV and Bacteroides type gram-negative bacilli | >30 (4) |
| 5–30 (3) | |
| 1–4 (2) | |
| <1 (1) | |
| 0 (0) | |
| Curved gram-variable bacilli | >5 (2) |
| <1–4 (1) | |
| 0 (0) |
| Hay-Ison criteria (Gram stain) | Grade |
|---|---|
| Predominance of Lactobacillus type gram-positive bacteria | 1. Normal |
| Mixed microbiota of a Lactobacillus and GV and Mobiluncus type gram-negative bacilli | 2. Intermediate |
| Predominance of GV and/or Mobiluncus with few/no Lactobacillus | 3. BV |
National Institutes of Health (NIH): Diagnosis of BV with the Nugent criteria (at least a score of 7) and one positive Amsel criteria, regardless of the symptoms.
The presence of polymorphonuclear leukocytes suggests the coexistence of another process such as cervicitis.
The Amsel criteria generate problems of overdiagnosis, as there would be many asymptomatic women diagnosed with BV. In a study in which 29% of women presented with BV according to the Amsel criteria, only 15.7% were symptomatic.20 However, this group of women had more complications associated with BV, regardless of whether or not they had symptoms.
The Nugent criteria have high diagnostic accuracy and reliability, with high inter- and intra-centre and interobserver reproducibility. However, the identification of microorganisms in the clue cells in the Gram stain is not always accurate. It has been demonstrated using quantitative PCR and FISH that the curved gram-negative bacilli that were believed to represent Mobiluncus spp. are more likely to be (in a factor of 100 to 1) BVAB1.21 In addition, a correlation between a greater score in accordance with the Nugent criteria and the presence of more symptoms in women has not been observed, although there are few studies conducted in this regard.18
For this reason, the National Institutes of Health (NIH) working group recommends using a combination of the Nugent criteria with at least a score of 7 and one positive Amsel criteria for the definition of BV, regardless of the symptoms.22
- (c)
Another alternative is the visualisation of the biofilm in desquamated cells in the urinary sediment with FISH with a diagnostic accuracy of 0.94, a SEN of 83% and a SPE of 97%, a positive predictive value of 83% and a negative predictive value of 97%.21 This method is appropriate for clinical and epidemiological studies, as the sample is easy to obtain.
- (d)
Currently, the culture of GV is not recommended nor is the use of specific probes for GV. The Gram stain has shown greater SPE than the culture in recent trials based on the study of the microbiome. The GV culture does not suggest nor test for BV or the need for its treatment, as 50% of asymptomatic women present with a positive culture. The FDA recently approved the Max Vaginal Panel (Becton-Dickinson, New Jersey, USA) for the study of the microbiome, which makes it possible to carry out two to 24 tests simultaneously. It enables the detection of L. crispatus, L. jensenii, GV, AV, BVAB2, Megasphaera type 1, Candida group (C. albicans, C. parapsilosis, C. dubliniensis, C. glabrata and C. krusei) and TV.23
The concept of BV as a syndrome was established many years ago, but FISH techniques have enabled biofilms where GV is the predominant microorganism to be seen, followed by AV and, to a lesser extent, by Bacteroides, Veillonella, Ruminococcus and the genus Streptococcus.12
