Serological diagnosis of acute phase infections implies the detection of IgM specific response, an effective marker of primary infection, but with less clinical significance in reactivations or reinfections. The aim of this article is to provide an updated view of the rapid diagnosis in serology by detecting the IgM isotype and reviewing its applications and limitations. Point-of-care (PoC) tests are analysed. PoC tests are used in geographical areas where traditional tests are not available, as well as in other circumstances where their use brings the diagnosis directly to the target population. Likewise, their use reduces the response time between taking the sample and the diagnosis, making it easier to make clinical decisions. PoC assays have proven cost-effective, especially in preventing vertical transmission of syphilis and HIV infection.
El diagnóstico serológico de las infecciones en fase aguda implica la detección de la respuesta IgM específica, marcador eficaz de infección primaria, aunque con menos validez en las reactivaciones o reinfecciones. El objetivo de este artículo es proporcionar una visión actualizada del diagnóstico rápido en serología mediante la detección del isotipo IgM y revisar sus aplicaciones y limitaciones. Se analizan especialmente ensayos Point-of-Care (PoC) utilizados en áreas geográficas donde las pruebas tradicionales son inaccesibles, y en otras circunstancias donde su empleo acerca el diagnóstico a la población diana, debido a que pueden efectuarse en centros no sanitarios. Asimismo, su empleo disminuye el tiempo transcurrido entre la toma de muestra y el diagnóstico, facilitando al clínico la toma de decisiones. Los ensayos PoC han probado su coste-efectividad, especialmente en la prevención de la transmisión vertical de la sífilis y en la infección por el VIH.
Diagnostic microbiology of acute infections involves the detection of microorganisms by direct methods, such as cultures and molecular techniques, or indirect methods, such as serology. In terms of serological methods, the fastest way to obtain an aetiological diagnosis is the detection of specific IgM response. This is an effective marker of primary infection despite its limited usefulness in reactivations and reinfections due to their fugacity and low-intensity manifestation. Nevertheless, demonstrating seroconversion equates to a definitive diagnosis, albeit delayed owing to the time taken to conduct a parallel study of samples obtained during both the acute and convalescent phases.
Serum or plasma clinical samples, usually in liquid phase, tend to be used for serological testing, although blood spots on filter paper are also sometimes used. Other bodily fluids, such as cerebrospinal fluid (for central nervous system infections), saliva and urine may also be used.
Serological techniquesThe classic serological techniques (such as neutralisation, complement fixation and haemagglutination inhibition) detect total antibodies and therefore do not offer a rapid diagnosis. Pre-analytical processes are generally required to eliminate non-specific reactants and the techniques are not automated, making them laborious to carry out. Solid-phase techniques, on the other hand, such as enzyme immunoassay (ELISA), immunofluorescence (IF), immunochemiluminescence (ICL) or immunochromatography (IC) can identify class-specific antibodies, and diagnosis by means of IgM detection is fast. ELISA and ICL assays are available in automatic formats that require expensive equipment.
Rapid serological diagnosis: IgM detectionThis is achieved by indirect assays and capture assays. In indirect assays, the specific antibodies in the serum bind to the immobilised antigen in solid phase. The IgM isotype is recognised by anti-IgM antiserum, which conjugates with the assay-specific indicator to produce the reaction. In capture assays, the IgM in the sample binds to an anti-IgM antiserum. If the sample contains specific IgM, it is recognised by adding a conjugated or viral antigen, together with, or followed by, a conjugated antiserum. In both cases, the intensity of the signal generated is directly proportional to the concentration of specific IgM.
Limitations of IgM detectionThe validity of IgM as an indicator of recent infection may be compromised by reactivity not directly related to the infection.1
- a)
The presence of rheumatoid factor2 in individuals with specific IgG. Rheumatoid factor is an IgM autoantibody with anti-IgG specificity. Removing IgG from the sample eliminates this reactivity. Most commercial methods for the detection of IgM that are currently on the market include prior incubation with anti-lgG antiserum, or recommend their use as an option.
- b)
Certain viruses belonging to a single group share antigenic determinants, expressed as cross-reactivity when measuring IgM.3 This cross-reactivity is important when dealing with infectious agents that could cause similar symptoms, such as Epstein–Barr virus (EBV) and cytomegalovirus (CMV) in infectious mononucleosis (IM).
- c)
Multiple reactivity to numerous antigens caused by polyclonal stimulation of memory B cells gives rise to IgM reactivity that is unrelated to the acute infection.4 It manifests as IgM reactivity to pathogens that had already infected the patient, thereby hindering differential diagnosis.
- d)
IgM is often present in secondary infections (exogenous reinfection or latent virus reactivation). This poses significant problems in certain situations, such as dengue viral infection or CMV infection during pregnancy. In this case, the primary infection causes congenital infection more often than reactivation, making it extremely important to characterise the infection type in the presence of specific IgM.5
- e)
The persistence of IgM also leads to diagnostic complications, as is the case with Toxoplasma gondii infections. Due to the risk it poses to the foetus, its detection in serological testing during pregnancy means having to differentiate the specific IgM response to the acute primary infection.6
- f)
Finally, lack of IgM response is possible in immunosuppressed patients with certain infections, including congenital infection.7
In light of the above, assays that are capable of confirming or excluding IgM response, or capable of linking it to primary or secondary infection, are required. These include the establishment of antibody profiles or IgG avidity assays, the latter of which is an effective indicator of primary infection.
