SARS-CoV-2 is a new emerging virus and the cause of the current COVID-19 pandemic. Molecular methods to detect viral genetic material in respiratory samples represent the most commonly used approach to diagnosis. Measurement of specific antibody response can also be of help under certain circumstances. A multitude of serological tests have been placed on the market over a short period of time, but not all of them offer optimal sensitivity or specificity, and literature supporting their use in a clinical context is limited. Hence, it is important to conduct validation studies with clinical samples before using them in practice.
Enzyme-linked immunosorbent assay (ELISA) was the first test to emerge and has yielded good results.1 The Euroimmun Medizinische Labordiagnostika® (Lübeck, Germany) ELISA was the first test on the market to quantitatively measure IgG and IgA against the S1 protein.2 Other techniques, such as chemiluminescence immunoassay (CLIA), offer greater automation and can provide faster results with reduced reliance on human resources. DiaSorin's Liaison® SARS-CoV-2 S1/2 IgG test (Saluggia, Italy) detects antibodies against the SARS-CoV-2 S1 and S2 proteins using an automated CLIA system that has not been thoroughly evaluated in clinical practice. We evaluated the performance of this system using serum samples from a blood bank. Those serum samples came from patients who had recovered from COVID-19 and were potential hyperimmune plasma donors.
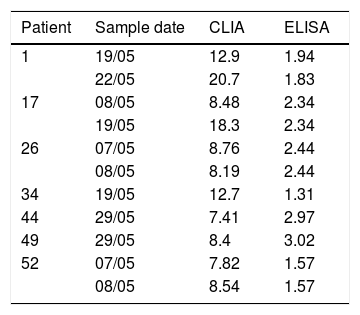
We studied a set of 91 sera from 57 patients, all with positive reverse transcription polymerase chain reaction (RT-PCR) results. Thirty-one patients had multiple extractions due to successive donations. All samples tested positive for IgG by ELISA (Euroimmun), with values ranging from 1.31 to >13 (cut-off point >1.1). With the cut-off point of >15 stipulated by the manufacturer, nine samples yielded discrepant results when tested by CLIA: seven were negative and two were inconclusive (Table 1). Based on these data, the sensitivity of DiaSorin's CLIA IgG test was 90.1%. In two of the patients with false negative results, the sample was collected closer to the time of infection.
To evaluate specificity, we used 11 serum samples from the blood bank with high titres for other respiratory pathogens (seven Mycoplasma pneumoniae, one Chlamydophila pneumoniae and one Coxiella burnetii) and another two serum samples from patients with pneumonia of unknown aetiology, all obtained prior to November 2019. All of these samples were negative, both by CLIA (with values <3.80 in all cases) and by ELISA. As a result, the specificity in our series was 100%.
In the few studies published to date, sensitivity ranges from 43.8% to 84% and specificity ranges from 94.9% to 97.9%.3–5 Sensitivity increases to 87.5% when more than two weeks have elapsed from the onset of symptoms or a positive PCR test.5 Some authors have proposed lowering the cut-off point to improve sensitivity,2,6 suggesting that this causes the performance of the Liaison® SARS-CoV-2 S1/2 IgG test to at least equal and possibly exceed the performance of ELISA.2 A recent study sponsored by the manufacturer (DiaSorin) proposed a cut-off point of nine. The study found that the reduced cut-off point increased sensitivity from 88.2% to 91.3% without significantly decreasing specificity.7 In applying this value, the sensitivity in our series would increase to 93.4%, with no loss of specificity.
In conclusion, DiaSorin's Liaison® system is easy to use and readily adapted to the clinical laboratory. We found that the sensitivity of the SARS-CoV-2 S1/2 IgG assay was 90.1% in sera from patients who had recovered from SARS-CoV-2 infection. We did not find any cross-reactivity with any other respiratory pathogens. The use of a lower cut-off point than the one proposed by the manufacturer may be advisable to increase sensitivity.
Despite the small number of samples analysed, we deemed it pertinent to publish the results of our study given the lack of information about this system available to date.
FundingNo funding was received for this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Infante Urrios A, Navarro Pérez L, Buñuel Adán F, Ortiz de la Tabla Ducasse V. Diagnóstico serológico del SARS-CoV-2. Rentabilidad diagnóstica de una prueba de quimioluminiscencia. Enferm Infecc Microbiol Clin. 2021;39:419–420.