Although traditionally it has been associated with the presence of GV, nowadays BV is associated with bacterial conglomerates, as shown in Table 5.1 Using molecular methods in the vaginal microbiota, vulva/labia, cervix and uterus, more than 250 bacterial species, yeasts, Chlamydia, Archaea, viruses and protozoa have been detected.24 In one study, up to six clusters or types of community statuses of the vaginal microbiota were observed using genome sequencing by Illumina (NGS): two associated with normal vaginal microbiota (one dominated by Lactobacillus iners and another by L. crispatus) and four associated with BV (dominated by P. bivia, GV, Lachnospiraceae or a mixture of different species).24 Similarly, other authors have described a cluster with the presence of Lactobacillus associated with normal microbiota, and another three groups with dysbiosis with the order Lachnospiraceae and genera Sneathia and Prevotella as dominant microorganisms.25 Finally, studies have been performed in pregnant and non-pregnant HIV-negative women, with five groups being described: (1) L. crispatus; (2) Lactobacillus gasseri; (3) L. iners; (4) Peptoniphilus, Prevotella and Anaerococcus spp. and a high quantity of GV or Ureaplasma; and (5) L. jensenii. Using transcriptomics, it has been observed that L. iners adapts to very different environments of BV.24,26
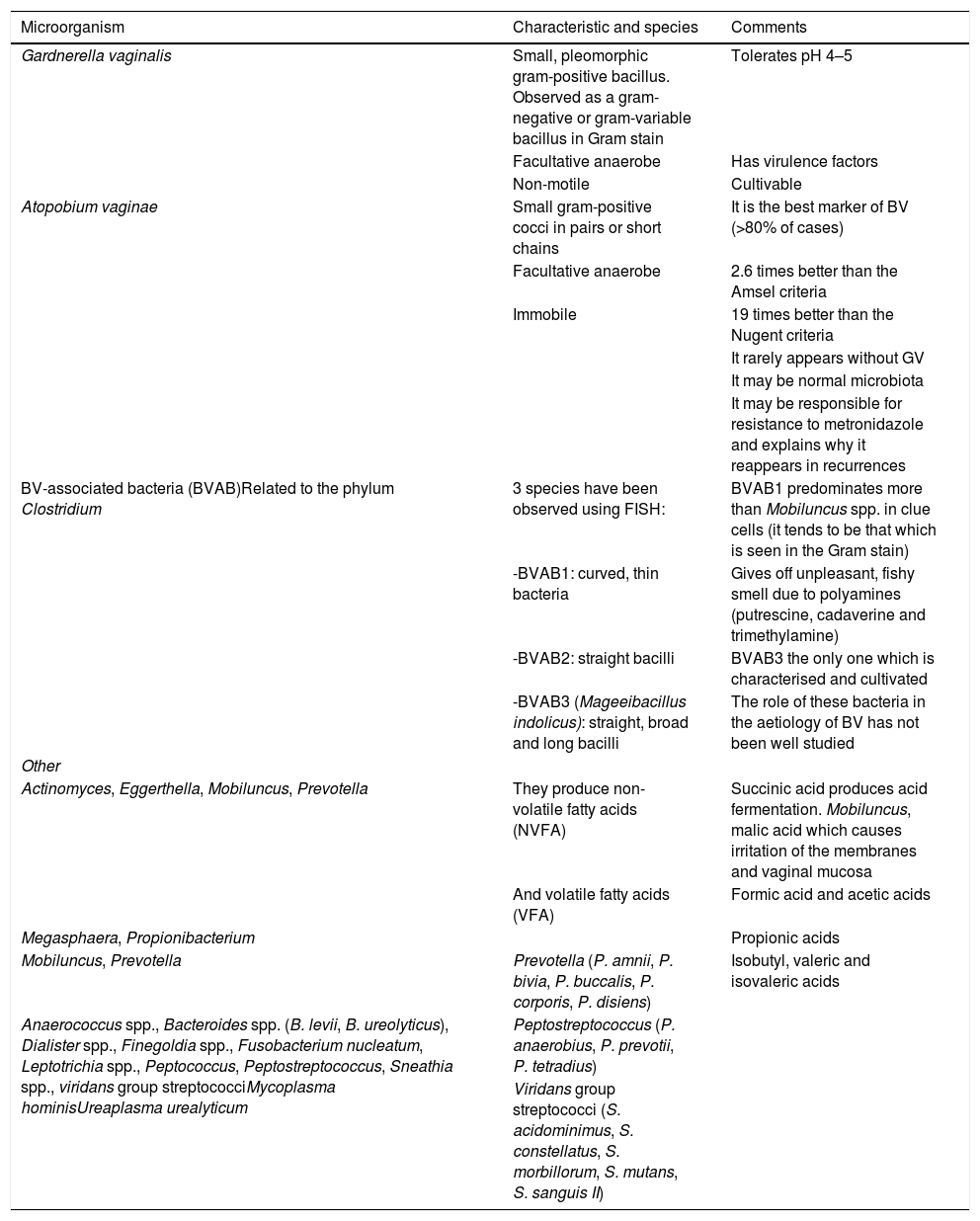
Microorganisms associated and/or found in bacterial vaginosis.
| Microorganism | Characteristic and species | Comments |
|---|---|---|
| Gardnerella vaginalis | Small, pleomorphic gram-positive bacillus. Observed as a gram-negative or gram-variable bacillus in Gram stain | Tolerates pH 4–5 |
| Facultative anaerobe | Has virulence factors | |
| Non-motile | Cultivable | |
| Atopobium vaginae | Small gram-positive cocci in pairs or short chains | It is the best marker of BV (>80% of cases) |
| Facultative anaerobe | 2.6 times better than the Amsel criteria | |
| Immobile | 19 times better than the Nugent criteria | |
| It rarely appears without GV | ||
| It may be normal microbiota | ||
| It may be responsible for resistance to metronidazole and explains why it reappears in recurrences | ||
| BV-associated bacteria (BVAB)Related to the phylum Clostridium | 3 species have been observed using FISH: | BVAB1 predominates more than Mobiluncus spp. in clue cells (it tends to be that which is seen in the Gram stain) |
| -BVAB1: curved, thin bacteria | Gives off unpleasant, fishy smell due to polyamines (putrescine, cadaverine and trimethylamine) | |
| -BVAB2: straight bacilli | BVAB3 the only one which is characterised and cultivated | |
| -BVAB3 (Mageeibacillus indolicus): straight, broad and long bacilli | The role of these bacteria in the aetiology of BV has not been well studied | |
| Other | ||
| Actinomyces, Eggerthella, Mobiluncus, Prevotella | They produce non-volatile fatty acids (NVFA) | Succinic acid produces acid fermentation. Mobiluncus, malic acid which causes irritation of the membranes and vaginal mucosa |
| And volatile fatty acids (VFA) | Formic acid and acetic acids | |
| Megasphaera, Propionibacterium | Propionic acids | |
| Mobiluncus, Prevotella | Prevotella (P. amnii, P. bivia, P. buccalis, P. corporis, P. disiens) | Isobutyl, valeric and isovaleric acids |
| Anaerococcus spp., Bacteroides spp. (B. levii, B. ureolyticus), Dialister spp., Finegoldia spp., Fusobacterium nucleatum, Leptotrichia spp., Peptococcus, Peptostreptococcus, Sneathia spp., viridans group streptococciMycoplasma hominisUreaplasma urealyticum | Peptostreptococcus (P. anaerobius, P. prevotii, P. tetradius) | |
| Viridans group streptococci (S. acidominimus, S. constellatus, S. morbillorum, S. mutans, S. sanguis II) |
There is high variability among these studies with regard to sampling, the storage of samples, the processing of samples, the DNA extraction kit, technical variations in PCR amplification and the use of different primers, as well as with regard to the statistical methods used. In addition, changes due to menstruation, sexual activity, spermicides, douches and antibiotics were not taken into account.
