The purpose of tuberculosis treatment is twofold: to provide an individual benefit centred on healing the patient with TB, and to provide a collective benefit to the community in which the patient resides. The different treatment regimens for tuberculosis sensitive to first-line antituberculosis drugs as well as resistant tuberculosis are examined and the peculiarities in the management of pulmonary and extrapulmonary tuberculosis are discussed.
El tratamiento de la enfermedad tuberculosa busca un doble beneficio: un beneficio individual, centrado en la curación del paciente afecto de tuberculosis, y un beneficio colectivo, de la comunidad en la cual reside el paciente. Se exponen los diferentes regímenes de tratamiento tanto de la tuberculosis sensible a fármacos antituberculosos de primera línea como de la tuberculosis resistente. Se comentan las peculiaridades en cuanto al manejo de la tuberculosis pulmonar y extrapulmonar.
First, it must be made clear that this article refers to the treatment of tuberculosis (TB) in general and to the treatment of TB disease in adults in particular.
Second, the different terms used in reference to resistant TB must be defined. Single-drug-resistant TB is TB caused by a strain resistant to a single first-line drug. Multidrug resistance is defined by resistance to more than one drug, apart from the combination of isoniazid (H) and rifampicin (R). Multidrug-resistant TB (MDR-TB) is defined as TB resistant to H and to R. TB is considered to be extensively drug-resistant (XDR-TB) when it is resistant not only to H and R, but also to at least one fluoroquinolone (FQ) as well as an aminoglycoside (amikacin, kanamycin) or capreomycin.
When a patient with tuberculous disease is treated, a dual benefit is sought: an individual (clinical) benefit, focused on curing the patient affected by TB, and a collective (public health) benefit, for the community in which the patient resides.
There are three essential objectives in TB treatment: (1) to rapidly decrease the number of TB bacilli in order to reduce morbidity and prevent patient death as well as decrease the capacity for infection of other people; (2) to prevent the development or worsening of resistant TB; and (3) to prevent relapses after completing treatment.
TB treatment consists of two phases: an initial intensive phase and a second continuation phase. The initial intensive phase is intended to fulfil objectives 1 and 2. To this end, it is very important to combine multiple drugs, especially drugs with bactericidal capacity that rapidly reduce bacilli reproduction. The antituberculosis drugs with the greatest bactericidal capacity are H and FQs. Tuberculous bacilli continuously undergo spontaneous mutations which may create resistance to a certain antituberculosis drug. If a clone is resistant to a specific drug, it will have a relative advantage over susceptible strains against this single drug, hence the importance of combining several drugs. When the bacillary load is higher, there is a greater risk of resistance developing; therefore, more drugs should be combined in the intensive phase of treatment. Objective 3 should be achieved by prolonging treatment over time and using drugs with a sterilising effect that are capable of eliminating persistent bacilli, which seem to restrict their metabolic activity and which cause the relapses that occur following antituberculosis treatment. The drug which plays the most important role in this regard and has demonstrated the greatest efficacy when preventing relapses is R. Pyrazinamide also has a substantial sterilising effect, although this activity is believed to be limited to special micro-environments of relatively increased acidity.1–4
Antituberculosis treatment should be started without delay in patients with a high likelihood of having TB, especially in those with serious, life-threatening disease, even before the results for sputum smear microscopy, molecular studies and cultures are available.
Resistance studies, both conventional phenotypic methods (antibiogram) and genotypic methods, are key to designing an effective treatment. At present, the use of rapid molecular tests (genotypic methods) applied directly to samples is recommended, since they shorten time to diagnosis and some of them provide additional information on susceptibility to drugs by using gene amplification techniques to detect mutations in genes which code resistance to antituberculosis drugs. Rapid molecular tests should be used for the resistance study at least in patients who are at higher risk of having resistant TB3: prior treatment of TB, especially if there has been poor adherence; contact with patients with resistant TB; and being from countries with a high incidence of MDR-TB (5% or higher). Knowing which drugs the patient has taken in the past is also extremely important. When a drug has been taken improperly for a month, the possibility that resistance to this drug has developed should be suspected.
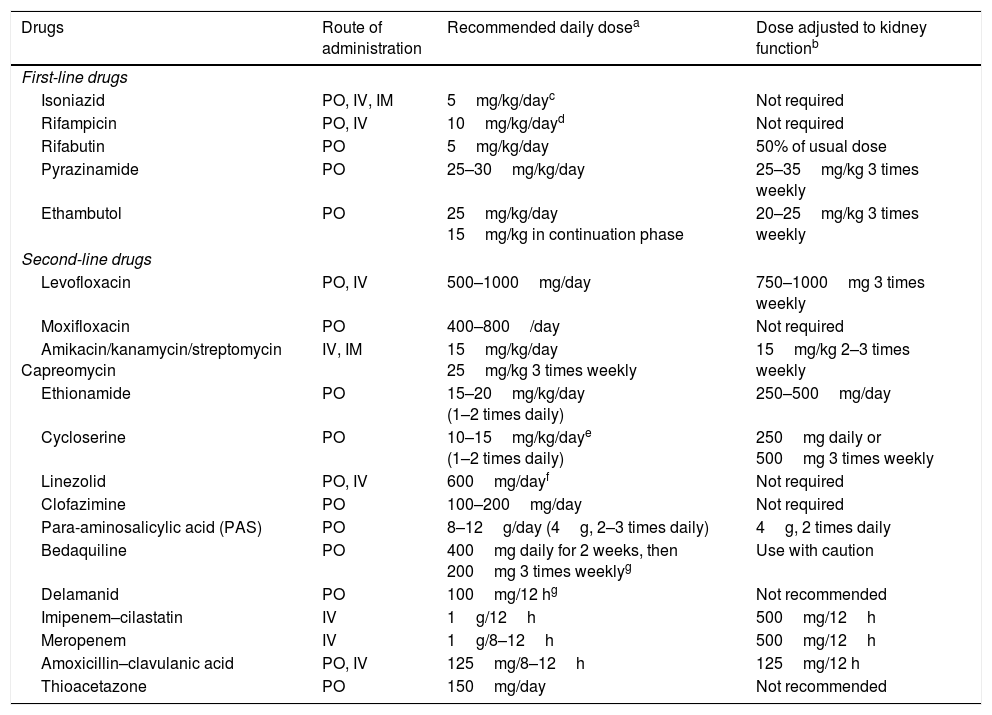
Drugs with activity against Mycobacterium tuberculosisThe first-line antituberculosis drugs (Table 1) are rifampicin (R), isoniazid (H), ethambutol (E) and pyrazinamide (Z). R, which is considered the key drug in the management of TB, belongs to the group of rifamycins. Rifabutin also belongs to this group and has the advantage of causing less induction of cytochrome P450 and therefore fewer drug interactions. Rifapentine is similar to R but has a longer half-life which enables weekly administration. It is not commercially available in Spain.
