Cancer patients may constitute a special risk group for the development of infective endocarditis (IE) because they are often subjected to invasive procedures. The aim of this study is to determine the differential clinical profile and prognosis of patients with IE and cancer.
MethodsA retrospective observational study was conducted on all patients consecutively diagnosed with IE in a single centre between 2005 and 2015. A comparative analysis was performed between patients with cancer and those free of disease, as well as a long-term follow-up.
ResultsThere were 208 IE cases, of which 32 had a cancer diagnosis. There were no significant differences in age (67.5 [59.2–74] vs 64 [51–74] years). The Charlson comorbidity index was same whether cancer was diagnosed or not (4 [2.2–5] vs 3.9 [2–5]). IE in cancer patients was mainly associated with health care (59.5% vs 24.4%, p<0.001). Staphylococcus aureus was the main causative agent (35%), and the tricuspid location was three times more common (18.8% vs 6.2%). Surgery was not performed in 18.7% of patients, despite having an indication, compared with 7.4% of patients without cancer. In-hospital mortality for cancer patients was 45.5%, and the probability of survival at one year was 40%.
ConclusionsIE in patients with cancer is predominantly caused by staphylococci, and has high early mortality. Although it is often related to health care, patients are limited from the therapeutic point of view.
Los pacientes con cáncer pueden constituir un especial grupo de riesgo para el desarrollo de endocarditis infecciosa (EI) debido a que frecuentemente están sometidos a maniobras invasivas. Nuestro objetivo es conocer cuál es el perfil diferencial y el pronóstico de los pacientes con EI y cáncer.
MétodosEstudio observacional retrospectivo de todos los pacientes con EI diagnosticados consecutivamente en un hospital entre 2005 y 2015. Se realiza un análisis comparativo entre los pacientes con enfermedad oncológica y sin ella, así como un seguimiento a largo plazo.
ResultadosSe diagnosticaron 208 casos de EI, de los cuales 32 sufrían enfermedad oncológica. No hubo diferencias significativas en cuanto a la edad (67,5 [59,2-74] vs. 64 [51-74] años), y la comorbilidad estimada por el índice de Charlson fue similar cuando no se consideró el propio diagnóstico de cáncer (4 [2,2-5] vs. 3,9 [2-5]). Se relacionó principalmente con la asistencia sanitaria (59,5% vs. 24,4%, p<0,001), predominó Staphylococcus aureus como agente causal (35%) y la localización tricuspídea fue 3 veces más frecuente (18,8% vs. 6,2%). Un 18,7% de pacientes no se intervinieron quirúrgicamente a pesar de tener indicación, frente al 7,4% de pacientes sin cáncer. La mortalidad intrahospitalaria alcanzó el 45,5% en pacientes con cáncer y la probabilidad de supervivencia al año fue del 40%.
ConclusionesLa EI en pacientes con cáncer está predominantemente causada por estafilococos y presenta una elevada mortalidad precoz. A pesar de que se relaciona frecuentemente con la asistencia sanitaria, los pacientes se ven limitados desde el punto de vista terapéutico.
Infective endocarditis (IE) is a serious disease whose epidemiology has evolved drastically in recent decades from being associated with young individuals with rheumatic valve disease to a complication that affects an increasing number of patients with a high comorbidity burden who undergo invasive tests and treatments.1 Cancer patients are, presumably, a special risk group, since they represent a growing proportion of patients treated in healthcare centres. Catheter-related staphylococcal bacteraemia is not the only risk2; endocarditis can be directly tumour-related and lead to cancer diagnosis, as in the case of colorectal cancer.3–5 However, some noninfective forms of endocarditis (non-bacterial thrombotic endocarditis) can confuse specialists and requires a more detailed diagnosis.
Even among medical specialists, a diagnosis of cancer still has connotations that can influence therapeutic decisions and cause clinicians to choose strategies they would not otherwise have considered.
The aim of our study has been to determine the clinical manifestations and differential microbiological profile of patients with IE and cancer, and to evaluate their short- and long-term prognosis.