In summary, molecular studies have demonstrated, as classic studies did previously using cultures, that there is a high presence of Lactobacillus in a healthy vagina and that it is not possible to associate it with one single microbiota composition.24 Therefore, different clusters of vaginal microbiota can be defined, each one correlated with the predominance of one or more microorganisms.9 If the Nugent criteria are compared with the vaginal microbiota defined molecularly, a good, although not a total, correlation is observed.27
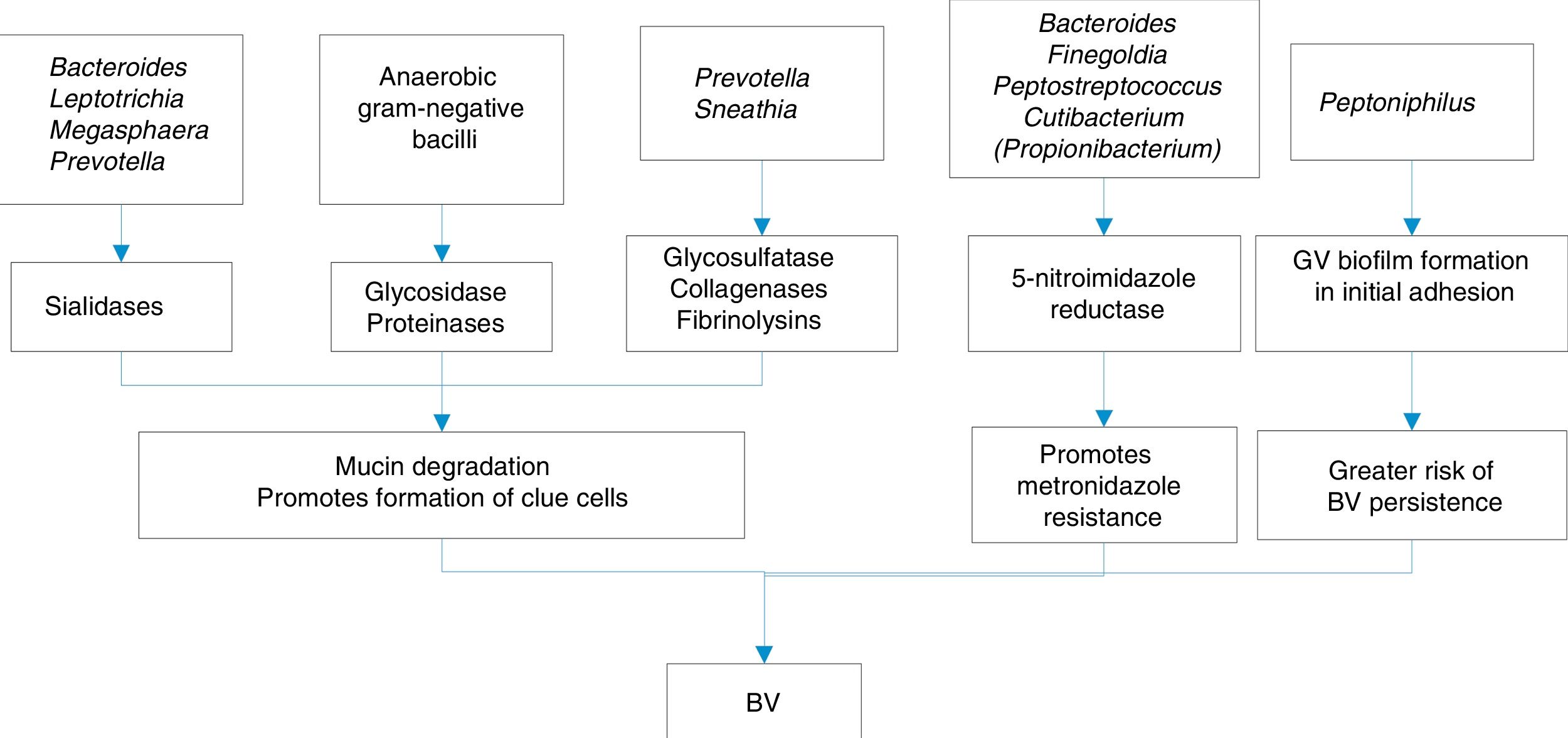
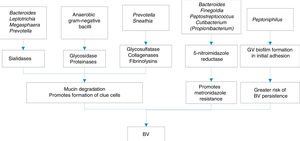
The substances released by this conglomerate of bacteria which make up the biofilms and which may be involved in BV are varied:
- (a)
Immunomodulatory such as haemolysins, volatile fatty acids (VFA) and non-volatile fatty acids (NVFA), proteases and sialidases. Sialidases inhibit serum IgA, increasing the risk of preterm birth due to cytokines, and also increase IL-1b, IL-8 and TNF-alpha, which, along with prolidase, confer greater susceptibility to HIV infections and herpes simplex virus type 2 (HSV-2).