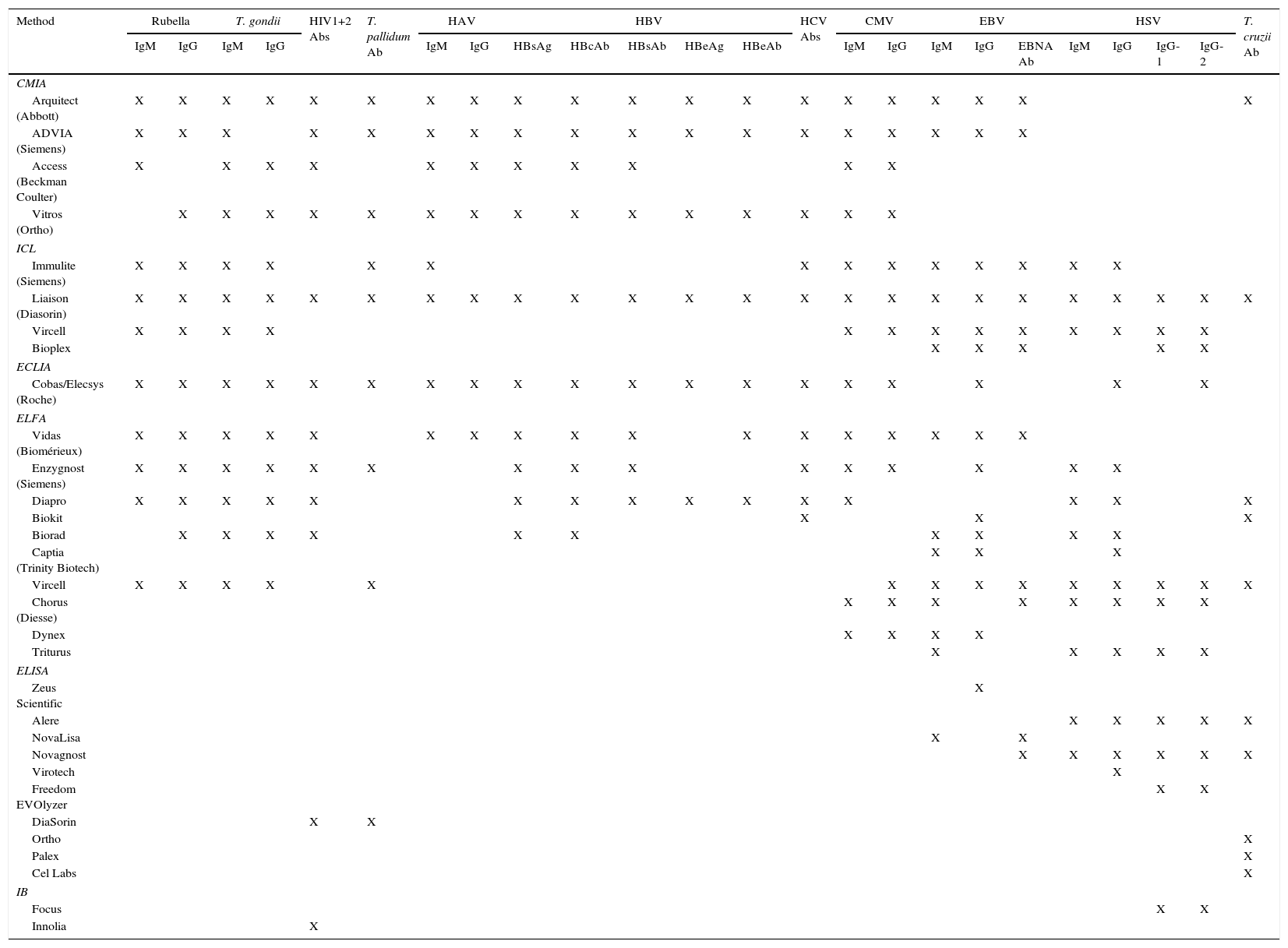
Rapid serological techniquesAutomated techniquesMost serological tests are available in automated formats, which are especially useful for particularly demanding tests. Their benefits include enhanced performance, accuracy, reduced response time, traceability and less manual work. In short, they are cost-effective. Their drawbacks include the high cost of both equipment and reagents, as well as technical and communication problems with the sample management systems. Table 1 presents a non-exhaustive list of automated assays used by Spanish laboratories, compiled with information from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) (https://www.seimc.org/controldecalidadseimc/).
Automated commercial assays used in Spain.