Route of administration, recommended daily dose and adjustment to kidney function for antituberculosis drugs.
| Drugs | Route of administration | Recommended daily dosea | Dose adjusted to kidney functionb |
|---|---|---|---|
| First-line drugs | |||
| Isoniazid | PO, IV, IM | 5mg/kg/dayc | Not required |
| Rifampicin | PO, IV | 10mg/kg/dayd | Not required |
| Rifabutin | PO | 5mg/kg/day | 50% of usual dose |
| Pyrazinamide | PO | 25–30mg/kg/day | 25–35mg/kg 3 times weekly |
| Ethambutol | PO | 25mg/kg/day 15mg/kg in continuation phase | 20–25mg/kg 3 times weekly |
| Second-line drugs | |||
| Levofloxacin | PO, IV | 500–1000mg/day | 750–1000mg 3 times weekly |
| Moxifloxacin | PO | 400–800/day | Not required |
| Amikacin/kanamycin/streptomycin Capreomycin | IV, IM | 15mg/kg/day 25mg/kg 3 times weekly | 15mg/kg 2–3 times weekly |
| Ethionamide | PO | 15–20mg/kg/day (1–2 times daily) | 250–500mg/day |
| Cycloserine | PO | 10–15mg/kg/daye (1–2 times daily) | 250mg daily or 500mg 3 times weekly |
| Linezolid | PO, IV | 600mg/dayf | Not required |
| Clofazimine | PO | 100–200mg/day | Not required |
| Para-aminosalicylic acid (PAS) | PO | 8–12g/day (4g, 2–3 times daily) | 4g, 2 times daily |
| Bedaquiline | PO | 400mg daily for 2 weeks, then 200mg 3 times weeklyg | Use with caution |
| Delamanid | PO | 100mg/12 hg | Not recommended |
| Imipenem–cilastatin | IV | 1g/12h | 500mg/12h |
| Meropenem | IV | 1g/8–12h | 500mg/12h |
| Amoxicillin–clavulanic acid | PO, IV | 125mg/8–12h | 125mg/12 h |
| Thioacetazone | PO | 150mg/day | Not recommended |
No studies have established ideal doses in obese patients (>20% their ideal weight); therefore, determinations of blood levels must be considered in these patients.
Recommended dose and frequency for patients with creatinine clearance <30ml/min and patients on haemodialysis.
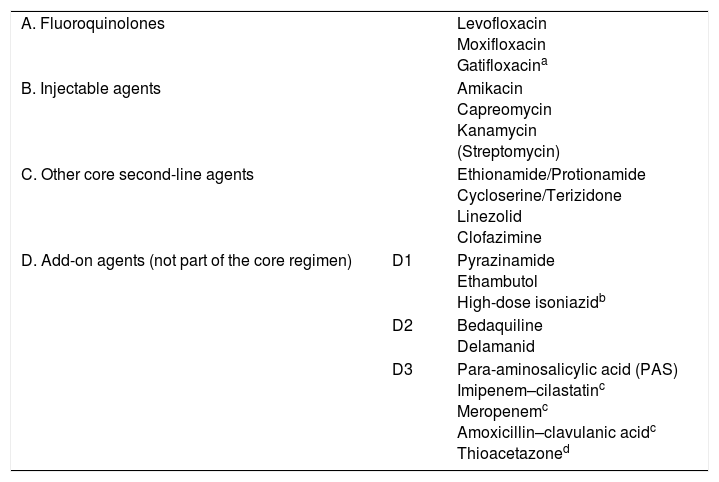
Recently, the WHO5 reclassified the second-line antituberculosis drugs in a functional table intended for designing treatment of MDR-TB (Table 2). Although streptomycin should be considered a first-line drug, it has been placed in the group of second-line injectables as it may be used as an injectable in the MDR-TB regimen if none of the other three agents can be used and if the strain is unlikely to be resistant to it. Streptomycin resistance in itself is insufficient to define XDR-TB. Moreover, the methods for study of this drug's resistance are not considered entirely reliable. Injectables are drugs with bactericidal capacity but hardly any sterilising capacity, which means they play a role in the intensive phase only. It should also be noted that they have cumulative toxicity which limits their prolonged use. FQs are key drugs and have an impact on the prognosis in the treatment of MDR-TB due to their bactericidal and sterilising effect and their limited toxicity. Recent years have seen a resurgence in the use of clofazimine (a drug used for leprosy) due to its probable sterilising action, particularly in cases in which Z is not effective. In the same group as clofazimine are other classic drugs such as cycloserine and thioamides (ethionamide/protionamide). The latter are more effective than cycloserine, but have cross-resistance with H when it is caused by mutation of the inhA gene. Linezolid is a good antituberculosis drug with bactericidal and sterilising capacity, but has the limitations of its high cost and substantial toxicity with prolonged administration.5,6 Bedaquiline and delamanid are the latest drugs to be included in the therapeutic arsenal, having demonstrated their efficacy in patients with MDR-TB and XDR-TB.6 Controversially, these drugs are not included in the group of main second-line drugs in the WHO classification,5 as other authors have suggested.6,7 This may have more to do with economics—they are expensive drugs—than efficacy, although some studies have shown the inclusion of these drugs to be economically viable.8 There may be cross-resistance between bedaquiline and clofazimine. The combination of carbapenems and clavulanic acid is probably set to play an increasingly important role, unlike para-aminosalicylic acid (PAS), which has virtually lapsed into disuse due to its poor tolerance and poor efficacy.