- (b)
Proinflammatory substances, such as lipopolysaccharide (LPS), which acts on cytokines, IL-1b, IL-6 and TNF-alpha, favouring preterm birth.
Some of the mechanisms of production of BV are shown in Fig. 1.
An alteration of the protein content of the cervicovaginal fluid has been detected in BV by proteome analysis: neutrophil elastase, kaliocin-1, defensin-1 of neutrophils, lambda-2 chain C regions of Ig and protein S100-A7. This seems to indicate that the alteration of the microbiota is capable of interfering with the immune response mechanisms.28
Regarding the role of GV, a phenotypic and genotypic heterogeneity has been observed, in addition to a variability in the virulence potential. Four GV clades have been described. It has been attempted to correlate them with the vaginal microbiota clusters. The hypothesis is that there is colonisation by different GV clades in the vagina which express different determinants of virulence.27 The role of some virulence factors such as haemolysin or vaginolysin (which is a cholesterol-dependent cytolysin), sialidase or prolidase is not clear. Furthermore, GV clade A is lipase-positive and clade B is sialidase-positive, which could indicate that different clades are involved either in the resolution of BV re-establishing the Lactobacillus, or, on the other hand, in the transition to infection by yeasts or AV.1
GV appears in the vagina in two forms: one in a scattered form (106–8 cells per gram in the vaginal fluid) and another in the infectious or transmissible form in the cohesive form a biofilm (1010,11 cells per gram).29 This pattern is also seen in men and in semen. It is not clear if it is different strains of GV, although all those which form part of the biofilms present genes involved in the synthesis of exopolysaccharide.14
Nor are there specific data on the cell surface receptors for GV, although various studies have demonstrated significant differences in the binding capacity and cytotoxicity between the pathogenic and non-pathogenic strains of this microorganism.30,31 Various GV ecotypes are capable of altering the adhesion of Lactobacillus, measured by cell-surface glycosaminoglycans, thanks to the production of glycosidases which degrade these receptors.31,32 In addition, the production of sialidases and vaginolysin is capable of altering the epithelial cells, therefore affecting the bound microbiota.33 Furthermore, lactobacilli are capable of affecting both the binding and the internalisation of GV, interfering with its pili and with fibronectin-binding proteins.30
How does the protection and alteration of the vaginal ecosystem occur?Lactobacilli play a key role in the protection of the vaginal mucosa and in the inhibition of colonisation by pathogenic bacteria through a series of mechanisms1:
- (a)
Adhesion to glycolipid receptors on the surface of vaginal epithelial cells. The presence of high volumes of Lactobacillus inoculum in the vaginal discharge of healthy premenopausal women (107–108CFU/g) prevents the binding of genitourinary pathogens to these receptors by means of a competitive exclusion mechanism.
- (b)
Coaggregation of Lactobacillus with pathogens and autoaggregation or quorum sensing.
Both mechanisms contribute to the formation of the biofilm in a healthy vagina and to the inhibition of the growth of pathogens. In a study of three vaginal populations of lactobacilli (Lactobacillus acidophilus, L. gasseri and L. jensenii), the autoaggregation ability measured by surface proteins or lipoproteins was demonstrated. In addition, the three strains adhered strongly to vaginal epithelial cells by glycoproteins (L. acidophilus and L. gasseri) and carbon hydrates (L. jensenii), while the lactobacilli recovered from other sources, such as dairy products, adhere in significantly lower numbers, which indicates that adherence is an idiosyncratic property of vaginal lactobacilli.34
- (c)
Vaginal acidification with the production of lactic acid. Metabolomic studies24 have observed the production of lactic acid, acetic acid, glycerol and others in a healthy vagina, while in BV there is 2-hydroxyvalerate, gamma-hydroxybutyrate or succinate. A diagnostic test could be developed to detect gamma-hydroxybutyrate in vaginal swabs with an elevated pH.
- (d)
The production of H2O2, bacteriocins and biosurfactants contributes to the inhibition of the growth of some pathogens.
On the other hand, the factors which alter the ecosystem would be:
- (a)
Excessive vaginal douches. Vaginal douches35 are defined as the use of a liquid solution in the vagina. It is estimated that they are used by 27–59% of women depending on ethnic, cultural and educational differences. Most studies advise against their use, although some see them as beneficial with the use of acetic acid to eliminate semen and the possible transmission of pathogens, unpleasant vaginal odour and to relive vaginal irritation. However, it is known that it increases the risk of developing BV an average of 2.1-fold (use of vaginal douches ≥once per month increases the risk 1.6-fold; ≥twice per month increases the risk 2.5-fold; and douches in the last two months increases the risk 2.9-fold).
- (b)
Use of spermicides (nonoxynol-9).
- (c)
Antibiotics.
- (d)
Phages (not tested). It is postulated that there is sexual transmission of phages with the ability to destroy the Lactobacillus population. Tobacco behaves as a facilitating factor, through the production of benzo[a]pyrene diol epoxide type promoters.