MethodsPatientsWe conducted a retrospective study of all patients consecutively diagnosed with IE in the University Hospital Nuestra Señora de Candelaria, in Santa Cruz de Tenerife, between January 2005 and September 2015. Our centre, with a catchment area of approximately 600,000 inhabitants, serves most of the island of Tenerife and is a reference centre for the islands of El Hierro and La Gomera.
All patients diagnosed with IE on admission or who develop the disease during their hospital stay are systematically incorporated into a database. The records include clinical, microbiological and ultrasound findings, and any complication arising over the course of the disease.
DefinitionsAll patients had to meet inclusion criteria of possible or definitive IE according to the modified Duke criteria.6 Recurrent endocarditis includes relapses (infection by the same bacteria causing the initial episode) and new infections by a different bacteria or by the same one 2 months after the end of antibiotic treatment.7 Acute IE is defined as infection that develops less than 2 weeks after the appearance of the first signs and symptoms. Comorbidity was measured using the Charlson index,8 and final score was adjusted by subtracting the cancer sub-score (Charlson-C). RIFLE9 criteria were used to define acute kidney failure. Heart failure was defined as class III and IV of the New York Heart Association (NYHA) classification. Cerebral or peripheral embolism was required to be confirmed by imaging tests (CT, MRI or ultrasound). Urgent surgery was defined as surgery that is not delayed more than 24h after the indication is confirmed. Surgical risk was calculated in patients with an indication for surgery according to the current guidelines of the European Society of Cardiology (ESC)10,11 using the EuroSCORE II scale.12 Antibiotic treatment was considered correct when it complied with current ESC guidelines and was assessed by an independent specialist, as previously published.13
Cancer patients include those with metastatic/non-metastatic disease, patients receiving curative chemotherapy (adjuvant or neoadjuvant), those receiving palliative chemotherapy and those receiving support without chemotherapy.
Neutropaenia was defined as a neutrophil count of less than 1000cells/mm3.
Study variables and statistical analysisWe compared the clinical, microbiological and ultrasound findings and in-hospital evolution (mortality and IE-related complications) of patients with IE already diagnosed with cancer or diagnosed during the endocarditis episode against those of patients with IE without a cancer diagnosis.
The Kolmogorov–Smirnov test was used to verify the normal distribution of continuous variables. These variables are expressed as mean (standard deviation) or median (interquartile range), as appropriate, and compared using the Student's t test or the Mann–Whitney U test.
Categorical variables are expressed as absolute number (percentage) and compared using the Chi-square test or Fisher's exact test, as appropriate.
In the endocarditis plus cancer group, the survival curve was calculated using the Kaplan–Meier method.
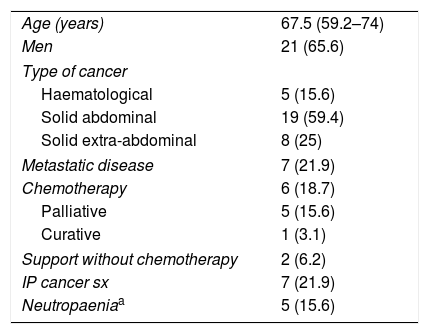
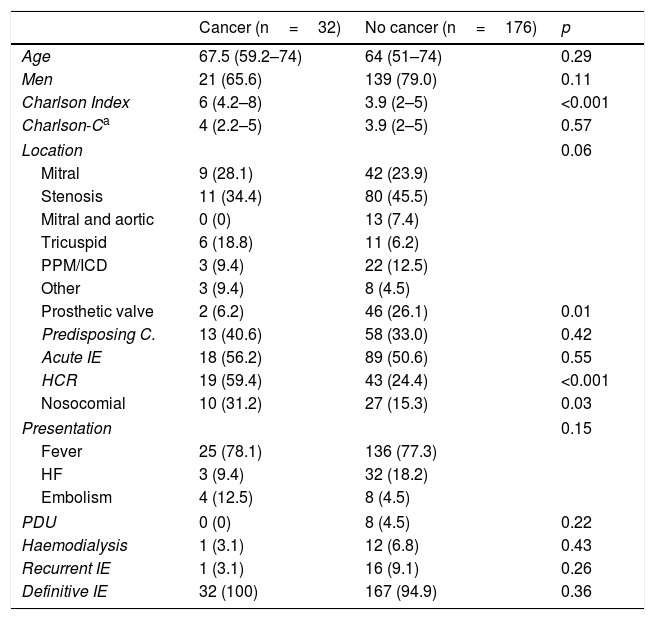
ResultsA total of 208 cases of IE were diagnosed during the study period, of which 32 also had a diagnosis of cancer. Table 1 shows the characteristics of the cancer patients. Solid abdominal tumours predominated, with 59.4% of cases; of these, 42% presented colorectal cancer. The majority of patients did not present advanced disease, 18.8% were receiving active treatment and neutropaenia was confirmed at the time of diagnosis of IE in only 15.6% of patients.