Drugs recommended for the treatment of multidrug-resistant tuberculosis (WHO, 2016).
| A. Fluoroquinolones | Levofloxacin Moxifloxacin Gatifloxacina | |
| B. Injectable agents | Amikacin Capreomycin Kanamycin (Streptomycin) | |
| C. Other core second-line agents | Ethionamide/Protionamide Cycloserine/Terizidone Linezolid Clofazimine | |
| D. Add-on agents (not part of the core regimen) | D1 | Pyrazinamide Ethambutol High-dose isoniazidb |
| D2 | Bedaquiline Delamanid | |
| D3 | Para-aminosalicylic acid (PAS) Imipenem–cilastatinc Meropenemc Amoxicillin–clavulanic acidc Thioacetazoned |
As TB treatment requires the administration of multiple drugs for several months, it is crucial that the patient play a significant role in decision-making with respect to treatment supervision and general care. The patient should be educated about TB and its treatment, including potential adverse effects; told what follow-up will be like; and engaged in discussion of management measures to prevent new cases of infection—all using terminology suited to the patient's culture, language and age.9 Ensuring that the patient adheres to the treatment regimen is key. Directly observed treatment (DOT), the practice of watching the patient swallow his or her antituberculosis medicines, has been widely used as a standard of practice in many TB programmes. A systematic review10 found no significant differences between self-administered treatment (SAT) and DOT following evaluation of several outcomes of interest, including mortality, termination of treatment and relapse. However, DOT was significantly associated with overall treatment success (the sum of the patients who were cured and the patients who completed treatment) and with a higher sputum conversion rate during treatment, compared to SAT. Moreover, participation in DOT may be advantageous for early recognition of adverse reactions and irregularities in treatment, as well as for establishing a stronger connection with the patient. DOT remains a standard of practice in most TB programmes and continues to be recommended by the WHO.4Table 3 describes the situations in which it may be most beneficial.
Situations in which directly observed treatment must be considered.
| Factors related to tuberculosis and treatment |
| Prior treatment of active tuberculosis or latent infection |
| Relapse or treatment failure |
| History of treatment non-adherence |
| Resistant tuberculosis |
| Use of intermittent treatment regimens |
| Factors related to patient characteristics |
| Adolescence |
| Poverty |
| History of or current drug abuse Chronic alcoholism |
| HIV infection |
| Psychiatric disorders |
| Cognitive impairment, frailty or substantial loss of vision |
| Residency in institutions (nursing homes, prisons, etc.) |
The recommended treatment regimen for TB, if resistance to first-line antituberculosis drugs is not suspected or has been ruled out, remains a combination of H, R, E and Z for two months, followed by H and R for another four months.1–4 When the likelihood of H-single-drug resistance exceeds 4%, E is added. E could be stopped if susceptibility to all other tuberculostatic drugs is confirmed at the start of or during treatment. One desirable objective in research studies on TB treatment is to attempt to establish shorter treatment regimens. Due to the major bactericidal activity of FQs against the TB bacillus, in recent years studies have been conducted in patients with drug-susceptible TB comparing the conventional regimen with another four-month regimen including FQs.11–13 Although four-month regimens that included FQs showed a faster sputum conversion rate, they had a higher incidence of relapses after 18 months of follow-up.
Daily doses or intermittent regimensThe most advisable regimen is once daily, seven days a week, in both the intensive phase and the continuation phase.1–4 There is good, extensive experience with intermittent treatment regimens of five days per week under a DOT regimen. Experts believe that five-day regimens may be an acceptable alternative in certain clinical and public-health situations, but always under a DOT regimen. The WHO does not recommend intermittent regimens of three weekly doses in either the intensive phase or the continuation phase.4 When the daily regimen is not possible, the United States guidelines1 do provide for intermittent administration three times per week in the continuation phase, and even in the intensive phase, in patients with no HIV infection, no cavitary lesions and negative sputum smear microscopy, always with DOT.
Use of fixed-dose combinations or individual drugsVarious studies have shown that the use of fixed-dose combinations is not more effective than the administration of individual drugs in terms of treatment failure, mortality, treatment adherence or adverse effects. However, patient satisfaction is greater with the use of fixed-dose combinations as the patient takes fewer tablets, which is why the WHO recommends their use4. Reducing the number of tablets to be taken could be particularly beneficial in patients with comorbidities such as HIV infection. However, it is worth noting that the dose must be adjusted in patients with drug intolerance or kidney or liver failure, which can only be made if drugs are administered individually. Finally, it is important for physicians to be familiar with the names of the various fixed-dose combinations given that they are similar, and because a prescribing error could have serious consequences as it could cause resistance or increased toxicity.
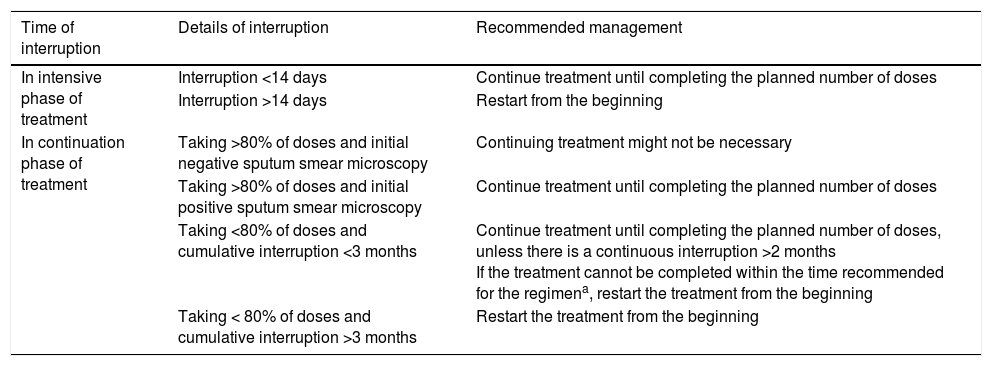
Treatment interruptionsTreatment interruptions are common in patients with TB. When a treatment interruption occurs, a decision should be made as to whether it is necessary to start again from the beginning or whether it is possible to carry on to completion. This decision depends on whether the interruption occurs in the intensive or the continuation phase of treatment, the duration of the interruption and the characteristics of the TB. In patients lost to follow-up, before starting treatment again, new sputum samples should be collected for study. Treatment interruptions during the intensive phase are more dangerous, since there is a greater bacillary population and a higher risk of developing resistance. Table 4 shows the recommendations regarding the management of treatment interruptions established in the 2016 United States guidelines.1
Management of treatment interruptions established in the 2016 United States guidelines (ATS, CDC and IDSA)1
| Time of interruption | Details of interruption | Recommended management |
|---|---|---|
| In intensive phase of treatment | Interruption <14 days | Continue treatment until completing the planned number of doses |
| Interruption >14 days | Restart from the beginning | |
| In continuation phase of treatment | Taking >80% of doses and initial negative sputum smear microscopy | Continuing treatment might not be necessary |
| Taking >80% of doses and initial positive sputum smear microscopy | Continue treatment until completing the planned number of doses | |
| Taking <80% of doses and cumulative interruption <3 months | Continue treatment until completing the planned number of doses, unless there is a continuous interruption >2 months If the treatment cannot be completed within the time recommended for the regimena, restart the treatment from the beginning | |
| Taking < 80% of doses and cumulative interruption >3 months | Restart the treatment from the beginning |
Patients with a diagnosis of pulmonary TB with negative cultures should undergo clinical and radiographic follow-up after two-three months of treatment. If there is clinical or radiographic improvement and no other aetiology is identified, antituberculosis treatment should be continued. If sputum samples were collected properly at the start of treatment, it should be interpreted as a form of paucibacillary TB and, in such case, it could be treated for only four months (two months of intensive phase with R, H, E and Z, and another two months of continuation phase with R and H). If there are doubts as to whether sputum samples were collected properly, the patient should be treated for six months1.