Three explanatory models of BV have been proposed,1 and all of them are in agreement in that there is: reduction of Lactobacillus spp., exposure and growth of BVAB and other BV-associated bacteria, and an increase in vaginal pH.
The three proposed models are: (a) depletion of Lactobacillus spp. model, (b) primary pathogen model and (c) polymicrobial pathogen model:
- (a)
In the first model, it is established that there is a reduction of H2O2-producing Lactobacillus spp., prior to the increase in vaginal pH which triggers the overgrowth of anaerobes producing BV. However, in opposition to this theory, there is the theory that there are healthy women without Lactobacillus and, furthermore, there are women in whom AV is isolated who produce lactic acid. According to this model, a treatment strategy would be the use of probiotics and acidifying agents.
- (b)
In the primary pathogen model, a pathogen such as GV would be introduced due to sexual activity which creates the conditions, due to its virulence factors and the formation of the biofilm, for the growth of an intermediate microbiota and, finally, the establishment of the BV-associated microbiota. Contrary to this model is the fact that GV is also detected in healthy women who are not sexually active, although it could be different genotypes and biotypes (e.g. biotype 5 is observed in healthy women). According to this model, agents which alter the GV biofilm could be used as treatment.
- (c)
In the polymicrobial pathogen model, it would be a set of BV bacteria which trigger colonisation after sexual activity, without the presence of an intermediate microbiota, with synergisms among them which would reduce the Lactobacillus population. In this case, and in opposition to this model, is the lower virulence of these other BV bacteria compared to that presented by GV. Treatment in this model should include agents which alter the polymicrobial biofilm.
- (d)
There are other proposed models or theories apart from the above-mentioned three:
- (d1)
BV-associated bacteria are internalised in the epithelial cells, meaning that microorganisms such as GV, AV and P. bivia would escape the defences and the action of antibiotics such as clindamycin and metronidazole. There is no evidence of this mechanism.
- (d2)
Alkaline semen would reduce the acidity of the vagina by increasing the pH after sexual intercourse, promoting the growth of GV. In this case, it could be defined as a sexually associated infection more than as an STI in the strict sense of the term.
- (d3)
Genetic polymorphisms would promote BV. There are no data.
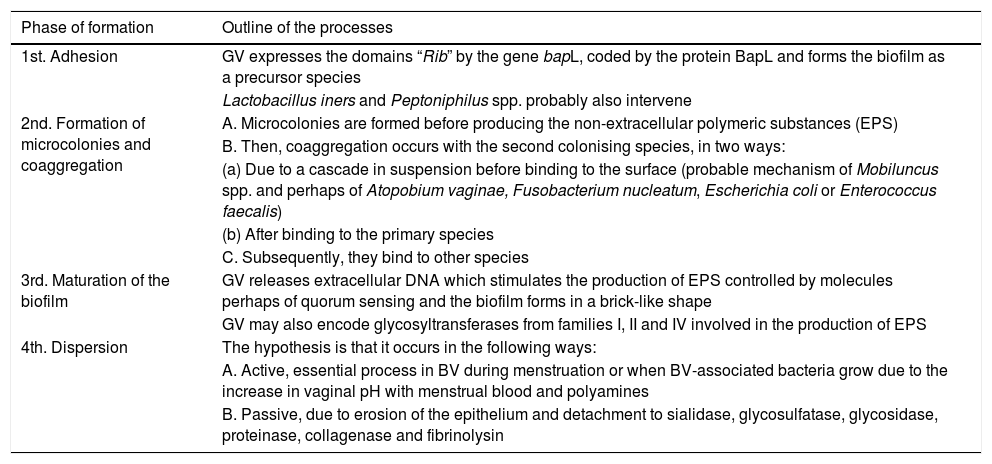
As in other locations, for example in the oral biofilm, the formation of the biofilm in BV has advantages for GV: it increases its tolerance to H2O2 five-fold and to lactic acid four to eight-fold.1 The stages of biofilm formation are detailed in Table 6.
Process and chronological stages of the formation of the biofilm in bacterial vaginosis.
| Phase of formation | Outline of the processes |
|---|---|
| 1st. Adhesion | GV expresses the domains “Rib” by the gene bapL, coded by the protein BapL and forms the biofilm as a precursor species |
| Lactobacillus iners and Peptoniphilus spp. probably also intervene | |
| 2nd. Formation of microcolonies and coaggregation | A. Microcolonies are formed before producing the non-extracellular polymeric substances (EPS) |
| B. Then, coaggregation occurs with the second colonising species, in two ways: | |
| (a) Due to a cascade in suspension before binding to the surface (probable mechanism of Mobiluncus spp. and perhaps of Atopobium vaginae, Fusobacterium nucleatum, Escherichia coli or Enterococcus faecalis) | |
| (b) After binding to the primary species | |
| C. Subsequently, they bind to other species | |
| 3rd. Maturation of the biofilm | GV releases extracellular DNA which stimulates the production of EPS controlled by molecules perhaps of quorum sensing and the biofilm forms in a brick-like shape |
| GV may also encode glycosyltransferases from families I, II and IV involved in the production of EPS | |
| 4th. Dispersion | The hypothesis is that it occurs in the following ways: |
| A. Active, essential process in BV during menstruation or when BV-associated bacteria grow due to the increase in vaginal pH with menstrual blood and polyamines | |
| B. Passive, due to erosion of the epithelium and detachment to sialidase, glycosulfatase, glycosidase, proteinase, collagenase and fibrinolysin |
The symptoms which appear in BV are: homogeneous discharge (90%) and fishy vaginal odour during menstruation, after sexual intercourse and with minimal itching or irritation. There is perivaginal irritation and rarely dysuria or dyspareunia, occasional abdominal pain, discharge at the introitus, there is no erythema or oedema of the labia and vulva.