| Method | Rubella | T. gondii | HIV1+2 Abs | T. pallidum Ab | HAV | HBV | HCV Abs | CMV | EBV | HSV | T. cruzii Ab | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgM | IgG | IgM | IgG | HBsAg | HBcAb | HBsAb | HBeAg | HBeAb | IgM | IgG | IgM | IgG | EBNA Ab | IgM | IgG | IgG-1 | IgG-2 | |||||
| CMIA | ||||||||||||||||||||||||
| Arquitect (Abbott) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| ADVIA (Siemens) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Access (Beckman Coulter) | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Vitros (Ortho) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| ICL | ||||||||||||||||||||||||
| Immulite (Siemens) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Liaison (Diasorin) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Vircell | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Bioplex | X | X | X | X | X | |||||||||||||||||||
| ECLIA | ||||||||||||||||||||||||
| Cobas/Elecsys (Roche) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| ELFA | ||||||||||||||||||||||||
| Vidas (Biomérieux) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Enzygnost (Siemens) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Diapro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Biokit | X | X | X | |||||||||||||||||||||
| Biorad | X | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Captia (Trinity Biotech) | X | X | X | |||||||||||||||||||||
| Vircell | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Chorus (Diesse) | X | X | X | X | X | X | X | X | ||||||||||||||||
| Dynex | X | X | X | X | ||||||||||||||||||||
| Triturus | X | X | X | X | X | |||||||||||||||||||
| ELISA | ||||||||||||||||||||||||
| Zeus Scientific | X | |||||||||||||||||||||||
| Alere | X | X | X | X | X | |||||||||||||||||||
| NovaLisa | X | X | ||||||||||||||||||||||
| Novagnost | X | X | X | X | X | X | ||||||||||||||||||
| Virotech | X | |||||||||||||||||||||||
| Freedom EVOlyzer | X | X | ||||||||||||||||||||||
| DiaSorin | X | X | ||||||||||||||||||||||
| Ortho | X | |||||||||||||||||||||||
| Palex | X | |||||||||||||||||||||||
| Cel Labs | X | |||||||||||||||||||||||
| IB | ||||||||||||||||||||||||
| Focus | X | X | ||||||||||||||||||||||
| Innolia | X | |||||||||||||||||||||||
CMIA: chemiluminescent microparticle immunoassay; ECLIA: electrochemiluminescence immunoassay; ELFA: enzyme-linked fluorescence assay: ELISA: enzyme-linked immunosorbent assay; IB: immunoblot; ICL: immunochemiluminescence.
https://www.seimc.org/contenidos/ccs/analisisderesultados/2015/ccs-ar-t2015-3-Serologia3b.pdf.
https://www.seimc.org/contenidos/ccs/analisisderesultados/2015/ccs-ar-t2015-3-Serologia3a.pdf.
https://www.seimc.org/contenidos/ccs/analisisderesultados/2015/ccs-ar-t2015-2-Serologia.pdf.
https://www.seimc.org/contenidos/ccs/analisisderesultados/2015/ccs-ar-t2015-1-Serologia1b.pdf.
https://www.seimc.org/contenidos/ccs/analisisderesultados/2014/ccs-ar-t2014-3-SerologiaA.pdf.
Point-of-Care (PoC) assays are performed outside the central laboratory at the patient's bedside, using material and equipment that can be easily transported. Results are either available within minutes or in up to one hour. Specially trained personnel are not required to conduct the assay or to interpret the results. Their uptake is higher in developing countries, taking advantage of their cost-effectiveness to prevent the vertical transmission of syphilis and human immunodeficiency virus (HIV) infection. However, they have also found a role in developed countries as they facilitate the diagnosis of the target population and can be performed outside of healthcare centres.
Immunochromatographic (IC) techniques, also known as lateral flow assays, are currently used to detect IgM and IgG antibodies. The sample passes through a nitrocellulose or nylon membrane where the reaction takes place. The antigens specific to the target antibodies are immobilised on the reaction membrane and bind to any antibodies present. The sample flows to the conjugate pad (antiserum to the target isotype) containing colloidal gold, which changes colour in the presence of antibodies. A control zone is also usually included to ensure that the reaction has taken place successfully. Results are obtained in 15–30mins.
Infectious mononucleosis (IM)In young adults and immunocompetent adolescents, the most common clinical manifestation of Epstein–Barr virus (EBV) primary infection is IM. Serological methods such as the heterophile antibody (HA) test and the EBV antibody test are used to establish a microbiological diagnosis. These can differentiate the symptoms associated with EBV from those associated with CMV, HIV or T. gondii.
HAs detected in the acute phase of the disease would confirm the diagnosis of IM caused by EBV, as they are not usually present in other conditions and tend to decline rapidly in 2 or 3 months. They are present in 80–90% of patients with IM over the age of 10 years, but the positivity rate is less than 50% for patients below this age, and fail to develop in almost all infants under the age of 3 years.
There are many HA tests available on the market, including variations of the Paul-Bunnell test, latex-particle agglutination tests and IC. Sensitivity ranges from 80% to 95% depending on the age group being tested, while specificity is around 100%.8 HA false positives are rare but are most likely to occur in patients with autoimmune diseases, leukaemia or certain infections including CMV, rubella or HIV. The HA test is usually a manual process, but there are automated multiple detection systems that simultaneously detect HA and EBV viral capsid antigen (VCA) IgM, which yield similar results to traditional techniques.9,10
Specific response must be investigated in HA-negative patients, particularly VCA IgM, which is the first to appear and is only detectable for up to 6 months, as well as anti-VCA IgG, which persists throughout a patient's lifetime, and antibodies to Epstein–Barr nuclear antigen (EBNA), which are not seen in the acute phase. However, anti-VCA IgM is undetectable in 10% of children with primary infection. Infection is confirmed by the presence of anti-VCA IgG in the absence of anti-EBNA reactivity. If IM is suspected in HA-negative patients, testing for the 3 specific markers will facilitate a conclusive diagnosis in more than 90% of cases. Cross-reactivity with various viruses, including CMV, HHV-6, parvovirus B19 and rubella virus, should be considered.