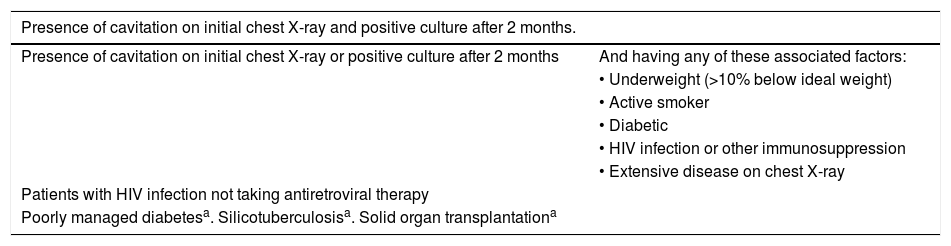
Prolonging the continuation phase in pulmonary tuberculosisA meta-analysis demonstrated that the rate of TB relapse in patients with susceptible strains was less than 1% when patients were treated with R regimens prolonged for at least eight months, versus 4% when patients were treated for six months.2 Several studies have identified various risk factors for having a higher percentage of relapses; in these cases, patients could benefit from prolonging treatment.14–17Table 5 shows the scenarios in which treatment of drug-susceptible TB should be prolonged to nine months.
Indications for prolonging treatment of drug-susceptible pulmonary tuberculosis to 9 months.
| Presence of cavitation on initial chest X-ray and positive culture after 2 months. | |
|---|---|
| Presence of cavitation on initial chest X-ray or positive culture after 2 months | And having any of these associated factors: |
| • Underweight (>10% below ideal weight) | |
| • Active smoker | |
| • Diabetic | |
| • HIV infection or other immunosuppression | |
| • Extensive disease on chest X-ray | |
| Patients with HIV infection not taking antiretroviral therapy | |
| Poorly managed diabetesa. Silicotuberculosisa. Solid organ transplantationa | |
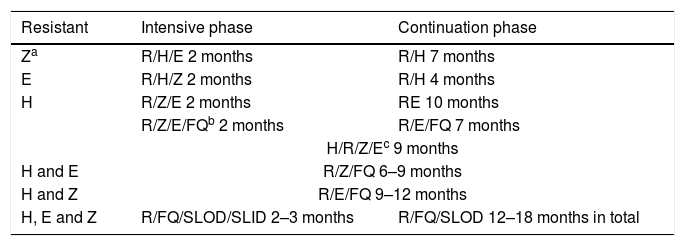
Table 6 shows the recommended therapeutic regimens in cases of single-drug resistance or multidrug-resistance. These regimens also apply to patients in whom any drug cannot be used due to serious adverse effects. Although FQs have not demonstrated efficacy in shortening treatment of drug-susceptible TB, one clinical trial13 showed that moxifloxacin could replace H in a six-month therapeutic regimen. Therefore, FQs could play a role in cases of single-drug resistance to H or H intolerance.
Proposed treatment regimens for resistance to first-line antituberculosis drugs (except rifampicin).
| Resistant | Intensive phase | Continuation phase |
|---|---|---|
| Za | R/H/E 2 months | R/H 7 months |
| E | R/H/Z 2 months | R/H 4 months |
| H | R/Z/E 2 months | RE 10 months |
| R/Z/E/FQb 2 months | R/E/FQ 7 months | |
| H/R/Z/Ec 9 months | ||
| H and E | R/Z/FQ 6–9 months | |
| H and Z | R/E/FQ 9–12 months | |
| H, E and Z | R/FQ/SLOD/SLID 2–3 months | R/FQ/SLOD 12–18 months in total |
E: ethambutol; FQ: fluoroquinolone; H: isoniazid; R: rifampicin; SLID: second-line injectable drug; SLOD: second-line oral drug; Z: pyrazinamide.
Isolated R resistance (without H resistance) is very rare. Furthermore, resistance to R is what determines the prognosis of MDR-TB. Hence, patients with isolated resistance to R should be managed as if they had MDR-TB,5 with the proviso that H should be carefully considered when designing a therapeutic regimen.
According to the 2016 WHO recommendations,5 conventional treatment of MDR-TB or isolated R resistance consists of a regimen of at least five drugs that are effective during the initial intensive treatment phase. This must include Z and four second-line drugs: one from group A, one from group B and at least two from group C (Table 1). If the minimum number of medicines effective against TB cannot be reached as specified above, an agent from group D2 and other agents from group D3 can be added to make a total of five. Adding high-dose H and/or E to this regimen could also be considered, but these should never be counted among the five recommended drugs. Z should be routinely added unless there is confirmed resistance or risk of toxicity. An intensive phase (with an injectable) lasting eight months (six months from sputum conversion) is recommended for most patients with MDR-TB, and a total treatment duration of around 20 months is recommended in previously untreated patients. The duration may be modified based on the treatment response. The association between treatment success and treatment duration is less clear in patients who have been treated, although the likelihood of treatment success seems to peak between 27.6 and 30.5 months.