The following are described as complications: chorioamnionitis, endometritis, salpingitis and pelvic inflammatory disease [PID] (BV treatment reduces PID after induced abortion). In pregnant women it can trigger: spontaneous abortion, premature rupture of membranes, preterm birth, premature baby, low birthweight, postpartum endometritis and infections of the postoperative wound.9 It also increases the risk of acquiring other STIs (Chlamydia trachomatis, Neisseria gonorrhoeae, HSV-2 and HIV) and transmission of HIV to male partners. There is also a negative impact on self-esteem, sexual relationships and quality of life.
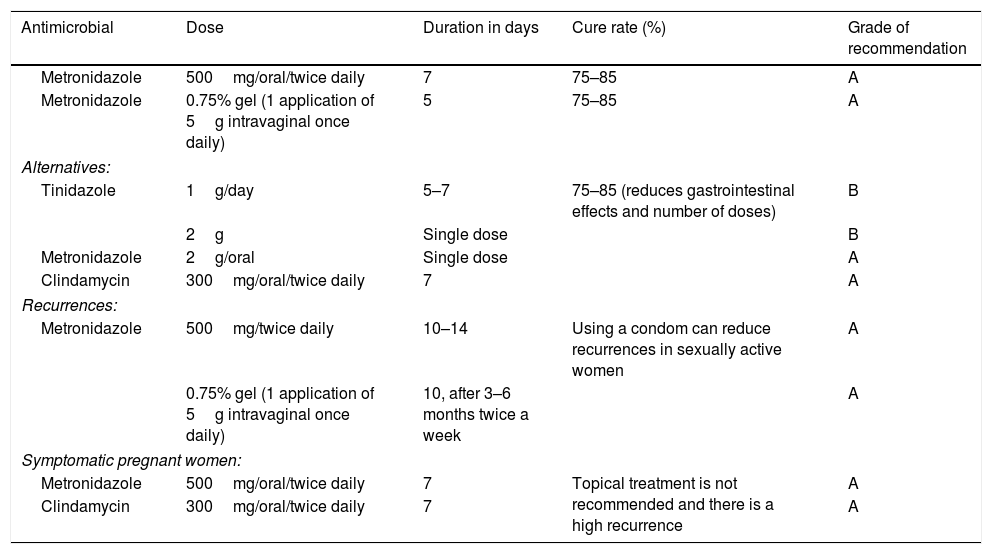
TreatmentStandard treatment is carried out with metronidazole or clindamycin (Table 7). In the short-term, a cure rate of 80–90% is obtained, although 50–70% of women have a recurrence after three to six months. In the long-term, a cure rate of up to 80% is achieved. It is therefore worth confirming the diagnosis in these cases. It is not clear if recurrence is due to (a) resistance (it does not seem probable as metronidazole is active against anaerobic gram-negative bacteria, Mobiluncus mulieris and less active against GV, anaerobic cocci and Mobiluncus curtisii) and inactive against Mycoplasma hominis and AV; (b) recurrence or reinfection, which seems more likely, due to various possible factors although none demonstrated reliably: (b1) an exogenous source (male or female sexual partner), (b2) endogenous source (rectal reservoir), (b3) formation of biofilms, (b4) risk factors such as vaginal douches and smoking, (b5) recolonisation failure or (b6) activation of phages.23 There is no evidence of complications associated with BV, meaning that standard treatment is only recommended in symptomatic women.
Established treatments of bacterial vaginosis.