ELISA, ICL and enzyme-linked fluorescence assay (ELFA) have largely replaced IF, traditionally the gold standard, in most microbiology laboratories. They offer good sensitivity and specificity, both in terms of individual markers and for establishing antibody profiles.11,12
There are rapid immunofiltration assays that can detect IgM against the ZEBRA protein (BamHI Z EBV replication activator) together with anti-VCA IgM. Results are obtained in less than 3min and their sensitivity ranges from 75% to 90%, with a specificity of 95–100%.13 A similar test is available to detect anti-VCA IgG antibodies and anti-EBNA-1 simultaneously.
SyphilisSyphilis is serologically diagnosed using a non-treponemal test (NTT), usually the Rapid Plasma Reagin (RPR) test or the Venereal Disease Research Laboratory (VDRL) test, combined with a treponemal test (TT), usually employing ELISA or ICL techniques. NTTs determine infection activity and have traditionally been used as screening tests. TTs are more specific and can detect past, latent and current infection without differentiating between them, as they remain positive after treatment. So-called “reverse sequence screening” has been introduced in recent years, which requires the study to be started with a TT. This enables cases of latent syphilis to be detected in populations with low prevalence of the infection that could not be diagnosed using traditional screening methods.
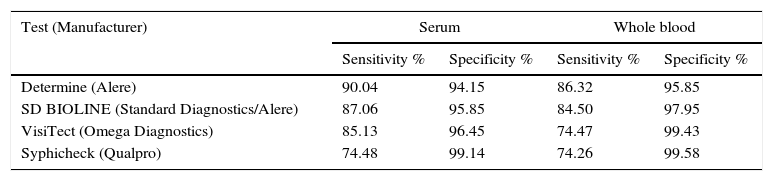
Most PoC assays used for syphilis screening only detect antibodies against treponemal antigens (TA). They are IC assays that use lateral flow technology and one or more recombinant TAs (TpN15, TpN17, TpN47). They can detect IgM, IgG and IgA. They test serum and plasma and some also use whole blood and oral fluids. According to studies conducted in various geographical areas, they offer good sensitivity and specificity, although sensitivity is higher in serum.14,15Table 2 shows the sensitivity and specificity data compiled from a recent meta-analysis.16
Sensitivity and specificity of the most widely used rapid diagnostic tests for syphilis in serum and whole blood samples.
| Test (Manufacturer) | Serum | Whole blood | ||
|---|---|---|---|---|
| Sensitivity % | Specificity % | Sensitivity % | Specificity % | |
| Determine (Alere) | 90.04 | 94.15 | 86.32 | 95.85 |
| SD BIOLINE (Standard Diagnostics/Alere) | 87.06 | 95.85 | 84.50 | 97.95 |
| VisiTect (Omega Diagnostics) | 85.13 | 96.45 | 74.47 | 99.43 |
| Syphicheck (Qualpro) | 74.48 | 99.14 | 74.26 | 99.58 |
Adapted from Jafari et al.16.
New assays have been developed to enhance rapid diagnosis. Some detect antibodies to both TA and non-treponemal antigens (NTA), while others detect a range of infections using the same device, generally HIV and hepatitis C virus (HCV). The aim of the former is to differentiate between active and past infection to avoid unnecessary treatment. The Dual Path Platform (DPP) Syphilis Screen & Confirm assay (Chembio Diagnostic Systems Inc., Medford, New York) is the only commercial assay that can detect both antibody types simultaneously using recombinant TAs (T1 line) and synthetic NTAs (T2 line). It has been evaluated by the US Centres for Disease Control and Prevention using the Treponema pallidum particle agglutination (TPPA) and RPR assays, respectively, as reference techniques.17 Compared to the RPR test, the reactive concordance was 98.4% when the RPR titres of sera were ≥1:2, and the non-reactive concordance was 98.6%. Concerning anti-TAs (T1), the reactive and non-reactive concordances were 96.5% and 95.5%, respectively. In an attempt to improve the sensitivity of this assay, an electronic reader has been developed that measures the density of the test lines. This could be the basis of a new quantitative assay.
Cost-effectiveness studies demonstrate the utility of DPP for screening pregnant women for syphilis in high-risk populations of developing countries, recommending their use in primary care centres and in areas with limited resources where the infection is prevalent and where the likelihood of patient diagnosis and follow-up is remote.18
From a clinical perspective, the DPP assay correctly identifies 93% of active infections, 28% of past infections and rules out infection in 78% of cases. The sensitivity of the T2 line appears to be correlated to the RPR titres. Overall concordance between DPP and the reference serological assays is higher in patients with active infection and when the RPR titre is ≥1:16, while concordance diminishes in cases of past infection and with a low antibody titre. It can also be used to diagnose other treponemal diseases.19,20
The most used multiple assays are those that simultaneously detect syphilis and HIV infection due to the synergic relationship between the two pathogens. Sensitivity and specificity are good for both microorganisms.21,22
Human immunodeficiency virusHIV infection affects more than 40 million people worldwide, 85% of whom live in developing countries and Sub-Saharan Africa in particular. In Europe and the United States of America, it is estimated that between 15% and 30% of people infected by the virus are unaware of their condition. Two major challenges must be overcome in order to control HIV infection: reduce transmission, which mainly occurs in the first weeks of the infection when the plasma viral load is at its peak; and reduce the time taken to inform the doctor and the patient after a positive result is obtained.