The 2016 WHO recommendations5 advocated a shorter regimen for patients with MDR-TB based on reported success with a “nine-month Bangladesh regimen”.18 This regimen consists of an initial four-month intensive phase (which may be prolonged if sputum smear microscopy is positive) with kanamycin (amikacin or capreomycin can be used), high-dose moxifloxacin, clofazimine, ethionamide (or protionamide), Z, E and high-dose H and a subsequent five-month continuation phase with high-dose moxifloxacin, clofazimine, E and Z. This short regimen (with a total duration of 9–12 months) may be suitable for patients with MDR-TB who meet the following criteria: no extrapulmonary disease, no pregnancy, access to all the antituberculosis drugs in the regimen and no resistance (except to H) or prior exposure to these drugs for more than one month. This short regimen may not be applicable in certain regions of the world (such as various countries in Europe) due to the prevalent pattern of resistance in these areas.19 In the United States, some experts recommend using the shortened regimen in patients with minimal radiographic disease or a low bacillary load.
Cases of MDR-TB, and XDR-TB in particular, should be managed by medical staff with experience in administering complicated antituberculosis regimens. The design of XDR-TB regimens must follow the rational classification used in MDR-TB, with efforts made to use as many new drugs as possible20.
In patients with MDR-TB or XDR-TB, elective partial pulmonary resection (lobectomy or wedge resection) may be indicated together with medical treatment when the lesion is quite localised, there is a good respiratory reserve which enables surgery to be tolerated and medical treatment is believed to be insufficient due to the pattern of resistance.5
New treatment regimensMultiple clinical trials are being conducted (https://www.tballiance.org/portfolio) with new drugs and regimens for both drug-susceptible and resistant TB which enable the duration of treatment to be shortened. This includes modifications of classic drugs (for example, higher doses of R or quinolones) and the use of the latest commercially available drugs (bedaquiline or delamanid) or others such as pretomanid (derived from metronidazole, like delamanid).
Extrapulmonary tuberculosisThe principles underlying the treatment of pulmonary TB also apply to extrapulmonary TB. The above-mentioned regimens are used in extrapulmonary resistant TB. In extrapulmonary TB without suspected or confirmed resistance to first-line antituberculosis drugs, the same regimen as in pulmonary TB is recommended.
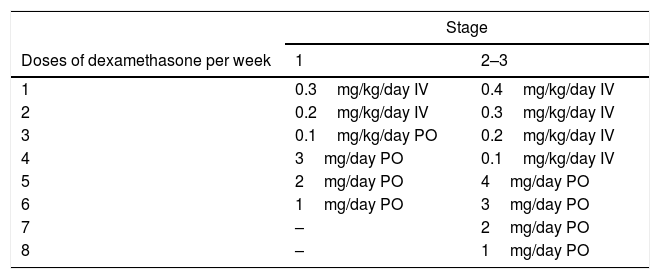
The exception is tuberculous meningitis, where, in the absence of conclusive clinical trials, most experts and associations recommend prolonging treatment to 12 months (two months of R, H, Z and E to continue with 10 months of R and H). As E21 crosses the blood–brain barrier poorly even with inflamed meninges, some clinicians have suggested using an FQ instead of E as a fourth drug. Aminoglycosides are also useful in MDR-TB, as they have good penetration during acute inflammation. In patients with MDR-TB, long 18–24-month regimens are recommended. According to expert opinion,1 lumbar puncture should be considered to monitor changes in cell count, glucose and proteins. It should always be accompanied by treatment with corticosteroids, since not using them increases mortality. Table 7 shows the regimen recommended by the 2016 United Kingdom NICE guidelines.3 Surgery should be considered in cases of elevated intracranial pressure or complications such as hydrocephaly or tuberculous brain abscesses.22 Early antituberculosis treatment and adjuvant treatment with glucocorticoids improve survival; notwithstanding this, nearly a third of patients with tuberculous meningitis die. The question of whether intensification of antituberculosis treatment for the first two months with high-dose R (15mg/kg/day) and levofloxacin (20mg/kg/day) would decrease the rate of death among patients has been studied. However, this strategy was not associated with a higher survival rate.23
Proposed corticosteroid regimen in patients with central nervous system tuberculosis (NICE, 2016).
| Stage | ||
|---|---|---|
| Doses of dexamethasone per week | 1 | 2–3 |
| 1 | 0.3mg/kg/day IV | 0.4mg/kg/day IV |
| 2 | 0.2mg/kg/day IV | 0.3mg/kg/day IV |
| 3 | 0.1mg/kg/day PO | 0.2mg/kg/day IV |
| 4 | 3mg/day PO | 0.1mg/kg/day IV |
| 5 | 2mg/day PO | 4mg/day PO |
| 6 | 1mg/day PO | 3mg/day PO |
| 7 | – | 2mg/day PO |
| 8 | – | 1mg/day PO |
Stage 1: Glasgow score of 15 without neurological signs and patient alert and orientated.
Stage 2: Glasgow score of 11–14 or 15 with neurological signs.
Stage 3: Glasgow score of 3–10 with or without neurological signs.
It is recommended that signs and symptoms suggestive of central nervous system impairment be examined in patients diagnosed with disseminated TB.3
Patients with spinal TB should be treated with a six-month regimen, unless there is direct spinal cord impairment, in which case the regimen should be prolonged to 12 months.3 In these patients, surgical debridement has not been shown to offer a clinical benefit over medical treatment, unless there is a poor response to medical treatment with persistence of infection, instability of the spine or evidence of spinal cord compression.3
Susceptible lymph node TB is also treated for six months. It should be noted that while affected lymph nodes may enlarge during treatment or new affected lymph nodes may appear during or after treatment, this does not indicate treatment failure or relapse.24 If active TB happens to be diagnosed in a lymph node removed during surgery, it should be treated with the standard six-month regimen.3
Susceptible pleural TB is also treated with a six-month regimen. The routine use of corticosteroids is not indicated. Tuberculous empyema is a rare complication in which active infection occurs in the pleural space with a large number of bacilli. It tends to occur due to rupture of a cavitation in the pleural space, in which case drainage would be indicated.25
Susceptible tuberculous pericarditis should also be treated with a six-month regimen. The use of corticosteroids combined with tuberculosis treatment has always been recommended. However, recent studies have not supported the routine use of corticosteroids and this is much disputed in the different guidelines.1,3 The English NICE guidelines3 always recommend the use of corticosteroids in tuberculous pericarditis. The United States guidelines1 only recommend this in patients with substantial pericardial effusion, high levels of markers and inflammatory cells or early signs of constriction. Should corticosteroids be needed, it is advisable to start with a dose of 60mg/day of prednisolone and gradually taper the dose until stopping it altogether in two-three weeks.