| Antimicrobial | Dose | Duration in days | Cure rate (%) | Grade of recommendation |
|---|---|---|---|---|
| Metronidazole | 500mg/oral/twice daily | 7 | 75–85 | A |
| Metronidazole | 0.75% gel (1 application of 5g intravaginal once daily) | 5 | 75–85 | A |
| Alternatives: | ||||
| Tinidazole | 1g/day | 5–7 | 75–85 (reduces gastrointestinal effects and number of doses) | B |
| 2g | Single dose | B | ||
| Metronidazole | 2g/oral | Single dose | A | |
| Clindamycin | 300mg/oral/twice daily | 7 | A | |
| Recurrences: | ||||
| Metronidazole | 500mg/twice daily | 10–14 | Using a condom can reduce recurrences in sexually active women | A |
| 0.75% gel (1 application of 5g intravaginal once daily) | 10, after 3–6 months twice a week | A | ||
| Symptomatic pregnant women: | ||||
| Metronidazole | 500mg/oral/twice daily | 7 | Topical treatment is not recommended and there is a high recurrence | A |
| Clindamycin | 300mg/oral/twice daily | 7 | A | |
In general, there is a 58–92% cure rate after one month. No nitroimidazole has demonstrated superiority over others from the same family. The combination of oral use plus vaginal use seems most efficient (80–86% cure rate versus 75–86% without the combination). Oral or local use of clindamycin or metronidazole presents the same cure rates.36
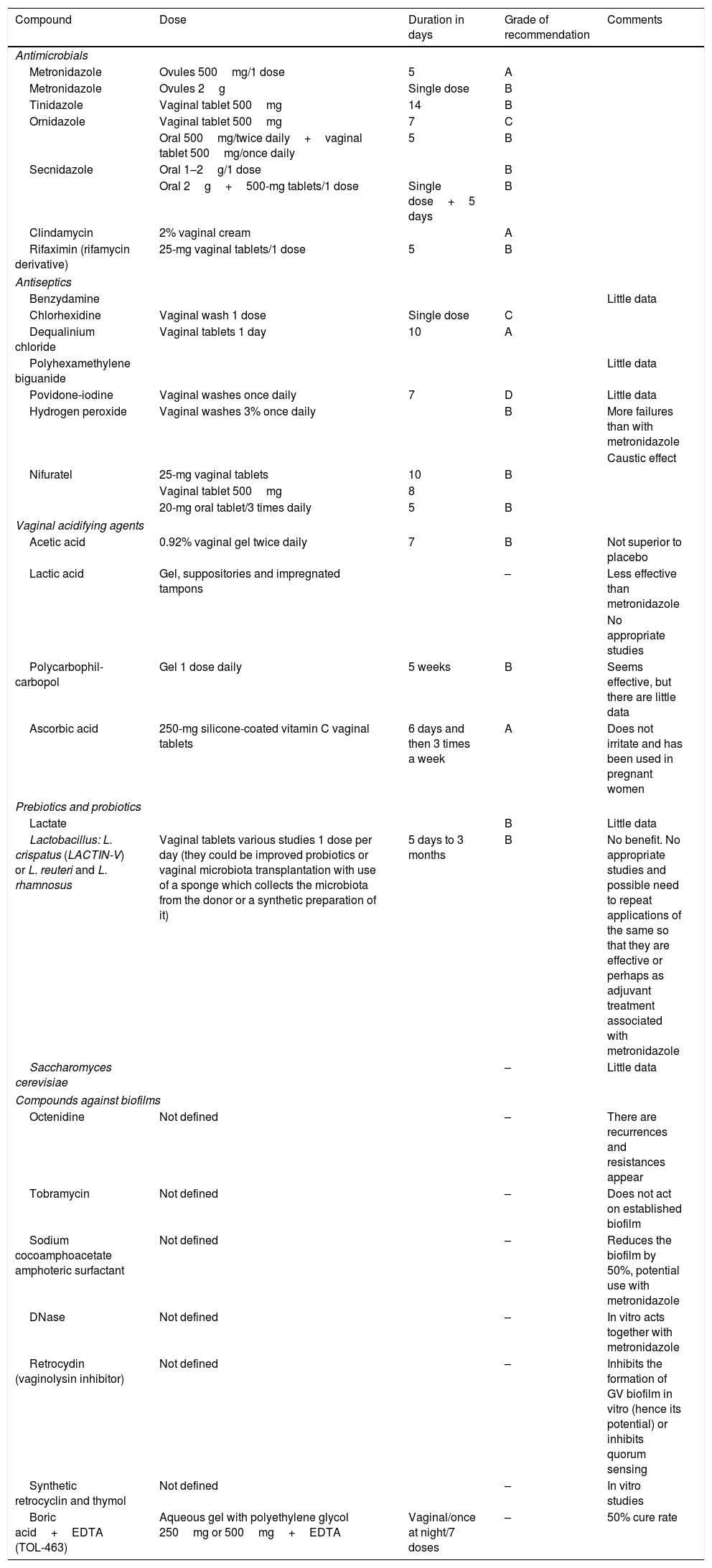
Due to these cure rates, different treatment regimens or new molecules which would act in light of this new knowledge on how pathogenesis occurs in BV have been sought, such as the case of biofilm formation (Table 8).23,35–38
Alternative treatments tested in bacterial vaginosis.