HIV infection is generally diagnosed by detecting specific antibodies. Both screening and confirmatory serological assays may be used. Screening assays that simultaneously detect the presence of HIV-1 and HIV-2 antibodies and the HIV-1 p24 antigen (fourth generation) have been developed in an attempt to improve the diagnosis of acute infections. These formats shorten the window period and look to identify the infection quicker than a positive Western Blot. They use automated ELISA or ICL techniques and offer a high degree of sensitivity (99.9–100%), with specificity in excess of 99%.23,24 A multiple assay that can simultaneously detect and differentiate the HIV-1 p24 antigen and the specific antibodies to HIV-1 (groups M and O) and HIV-2 in serum and plasma samples has recently been marketed, with 100% sensitivity and 99.5% specificity.25
The confirmatory assay used in the event of a positive screening test will depend on whether the traditional or Clinical and Laboratory Standards Institute algorithm has been chosen.26,27 A rapid differentiation and confirmatory system specific to anti-HIV-1 and anti-HIV-2 antibodies is currently available. This is an IC assay that includes recombinant antigens or synthetic peptides specific for HIV-1 (gp160, gp41, p31, p24) and for HIV-2 (gp140, gp36).28 Although results must be visually read and interpreted, the automated reader ensures objectivity. Results are obtained in less than 30min. It is able to effectively discriminate between HIV-1 and HIV-2, reduce the number of indeterminates and generally discriminate better than LIA.29
The administration of antiretroviral prophylaxis following occupational exposure to blood or fluids, the need for rapid results in emergencies or during childbirth and the need for detection in non-clinical settings have led to the development of PoC assays. The World Health Organisation recommends the use of Point-of-Care assays in resource-poor settings. Studies conducted in Sub-Saharan Africa have shown that beginning treatment during the first visit on the day of diagnosis increases acceptance of antiretroviral therapy by 36% and viral suppression by 26%.30 Although the sensitivity and specificity of these tests is good, it is inferior to the immunoassays currently performed in conventional diagnosis, despite using similar antigens. Its sensitivity diminishes in acute infections and may yield false negatives if specific antibodies have not yet developed. A recent meta-analysis found that Point-of-Care assays performed in developing countries offer 97.4% sensitivity compared to nucleic acid amplification techniques, and 98.8% sensitivity if the reference was a fourth-generation immunoassay, with specificity exceeding 99.6% in both cases.31
Point-of-Care assays can be performed using oral fluid, capillary blood collected by skin puncture, serum or plasma. Although specificity is similar, their sensitivity in saliva is approximately 2% lower than in blood.32 Fourth-generation Point-of-Care assays have been released in the last few years. They are believed to be less sensitive than the standard combined test. They can be used to detect established HIV-1 and HIV-2 infection,33 but they offer low sensitivity in acute infections. When the components of the assay were evaluated separately, the HIV-1 p24 antigen produced a sensitivity of 12.3%, while the pooled sensitivity of the antibody component was 97.3%.34
Positive Point-of-Care assay results must be confirmed. In developing countries, each strategy implemented must adopt its own quality control procedure with the resources at its disposal.
HepatitisOf all the viruses for which the primary tissue tropism is the liver, only hepatitis B (HBV), hepatitis C (HCV) and hepatitis E (HEV) are discussed due to the clinical implications of rapid diagnosis. There are numerous serological diagnosis plasma and serum tests for viral hepatitis available on the market. They use various methods and platforms and their sensitivity and specificity are very good. There are also rapid diagnostic tests available, most of which are based on IC immunoadhesion or immunofiltration assays with results available in a matter of minutes. Immunofiltration assays offer good specificity, but their sensitivity is variable and always inferior to fluorescent assays, ELISA and ICL.35 In our setting, Point-of-Care assays tend to be used in emergencies, but they may play a pivotal role in developing countries.
Hepatitis B virus. The surface antigen of the hepatitis B virus (HBsAg) is a very early marker. It is detectable in the incubation period and particularly in the acute and chronic phases of the infection, which are the phases targeted by the rapid diagnostic tests. Quantifying HBsAg may help to identify true inactive carriers of the infection, monitor disease progression and predict treatment response with interferon.
Among other criteria, an assay designed to measure HBsAg must be able to detect at least 0.25ng/ml of this protein. Commercial ICL or ELISA immunoassay kits can reach a limit of detection < 0.13IU/ml (equivalent to <0.5ng/ml), while the lower limit of detection of most rapid diagnostic tests ranges from 0.5 to 2IU/ml (1–3ng/ml). Their capacity to detect new mutant strains of the virus also varies, which makes it very important to understand the analytical characteristics of each rapid assay in order to make an informed choice.36,37 They are generally performed on whole blood, serum or plasma samples. A recent meta-analysis found the sensitivity of the various commercial PoC assays to be extremely variable (43.5–99.8%). However, studies conducted in developed countries found greater homogeneity than studies conducted in developing countries. Specificity values have not been found to vary as much (90–100%).38
Hepatitis C virus. IC-based PoC assays generally use recombinant antigens from the core region of the NS3 and NS4 non-structural regions, although some also include NS5. The assays may be performed on blood, serum, plasma or saliva. Sensitivity varies depending on the test and type of sample used, with saliva offering the lowest sensitivity, although this appears to be independent of the infecting genotype. A recent meta-analysis39 and other studies40–42 found that the OraQuick (OraSure Technologies, USA) offers the greatest sensitivity and specificity. It has been approved by the US Food and Drug Administration as a rapid anti-HCV antibody screening test in fingerstick and venipuncture blood samples for people over the age of 15 years. Multiple assays that can simultaneously detect and differentiate antibodies to HCV and other pathogens such as HIV, HBV or T. pallidum are also available (Table 3). Their sensitivity and specificity are similar or slightly inferior to anti HCV assays, although specificity is high. They could be used in sexually transmitted infection screening programmes, although it should be noted that false negatives have been reported in HIV-infected patients, rendering them less useful in areas with a high HIV prevalence.43
Hepatitis E virus. The specific IgM isotype is a marker of acute infection and confirms the infection. Commercial serological assays to diagnose HEV can detect infections caused by 4 genotypes: genotypes 1–2 in developing countries and 3–4 in industrialised countries, although their effectiveness may vary depending on the infecting genotype. These are ELISA or IC assays based on peptides of the ORF2/ORF3 region or recombinant HEV antigens.
