Nasal carriage of methicillin-resistant Staphylococcus aureus (MRSA) plays a pivotal role in the epidemiology and pathogenesis of MRSA infection.1 Although mupirocin has emerged as the agent of choice for elimination of S. aureus nasal carriage,3 several studies have identified high rates of MRSA resistant to mupirocin.3,4 Therefore, the development of eradication therapy for MRSA nasal carriage is needed.
Previously, it has been demonstrated that the 40-kDa fetidin of the earthworm Eisenia fetida suppressed the growth of Bacillus megaterium.5 Moreover, the extract of Lumbricus rubellus, another species of earthworm, displays more potent antibacterial activity in vitro against S. aureus than E. fetida.6 Hence, this study aimed to investigate the antibacterial activity of the 40-kDa protein of L. rubellus in a model of long-term MRSA nasal carriage. Besides, the innate immune response, secretory IgA (sIgA) levels, was also evaluated.
L. rubellus was obtained from a local store in Malang, Indonesia. Total proteins of L. rubellus were extracted and characterized by SDS-PAGE. The 40-kDa protein band of L. rubellus was cut and eluted, yielding a concentration of 3550mg/L.7–10 The bacterial isolate, MRSA, was provided by the Department of Clinical Microbiology, Faculty of Medicine, Brawijaya University. For in vivo experiments, thirty female Balb/c mice (8–12 weeks of age) were randomly divided into six groups (5 mice/group), such as negative control, positive control, and infected mice treated with mupirocin and three different doses of the 40-kDa protein of L. rubellus. The study was approved by the ethics committee of Brawijaya University (Ref. No. 26-KE).
The model of long-term MRSA nasal carriage was performed as previously described.2 Briefly, mice were injected with hydrocortisone subcutaneously (100mg/kg/day at days 0 and 4), followed by intranasal infections of MRSA (10μL containing 3×104CFU) on days 5, 7, 9, 30, 32 and 34. On day 35, nasal swab specimens were inoculated on CHROMagar to confirm the nasal carriage of MRSA. The control treatment group was administered with 2% mupirocin (Bactroban, 125mg diluted in 240mL saline11), while the treatment groups were treated with the 40-kDa protein of L. rubellus (diluted in saline at a concentration of 1:10, 1:50, and 1:100 from the total protein obtained previously) in a volume of 10μL to the respective nares for 5 days starting from day 36 (Fig. 1A). Negative controls were treated with saline only. The nasal swabs were conducted 24h after the last administration of mupirocin or 40-kDa protein of L. rubellus. Swabs were then cultured in phenol red broth at 37°C. The next day, 10μL of broth at a concentration of 10−1CFU/mL were inoculated on CHROMagar, incubated at 37°C for 24h, and then bacterial numbers were counted. No colonization of MRSA was observed in negative controls.
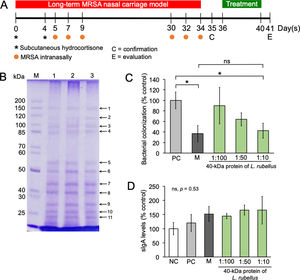
Effect of the 40-kDa protein of Lumbricus rubellus in the long-term MRSA nasal carriage model. (A) Experimental timeline; (B) Protein profile of L. rubellus; M, marker; lane 1–3, replication 1–3; (C, D) MRSA colonization and the level of sIgA in the nasal mucosa after treatment with mupirocin or 40-kDa protein of L. rubellus, respectively. NC, negative control; PC, positive control; M, mupirocin; ns, not significant. (*) Indicates a significant difference between the two groups indicated in the graphs (p<0.05).
For quantification of sIgA levels, mice were sacrificed. Nasal mucosa was scrapped, diluted in 5mL PBS containing a protease inhibitor cocktail (25μg/mL) and centrifuged at 12,000rpm (4°C) for 15min. Supernatants were purified with 40% ammonium sulfate. Suspensions were then diluted in 1mL PBS and used for ELISA of sIgA.12 All experiments were done in duplicate, resulting in similar findings. The numbers of bacterial colonization and sIgA levels were analyzed by one-way ANOVA, followed by Tukey's post hoc test using StatPlus. Significant differences were accepted when p<0.05.
We identified eleven conserved protein bands from L. rubellus (Fig. 1B). Among them, low molecular protein bands (50, 40, 27, 25, and 22-kDa) displayed high concentration profiles. Mupirocin and a high dose of the 40-kDa protein of L. rubellus significantly inhibited MRSA colonization (p=0.03 and p=0.04, respectively) in the nasal mucosa of mice (Fig. 1C), thereby implying that the 40-kDa protein of L. rubellus was able to eradicate MRSA colonization as effectively as mupirocin. No significant antibacterial activity was observed from the other protein bands (data not shown). Although we failed to show that neither mupirocin nor the 40-kDa protein of L. rubellus were able to stimulate sIgA secretion (p=0.35) (Fig. 1D), both groups tended to have higher levels of sIgA, suggesting that the level of sIgA would initially be beneficial to protect the nasal mucosa from MRSA invasion. Altogether, the 40-kDa protein of L. rubellus exhibits potent activity anti-MRSA in vivo.
Funding statementNone to declare.
Conflict of interestNone to declare.
The authors would like to thank to Alif Fakhrurrozi and Budi Darmansyah for their help in this project.