| Compound | Dose | Duration in days | Grade of recommendation | Comments |
|---|---|---|---|---|
| Antimicrobials | ||||
| Metronidazole | Ovules 500mg/1 dose | 5 | A | |
| Metronidazole | Ovules 2g | Single dose | B | |
| Tinidazole | Vaginal tablet 500mg | 14 | B | |
| Ornidazole | Vaginal tablet 500mg | 7 | C | |
| Oral 500mg/twice daily+vaginal tablet 500mg/once daily | 5 | B | ||
| Secnidazole | Oral 1–2g/1 dose | B | ||
| Oral 2g+500-mg tablets/1 dose | Single dose+5 days | B | ||
| Clindamycin | 2% vaginal cream | A | ||
| Rifaximin (rifamycin derivative) | 25-mg vaginal tablets/1 dose | 5 | B | |
| Antiseptics | ||||
| Benzydamine | Little data | |||
| Chlorhexidine | Vaginal wash 1 dose | Single dose | C | |
| Dequalinium chloride | Vaginal tablets 1 day | 10 | A | |
| Polyhexamethylene biguanide | Little data | |||
| Povidone-iodine | Vaginal washes once daily | 7 | D | Little data |
| Hydrogen peroxide | Vaginal washes 3% once daily | B | More failures than with metronidazole | |
| Caustic effect | ||||
| Nifuratel | 25-mg vaginal tablets | 10 | B | |
| Vaginal tablet 500mg | 8 | |||
| 20-mg oral tablet/3 times daily | 5 | B | ||
| Vaginal acidifying agents | ||||
| Acetic acid | 0.92% vaginal gel twice daily | 7 | B | Not superior to placebo |
| Lactic acid | Gel, suppositories and impregnated tampons | – | Less effective than metronidazole | |
| No appropriate studies | ||||
| Polycarbophil-carbopol | Gel 1 dose daily | 5 weeks | B | Seems effective, but there are little data |
| Ascorbic acid | 250-mg silicone-coated vitamin C vaginal tablets | 6 days and then 3 times a week | A | Does not irritate and has been used in pregnant women |
| Prebiotics and probiotics | ||||
| Lactate | B | Little data | ||
| Lactobacillus: L. crispatus (LACTIN-V) or L. reuteri and L. rhamnosus | Vaginal tablets various studies 1 dose per day (they could be improved probiotics or vaginal microbiota transplantation with use of a sponge which collects the microbiota from the donor or a synthetic preparation of it) | 5 days to 3 months | B | No benefit. No appropriate studies and possible need to repeat applications of the same so that they are effective or perhaps as adjuvant treatment associated with metronidazole |
| Saccharomyces cerevisiae | – | Little data | ||
| Compounds against biofilms | ||||
| Octenidine | Not defined | – | There are recurrences and resistances appear | |
| Tobramycin | Not defined | – | Does not act on established biofilm | |
| Sodium cocoamphoacetate amphoteric surfactant | Not defined | – | Reduces the biofilm by 50%, potential use with metronidazole | |
| DNase | Not defined | – | In vitro acts together with metronidazole | |
| Retrocydin (vaginolysin inhibitor) | Not defined | – | Inhibits the formation of GV biofilm in vitro (hence its potential) or inhibits quorum sensing | |
| Synthetic retrocyclin and thymol | Not defined | – | In vitro studies | |
| Boric acid+EDTA (TOL-463) | Aqueous gel with polyethylene glycol 250mg or 500mg+EDTA | Vaginal/once at night/7 doses | – | 50% cure rate |
In the case of the use of lactobacilli, the ideal lactobacilli strains with probiotic potential would be those which produce lactic acid, H2O2, which form biofilms and whose minimum inhibitory concentration (MIC) for metronidazole, clindamycin and nonoxynol-9 is high. Endogenous lactobacilli from the vagina have not presented carcinogenetic potential and do not code for antibiotic resistance determinants transmissible to the present microbiota, despite containing numerous plasmids. Furthermore, lactobacilli which produce genital conditions have not been reported, and very rarely are they the cause of infections in other anatomical regions.39–41
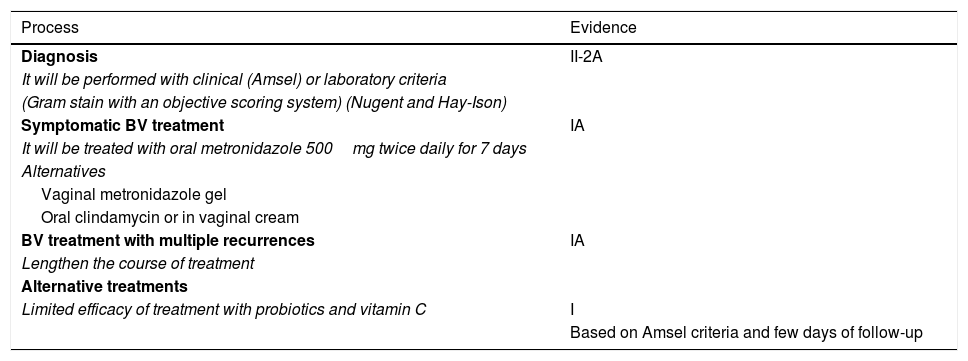
Table 9 shows current evidence of diagnosis and BV treatment.36
Evidence in the diagnosis and treatment of bacterial vaginosis.
| Process | Evidence |
|---|---|
| Diagnosis | II-2A |
| It will be performed with clinical (Amsel) or laboratory criteria | |
| (Gram stain with an objective scoring system) (Nugent and Hay-Ison) | |
| Symptomatic BV treatment | IA |
| It will be treated with oral metronidazole 500mg twice daily for 7 days | |
| Alternatives | |
| Vaginal metronidazole gel | |
| Oral clindamycin or in vaginal cream | |
| BV treatment with multiple recurrences | IA |
| Lengthen the course of treatment | |
| Alternative treatments | |
| Limited efficacy of treatment with probiotics and vitamin C | I |
| Based on Amsel criteria and few days of follow-up |
In conclusion, an important breakthrough in the knowledge of these two clinical entities has occurred, which enables better management and diagnosis in light of new studies on the vaginal microbiome.
Conflicts of interestNone declared.
Please cite this article as: Vazquez F, Fernández-Blázquez A, García B. Vaginosis. Microbiota vaginal. Enferm Infecc Microbiol Clin. 2019;37:592–601.