Susceptible renal TB should be treated with the conventional six-month regimen. Procedures involving intraurethral catheters or nephrostomies are sometimes required to resolve obstructive urinary tract conditions. Nephrostomy should be considered in non-functioning or poorly functioning kidneys, especially in cases of hypertension or persistent pain. Genital TB is also managed with the standard treatment, although sometimes surgery is needed due to large residual Fallopian-tube or ovarian abscesses.26
Due to a lack of studies, intermittent regimens are not recommended in extrapulmonary TB.
Special situationsHIV infectionAntiretroviral treatment (ART) raises the possibility of interactions, especially with treatment with R; in some cases, treatment with rifabutin may be valid. In case of unfamiliarity with ART interactions, it is recommended to visit one of the websites available on interactions (https://www.cdc.gov/tb/publications/guidelines/tb_hiv_drugs/default.htm, http://www.hiv-druginteractions.org, http://www.interaccionesvih.com). The duration of treatment of drug-susceptible pulmonary TB in patients taking ART must be six months; it is recommended that treatment be prolonged by three months in the continuation phase in special cases in which the patient is not receiving ART. The use of intermittent treatment regimens in patients with HIV has a higher rate of relapse as well as development of resistance, especially in more immunosuppressed patients; therefore, these regimens are not recommended.
All patients with TB and HIV infection should take ART, regardless of CD4 levels.1–4 If the patient was not previously taking ART, it should be started in the first two weeks of tuberculosis treatment in those with a CD4 count <50 cells/μl.1,3,4 In patients with a CD4 count ≥50 cells/μl, there is a certain amount of disagreement: the 2016 United States guidelines1 recommend starting ART after 8–12 weeks, whereas the 2017 WHO guidelines4 recommend doing so within the first eight weeks. In patients with tuberculous meningitis, ART should not be started in the first eight weeks of TB treatment as it increases mortality.1,3,4
Due to so-called immune reconstitution inflammatory syndrome (IRIS), patients with HIV infection and TB may have a paradoxical response with the start of treatment involving a worsening of TB signs or the onset of new complications such as development of pleural effusion, intra-abdominal or retroperitoneal abscesses, or expansion of central nervous system lesions which may or may not have been previously diagnosed. IRIS requires a differential diagnosis with TB treatment failure due to resistance to antituberculosis drugs or other opportunistic diseases. The management of IRIS is symptomatic. Anti-inflammatory agents such as ibuprofen are used to treat mild forms. Treatment with corticosteroids is indicated in moderate and severe forms; prednisone is administered at a dose of 1.25mg/kg/day for two-four weeks and gradually tapered in the following 6–12 weeks.1
The elderlySome experts and associations1,2 recommend not using Z in patients over 65 or 75 years of age as this population is at higher risk of hepatotoxicity. In this case, the continuation phase with H and R should be prolonged to seven months.
Pregnancy and breast-feedingAll first-line tuberculostatic drugs cross the placenta but do not seem to have a teratogenic effect. Although R as well as H, Z and E are classified by the FDA as category C drugs,27 using Z in pregnant patients is much disputed in the United States.1 The WHO recommends the use of four first-line tuberculostatic drugs in pregnant women.4
The foetal effects of second-line antituberculosis drugs are not well-established but injectables and ethionamide or protionamide are not considered safe. There is a case series of multidrug-resistant pregnant women who achieved a positive outcome with the usual regimens.28,29
There is no contraindication for breast-feeding if the mother is being treated. Indeed, breast-feeding must be promoted when the patient is no longer contagious. The infant should take pyridoxine (1–2mg/kg/day) since H is detected in breast milk, albeit in small concentrations.1
Kidney diseaseThe doses of some drugs must be adjusted in patients with kidney failure (Table 5). The interval between doses should be increased in patients with a creatinine clearance <30ml/min or on haemodialysis (Table 1). Recommended doses are based on expert opinion, but in certain cases it is necessary to measure blood levels of drugs two-six h after administering them. In patients on haemodialysis, it is recommended that antituberculosis drugs be administered under DOT after dialysis sessions to prevent premature clearance of antituberculosis drugs. Patients on peritoneal dialysis may require close monitoring with serum levels of drugs being measured before and after peritoneal dialysis sessions due to a risk of toxicity.1
Liver diseaseThe likelihood of developing drug-induced hepatitis is greater in patients with advanced liver disease, hepatitis B or C or liver transplantation. As Z is one of the drugs that most commonly causes hepatitis, a therapeutic regimen for drug-susceptible TB maintaining the other three first-line drugs may be proposed. A therapeutic regimen without H may also be proposed (Table 6). Patients with more advanced liver disease may follow a therapeutic regimen with neither H nor Z in which R and E are used with an FQ, an injectable drug or cycloserine for 12–18 months, depending on disease extent and response. In situations of serious disease, it is sometimes necessary to use regimens with low hepatotoxicity like those used in MDR-TB when R, H and Z are suspended.
Treatment with TNF-α inhibitorsExpert opinion holds that treatment with TNF-α inhibitors should be maintained when active TB is diagnosed, whenever the clinical situation allows it. If it has been suspended, there is no consensus on when it may be resumed. However, small case series have suggested that it is safe to resume treatment with TNF-α inhibitors in patients with a good clinical response after completing at least two months of antituberculosis treatment.30
Follow-up of patients with tuberculosisSide effects of antituberculosis drugsBecause side effects of antituberculosis drugs are common, it is necessary to be familiar with them. They are often mild and may resolve with simple recommendations or symptomatic treatments. For example, patients who take H may experience reddening with or without flushing, palpitations or headache two-three h after eating tyramine-containing foods (such as cheese, wine, cured meats and soy sauce). These generally resolve with avoidance of the foods that precipitate them.