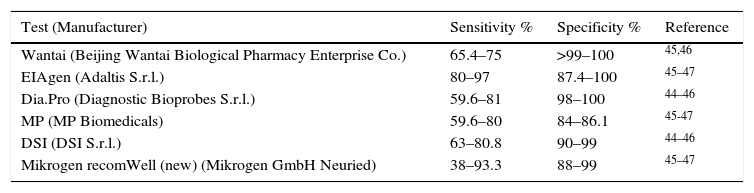
ELISA assays that detect IgM offer variable sensitivity depending on the population group studied, even when using the same technique. This is because sensitivity decreases in all immunosuppressed patients. Specificity ranges from 84% to 100% (Table 4).44–47 An ELISA assay designed to detect the HEV capsid antigen has recently been reported. It shows good concordance with HEV-RNA detection, which means it could be effective in detecting acute infection in both immunocompetent and immunosuppressed patients.48 PoC assays have been developed to detect anti-HEV IgM antibodies in whole blood, serum or plasma samples. They have proven to be useful in areas of endemicity and nonendemicity, although their sensitivity is also lower in immunocompromised patients.46,49
Sensitivity and specificity of ELISA tests to detect anti-HEV IgM.
| Test (Manufacturer) | Sensitivity % | Specificity % | Reference |
|---|---|---|---|
| Wantai (Beijing Wantai Biological Pharmacy Enterprise Co.) | 65.4–75 | >99–100 | 45,46 |
| EIAgen (Adaltis S.r.l.) | 80–97 | 87.4–100 | 45–47 |
| Dia.Pro (Diagnostic Bioprobes S.r.l.) | 59.6–81 | 98–100 | 44–46 |
| MP (MP Biomedicals) | 59.6–80 | 84–86.1 | 45-47 |
| DSI (DSI S.r.l.) | 63–80.8 | 90–99 | 44–46 |
| Mikrogen recomWell (new) (Mikrogen GmbH Neuried) | 38–93.3 | 88–99 | 45–47 |
The dengue virus (DENV), Zika virus (ZIKV) (Flaviviridae) and Chikungunya virus (CHIKV) (Togaviridae) are the three mosquito-borne arboviruses that pose challenges in our field, for various reasons: the Aedes genus of mosquito is common to all three viruses, of which A. albopictus can be found in Spain's Mediterranean region; they all cause similar symptoms (fever, exanthema and joint pain); and they are all endemic in the same geographical areas, making diagnosis in clinical laboratories difficult.
Serology is the method of choice for establishing a microbiological diagnosis 7 days after the initial onset of symptoms. When using serological assays to diagnose the infection during the acute phase, cross-reactivity between DENV, ZIKV and other flaviviruses, such as yellow fever virus, must be taken into consideration. A neutralisation test must be performed to unequivocally identify the serological response.
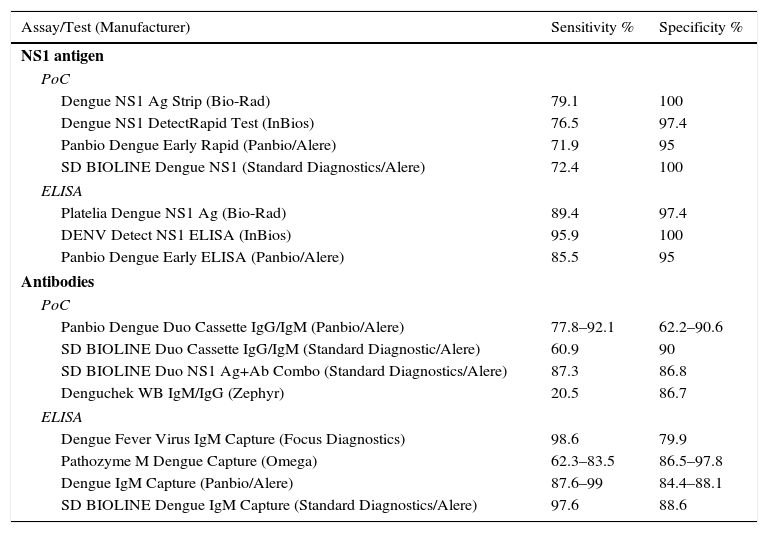
Dengue virus. Dengue is the most-common mosquito-borne disease in the world. There are 4 different serotypes: DENV1, DENV2, DENV3 and DENV4. Acquired immunity is serotype-specific and does not protect against other serotypes. The sensitivity of diagnostic microbiology tests for dengue depend on the time elapsed since infection, infection type (primary or secondary) and infecting virus. During the viraemic phase, the detection of viral RNA or non-structural NS1 protein are the main methods used to diagnose the disease. The NS1 antigen is a glycoprotein found in high concentrations in all DENV serotypes and detectable in serum during the early stage of the disease. It can be effectively detected in the first 2–4 days after the onset of symptoms and supports a diagnosis of acute infection due to its high specificity. There are numerous commercial ELISA and IC assays on the market that can detect the NS1 antigen in serum or plasma (Table 5).50 In DENV primary infection, the IgM isotype may be detectable from the third day after the onset of fever when the viraemic phase starts to subside. However, in secondary infection, IgG levels rapidly increase after the onset of symptoms, resulting in a low or undetectable IgM response. To distinguish primary infection from secondary infection, an ELISA assay must be performed to establish the IgM/IgG ratio.51
Dengue virus: sensitivity and specificity of rapid diagnostic tests and ELISA to detect NS1 and antibodies.
| Assay/Test (Manufacturer) | Sensitivity % | Specificity % |
|---|---|---|
| NS1 antigen | ||
| PoC | ||
| Dengue NS1 Ag Strip (Bio-Rad) | 79.1 | 100 |
| Dengue NS1 DetectRapid Test (InBios) | 76.5 | 97.4 |
| Panbio Dengue Early Rapid (Panbio/Alere) | 71.9 | 95 |
| SD BIOLINE Dengue NS1 (Standard Diagnostics/Alere) | 72.4 | 100 |
| ELISA | ||
| Platelia Dengue NS1 Ag (Bio-Rad) | 89.4 | 97.4 |
| DENV Detect NS1 ELISA (InBios) | 95.9 | 100 |
| Panbio Dengue Early ELISA (Panbio/Alere) | 85.5 | 95 |
| Antibodies | ||
| PoC | ||
| Panbio Dengue Duo Cassette IgG/IgM (Panbio/Alere) | 77.8–92.1 | 62.2–90.6 |
| SD BIOLINE Duo Cassette IgG/IgM (Standard Diagnostic/Alere) | 60.9 | 90 |
| SD BIOLINE Duo NS1 Ag+Ab Combo (Standard Diagnostics/Alere) | 87.3 | 86.8 |
| Denguchek WB IgM/IgG (Zephyr) | 20.5 | 86.7 |
| ELISA | ||
| Dengue Fever Virus IgM Capture (Focus Diagnostics) | 98.6 | 79.9 |
| Pathozyme M Dengue Capture (Omega) | 62.3–83.5 | 86.5–97.8 |
| Dengue IgM Capture (Panbio/Alere) | 87.6–99 | 84.4–88.1 |
| SD BIOLINE Dengue IgM Capture (Standard Diagnostics/Alere) | 97.6 | 88.6 |
The sensitivity of ELISA assays for the detection of IgM is higher than IC assays that detect the same antibody. Capture assays are recommended to improve sensitivity, especially in secondary dengue virus infection. IC assays are not effective due to their low sensitivity (6.4–65.3%).52 In all cases, sensitivity increases as the time since the onset of symptoms increases. In an attempt to improve the diagnosis of DENV infections, rapid diagnostic tests have been designed that use monoclonal antibodies that simultaneously detect IgM and IgG antibodies, while some also include the NS1 antigen. The pooled sensitivity and specificity in samples obtained between 4 and 14 days after the onset of symptoms is higher and enhances their diagnostic usefulness, particularly in areas with limited or no access to reference assays.53,54Table 5 shows the sensitivity and specificity of these assays.
In terms of other clinical samples, such as saliva or urine, the assays show good specificity but lack the required sensitivity to be a viable diagnostic option.
Chikungunya virus. Available rapid IC tests detect the IgM isotype in serum or plasma samples. Studies conducted with these tests in high-prevalence areas found a significantly lower sensitivity (1.9–50%) than comparative reference assays, especially in the acute phase of the disease, and this must be improved before they can be used in areas of endemicity or nonendemicity.55 Studies conducted on monoclonal antibodies to glycoprotein E of the CHIKV envelope have yielded promising results, and they may replace the recombinant antigens that are currently used in the not-too-distant future.56
Zika virus. IF assays are available that can simultaneously detect antibodies to ZIKV, DENV and CHIKV and reveal cross-reactions between ZIKV and DENV. An NS1 protein ELISA improves specificity. An IC assay with CE marking designed to qualitatively and simultaneously detect ZIKV IgM and IgG antibodies is commercially available, but its effectiveness has yet to be established.57
Chagas disease (American trypanosomiasis)Chagas disease affects more than 10 million people in Latin America and has spread to non-endemic areas, including Europe, North America, Asia and Australia, as a result of migration. In our setting, we are typically required to serologically diagnose the disease during its chronic phase, by detecting specific IgG anti-Trypanosoma cruzi antibodies. A definitive serological diagnosis is defined as concordance between at least two tests employing different techniques and using different antigens. In the event of a discrepancy, further testing should be undertaken to confirm and rule out other infections that could give rise to false-positive reactions.58
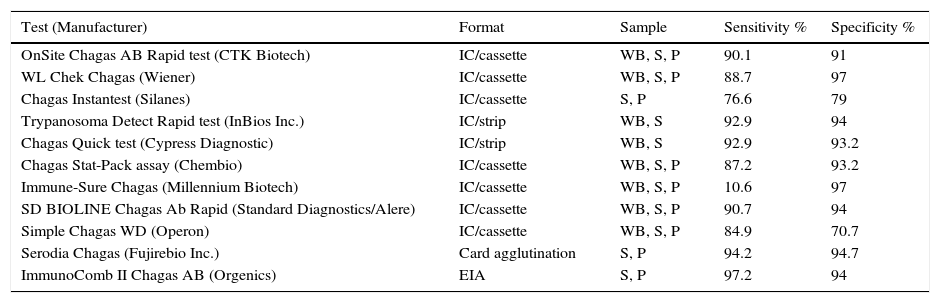
Conventional methods use a whole-parasite antigen. They are very sensitive but give rise to cross-reactions with other infections (malaria, leishmaniasis, syphilis, toxoplasmosis, hepatitis, IM, schistosomiasis) and also autoimmune diseases. To increase specificity, new serological methods and PoC assays have been developed that use recombinant antigens or synthetic peptides. As shown by a multicentre study conducted on samples extensively tested for T. cruzi infection in national reference laboratories in areas of endemicity and nonendemicity,59 the sensitivity and specificity of these assays vary greatly. The study concluded that most of the tests evaluated are useful for diagnosing this infection, but recognised that the sensitivity and specificity values obtained were somewhat lower than those published in other studies or claimed by the manufacturers. Table 6 summarises the characteristics of the assays tested.59
Sensitivity and specificity in different samples of commercial rapid diagnostic tests to detect anti-T. cruzi IgG.