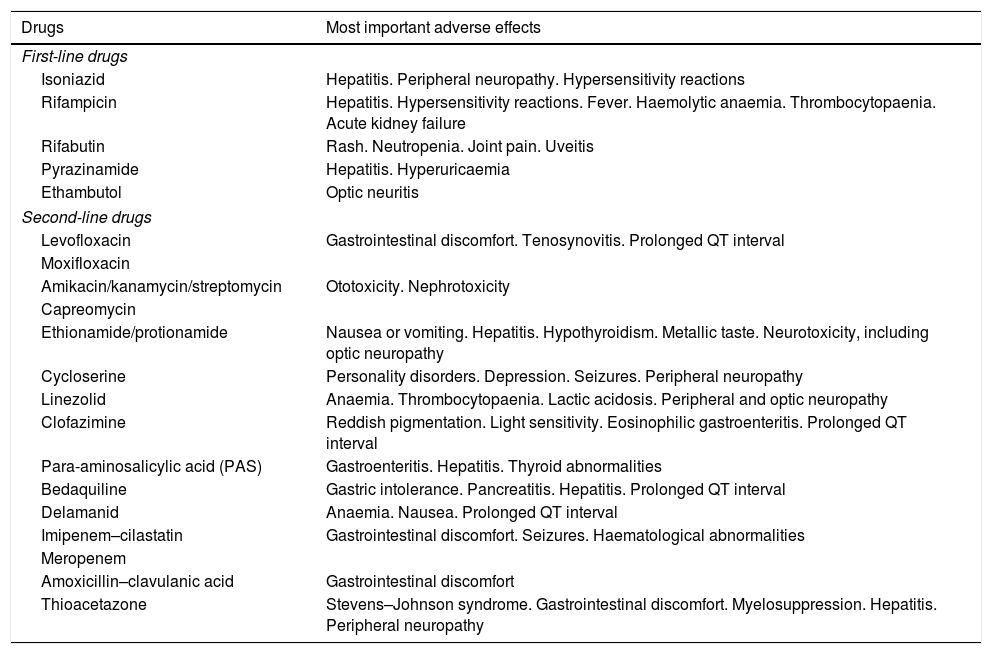
In other cases, side effects are serious and require certain drugs to be suspended and new treatment regimens to be followed. Table 8 shows the most important side effects of the antituberculosis drugs available at present.
Main adverse effects of antituberculosis drugs.
| Drugs | Most important adverse effects |
|---|---|
| First-line drugs | |
| Isoniazid | Hepatitis. Peripheral neuropathy. Hypersensitivity reactions |
| Rifampicin | Hepatitis. Hypersensitivity reactions. Fever. Haemolytic anaemia. Thrombocytopaenia. Acute kidney failure |
| Rifabutin | Rash. Neutropenia. Joint pain. Uveitis |
| Pyrazinamide | Hepatitis. Hyperuricaemia |
| Ethambutol | Optic neuritis |
| Second-line drugs | |
| Levofloxacin | Gastrointestinal discomfort. Tenosynovitis. Prolonged QT interval |
| Moxifloxacin | |
| Amikacin/kanamycin/streptomycin | Ototoxicity. Nephrotoxicity |
| Capreomycin | |
| Ethionamide/protionamide | Nausea or vomiting. Hepatitis. Hypothyroidism. Metallic taste. Neurotoxicity, including optic neuropathy |
| Cycloserine | Personality disorders. Depression. Seizures. Peripheral neuropathy |
| Linezolid | Anaemia. Thrombocytopaenia. Lactic acidosis. Peripheral and optic neuropathy |
| Clofazimine | Reddish pigmentation. Light sensitivity. Eosinophilic gastroenteritis. Prolonged QT interval |
| Para-aminosalicylic acid (PAS) | Gastroenteritis. Hepatitis. Thyroid abnormalities |
| Bedaquiline | Gastric intolerance. Pancreatitis. Hepatitis. Prolonged QT interval |
| Delamanid | Anaemia. Nausea. Prolonged QT interval |
| Imipenem–cilastatin | Gastrointestinal discomfort. Seizures. Haematological abnormalities |
| Meropenem | |
| Amoxicillin–clavulanic acid | Gastrointestinal discomfort |
| Thioacetazone | Stevens–Johnson syndrome. Gastrointestinal discomfort. Myelosuppression. Hepatitis. Peripheral neuropathy |
Gastrointestinal abnormalities are common and are often managed with antacids or proton pump inhibitors. Several antituberculosis drugs1,4 could cause gastrointestinal discomfort: R, H, ethionamide, PAS, linezolid, FQs, clofazimine and bedaquiline. While most antituberculosis drugs are best absorbed under fasting conditions, sometimes there is no alternative to taking them with some sort of food. In that case, it is recommended that they be small amounts of food without fat or sugar (glucose and lactose decrease H absorption). The absorption of FQs also markedly decreases when they are taken with medicines containing bivalent cations (calcium, iron or zinc).31
Hepatotoxicity must be ruled out in cases of persistent nausea and vomiting, especially when accompanied by abdominal pain. Drug-induced hepatitis is the most common serious side effect of first-line antituberculosis drugs. If GPT levels rise to more than five times the upper limit of normal or more than three times the upper limit of normal with symptoms, hepatotoxic antituberculosis drugs should be suspended. Once GPT levels fall below twice the upper limit of normal, the drugs may be reintroduced one at a time. The reintroduction of the drugs should be spaced out over the course of a week, to observe whether GPT levels increase again. During this process, a therapeutic regimen with a low likelihood of hepatotoxicity is sometimes followed. It is recommended to reintroduce R first since it causes less hepatotoxicity than H or Z. However, it should be noted that, with the standard regimen, asymptomatic elevation of transaminases occurs in up to 20% of patients and, in most cases, spontaneously resolves.1,32
Any antituberculosis drug could cause a rash. In most cases, it is mild and accompanied by pruritus without affecting the mucosae or presenting systemic symptoms such as fever, and can be symptomatically treated with antihistamines. If the patient experiences a serious erythematous rash, especially with mucosal involvement or fever, antituberculosis treatment must be stopped as the patient may have Stevens–Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). Systemic corticosteroids may be used to treat severe systemic reactions without worsening the course of TB.33 In most cases, the cause is difficult to determine.34 When the outbreak has substantially improved, medicines may be restarted one at a time two-three days apart to ascertain which drug is involved.