| Test (Manufacturer) | Format | Sample | Sensitivity % | Specificity % |
|---|---|---|---|---|
| OnSite Chagas AB Rapid test (CTK Biotech) | IC/cassette | WB, S, P | 90.1 | 91 |
| WL Chek Chagas (Wiener) | IC/cassette | WB, S, P | 88.7 | 97 |
| Chagas Instantest (Silanes) | IC/cassette | S, P | 76.6 | 79 |
| Trypanosoma Detect Rapid test (InBios Inc.) | IC/strip | WB, S | 92.9 | 94 |
| Chagas Quick test (Cypress Diagnostic) | IC/strip | WB, S | 92.9 | 93.2 |
| Chagas Stat-Pack assay (Chembio) | IC/cassette | WB, S, P | 87.2 | 93.2 |
| Immune-Sure Chagas (Millennium Biotech) | IC/cassette | WB, S, P | 10.6 | 97 |
| SD BIOLINE Chagas Ab Rapid (Standard Diagnostics/Alere) | IC/cassette | WB, S, P | 90.7 | 94 |
| Simple Chagas WD (Operon) | IC/cassette | WB, S, P | 84.9 | 70.7 |
| Serodia Chagas (Fujirebio Inc.) | Card agglutination | S, P | 94.2 | 94.7 |
| ImmunoComb II Chagas AB (Orgenics) | EIA | S, P | 97.2 | 94 |
P: plasma; S: serum; WB: whole blood.
Adapted from Sánchez-Camargo et al.59.
Molecular and serological techniques are the most widely-used methods to establish an aetiological diagnosis of lower respiratory tract infections caused by Mycoplasma pneumoniae. Serology continues to be a widely-used method to establish an aetiological diagnosis and the only way to determine epidemiological characteristics.
ELISA is the technique of choice in most microbiology laboratories because of its sensitivity, ability to discriminate between IgM and IgG responses and its potential for automation. ICL formats that enable the assay to be performed in isolation have been included in the diagnostic procedure. In terms of ELISA, the highest diagnostic sensitivity is achieved with a parallel study of acute phase and convalescent phase samples. In paediatric patients, the detection of specific IgM in samples obtained one week after the onset of symptoms is strongly indicative of M. pneumoniae infection; in adults, the presence of IgM is weak and fleeting. The sensitivity of these assays varies in the paediatric population from 77% to 92%, although this could fall even further depending on the commercial test chosen.60,61 The sensitivity of IgM assays may vary depending on the reference method with which they are compared. Molecular techniques tend to offer lower sensitivity than complement fixation tests. The specificity of these assays exceeds 95%. Individual membrane-based rapid diagnostic tests that can qualitatively detect IgM and which offer good sensitivity (90%) and specificity (93–96%) are also available.62,63 They can be used in emergencies and in laboratories with limited samples.
Quality and rapid serological diagnosisThe application of standard UNE-EN-ISO 15189:2013, which establishes the requirements for quality and competence particular to medical laboratories, ensures that assays are conducted in compliance with these criteria. The first aspect to consider is the suitability of the assay's characteristics (sensitivity and specificity) to meet its objective. Although manufacturers of equipment with a CE marking will provide an appropriate assessment, each laboratory should establish its own operating conditions and verify that the validated characteristics are met. For accreditation, the pre-analytical, analytical and post-analytical aspects of the test should be considered. As they are performed manually, the main source of error in PoC assays stems from sample identification and/or issuance of the results; this problem is minimised by automated assays (assuming that an automated sample identification system is included). Participating in interlaboratory comparisons is one of the critical aspects of assay accreditation. However, for some serological markers used in clinical diagnostic laboratories, intercomparison assays are not available often enough or do not involve a sufficient number of laboratories.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: García-Bermejo I, de Ory F. Diagnóstico rápido en serología. Enferm Infecc Microbiol Clin. 2017;35:246–254.