Several antituberculosis drugs may be associated with peripheral neuropathy: H, ethionamide, cycloserine and linezolid. Pyridoxine (vitamin B6) should be administered in patients at a higher risk of neuropathy: pregnant women, the elderly or patients with HIV infection, diabetes, alcoholism, malnourishment or chronic kidney disease.1,3 The recommended does is 25–50mg/day or 100mg/day in patients with established peripheral neuropathy and patients with MDR-TB taking a combination of several drugs which may cause neuropathy. Antituberculosis drugs associated with optic nerve toxicity include E, linezolid, ethionamide and H. Clofazimine toxicity causes pigmentary maculopathy and retinal degeneration.1,5
Finally, it should be noted that linezolid should not be administered together with selective serotonin reuptake inhibitors, tricyclic antidepressants or a diet rich in tyramine-containing foods due to a risk of developing serotonin syndrome.35
Microbiological studiesIn drug-susceptible TB, it is recommended to perform a sputum smear microscopy and sputum culture on a monthly basis until negative cultures are achieved for two consecutive samples. As noted above, it is very important to perform the study after completing the intensive phase in drug-susceptible pulmonary TB, in order to determine treatment duration. Treatment with E and Z should not be suspended following completion of the intensive phase of treatment of drug-susceptible TB until the result of the resistance studies is known, so as to avoid monotherapy with R in case of H resistance. In MDR-TB, it is recommended to perform a sputum smear microscopy and sputum culture on a monthly basis until negative cultures are achieved for two consecutive samples; subsequently, they could be performed every two months.
Sometimes, during treatment, a patient has positive sputum smear microscopies and subsequent negative cultures, due to the presence of dead bacilli. This usually happens in patients with large cavitations. For the same reason, the patient should not be monitored during follow-up with repeated molecular tests, as these tests detect genetic material from dead bacilli.1–4
Laboratory testing in follow-upIn the initial study it is recommended that serology be performed for HIV, hepatitis B and hepatitis C. It is not necessary to monitor transaminases or bilirubin, unless the patient has abnormal baseline values; symptoms of hepatotoxicity; abuse of alcohol or other hepatotoxic drugs; or a history of liver disease, viral hepatitis or HIV infection. In patients with MDR-TB, it is recommended that laboratory testing be performed on a monthly basis (initially, on a weekly basis). This varies depending on the patient's clinical condition, renal function, whether the patient is receiving treatment with injectables and the patient's complete blood count if he or she is taking linezolid. Thyroid function tests are to be performed every three months if the patient is receiving treatment with ethionamide or PAS.1–5
Other tests during follow-upPatients being treated with injectable drugs should have monthly hearing tests. As several drugs prolong the QT interval, closer follow-up should be performed depending on the dose and drug combination. If the patient is receiving normal doses of moxifloxacin, an ECG should be performed every two-three months.7 If the patient is being treated with bedaquiline, an ECG must be done at least in weeks two, 12 and 24. For patients on sustained treatment with E, linezolid or clofazimine, a monthly ophthalmological test of visual acuity and colour discrimination is recommended.5 At medical visits, a targeted medical history must be performed to detect side effects such as tinnitus and paraesthesia. In many cases, these are unrelated to the patient's medication. The clinician must also be alert to psychiatric abnormalities which may appear during treatment with cycloserine or other drugs, as such abnormalities have also been reported after administration of H, E or ethionamide.
Recurrent tuberculosis and treatment failuresRecurrent TB refers to cases in which a patient who has had a favourable clinical response and negative cultures while receiving antituberculosis treatment has clinical or radiological decline or positive cultures after completing treatment. In these cases, a distinction must be made between a relapse, development of resistant TB and reinfection in contexts with a high incidence or where infection management is lacking. True relapses are believed to consist of recurrent TB caused by the same strain which was previously identified, and are thought to occur when chemotherapy fails to sterilise the host tissues, thereby enabling endogenous worsening of the original infection.1 Most relapses occur within the first six to 12 months following the end of treatment.36 In most patients with TB susceptible to first-line drugs treated with DOT according to regimens that contain rifamycins, relapses occur with susceptible organisms and may be retreated with the standard regimen. However, the risk of acquired resistance is substantial in patients who did not have DOT or who received an intermittent regimen, especially in patients with HIV infection or who received a regimen which did not contain rifamycins. It should be noted that, if no initial test of susceptibility to antituberculosis drugs was performed, it is highly likely that the organisms were resistant from the start.37–39 Some experts advise caution in interpreting the results of the molecular tests used when recurrences are suspected, as false positives have been reported in molecular studies of resistance in previously treated patients.40
In Europe and according to the WHO, treatment failure is defined as the presence of positive cultures after five months (four months in the United States) of treatment in a patient receiving suitable chemotherapy.1,3,4 It should be noted that 90–95% of patients with drug-susceptible pulmonary TB, even with extensive pulmonary cavitation, will have negative cultures after three months of treatment with a standard regimen. Therefore, patients with persistently positive cultures should be carefully evaluated after three months of treatment. There are multiple reasons for treatment failure: poor treatment adherence, prescribing errors, previously undetected resistance to antituberculosis drugs, malabsorption of drugs (diarrhoea, prior stomach or small bowel resection, diabetic gastroparesis, etc.). Both in relapses with suspected drug resistance and in treatment failure, caution must be exercised when designing empirical treatments, in anticipation of new resistance studies to be conducted. More drug resistance may be generated if resistance study results are delayed. A single drug should never be added to a treatment regimen that has failed.5 These empirical treatments must be based on regimens for multidrug-resistant TB.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pascual-Pareja JF, Carrillo-Gómez R, Hontañón-Antoñana V, Martínez-Prieto M. Tratamiento de la enfermedad tuberculosa pulmonar y extrapulmonar. Enferm Infecc Microbiol Clin. 2018;36:507–516.