Characteristics of cancer patients.
| Age (years) | 67.5 (59.2–74) |
| Men | 21 (65.6) |
| Type of cancer | |
| Haematological | 5 (15.6) |
| Solid abdominal | 19 (59.4) |
| Solid extra-abdominal | 8 (25) |
| Metastatic disease | 7 (21.9) |
| Chemotherapy | 6 (18.7) |
| Palliative | 5 (15.6) |
| Curative | 1 (3.1) |
| Support without chemotherapy | 2 (6.2) |
| IP cancer sx | 7 (21.9) |
| Neutropaeniaa | 5 (15.6) |
Data are expressed as n (%) or median (interquartile range).
IP cancer sx: immediate postoperative cancer surgery.
Table 2 summarises the clinical characteristics by groups at the time of diagnosis. Both groups were well matched in terms of age, sex or predominant presenting symptom. Patients diagnosed with cancer presented a higher Charlson comorbidity index. However, this difference disappeared when the cancer diagnosis was eliminated from the score. There was a significantly higher proportion of healthcare-related cases, particularly nosocomial infections. There was a high percentage of patients with previous endocarditis in the group of patients without cancer: 14 cases of reinfection and 2 of relapse. The case of recurrence in the cancer patient was due to reinfection.
Differential clinical characteristics at the time of diagnosis.
| Cancer (n=32) | No cancer (n=176) | p | |
|---|---|---|---|
| Age | 67.5 (59.2–74) | 64 (51–74) | 0.29 |
| Men | 21 (65.6) | 139 (79.0) | 0.11 |
| Charlson Index | 6 (4.2–8) | 3.9 (2–5) | <0.001 |
| Charlson-Ca | 4 (2.2–5) | 3.9 (2–5) | 0.57 |
| Location | 0.06 | ||
| Mitral | 9 (28.1) | 42 (23.9) | |
| Stenosis | 11 (34.4) | 80 (45.5) | |
| Mitral and aortic | 0 (0) | 13 (7.4) | |
| Tricuspid | 6 (18.8) | 11 (6.2) | |
| PPM/ICD | 3 (9.4) | 22 (12.5) | |
| Other | 3 (9.4) | 8 (4.5) | |
| Prosthetic valve | 2 (6.2) | 46 (26.1) | 0.01 |
| Predisposing C. | 13 (40.6) | 58 (33.0) | 0.42 |
| Acute IE | 18 (56.2) | 89 (50.6) | 0.55 |
| HCR | 19 (59.4) | 43 (24.4) | <0.001 |
| Nosocomial | 10 (31.2) | 27 (15.3) | 0.03 |
| Presentation | 0.15 | ||
| Fever | 25 (78.1) | 136 (77.3) | |
| HF | 3 (9.4) | 32 (18.2) | |
| Embolism | 4 (12.5) | 8 (4.5) | |
| PDU | 0 (0) | 8 (4.5) | 0.22 |
| Haemodialysis | 1 (3.1) | 12 (6.8) | 0.43 |
| Recurrent IE | 1 (3.1) | 16 (9.1) | 0.26 |
| Definitive IE | 32 (100) | 167 (94.9) | 0.36 |
Data are expressed as n (%) or median (interquartile range).
Predisposing C.: existence of cardiopathy that predisposes to the development of IE; IE: infective endocarditis; HF: heart failure; PPM/ICD: pacemaker or implantable cardioverter-defibrillator; HCR: healthcare-related; PDU: parenteral drug users.
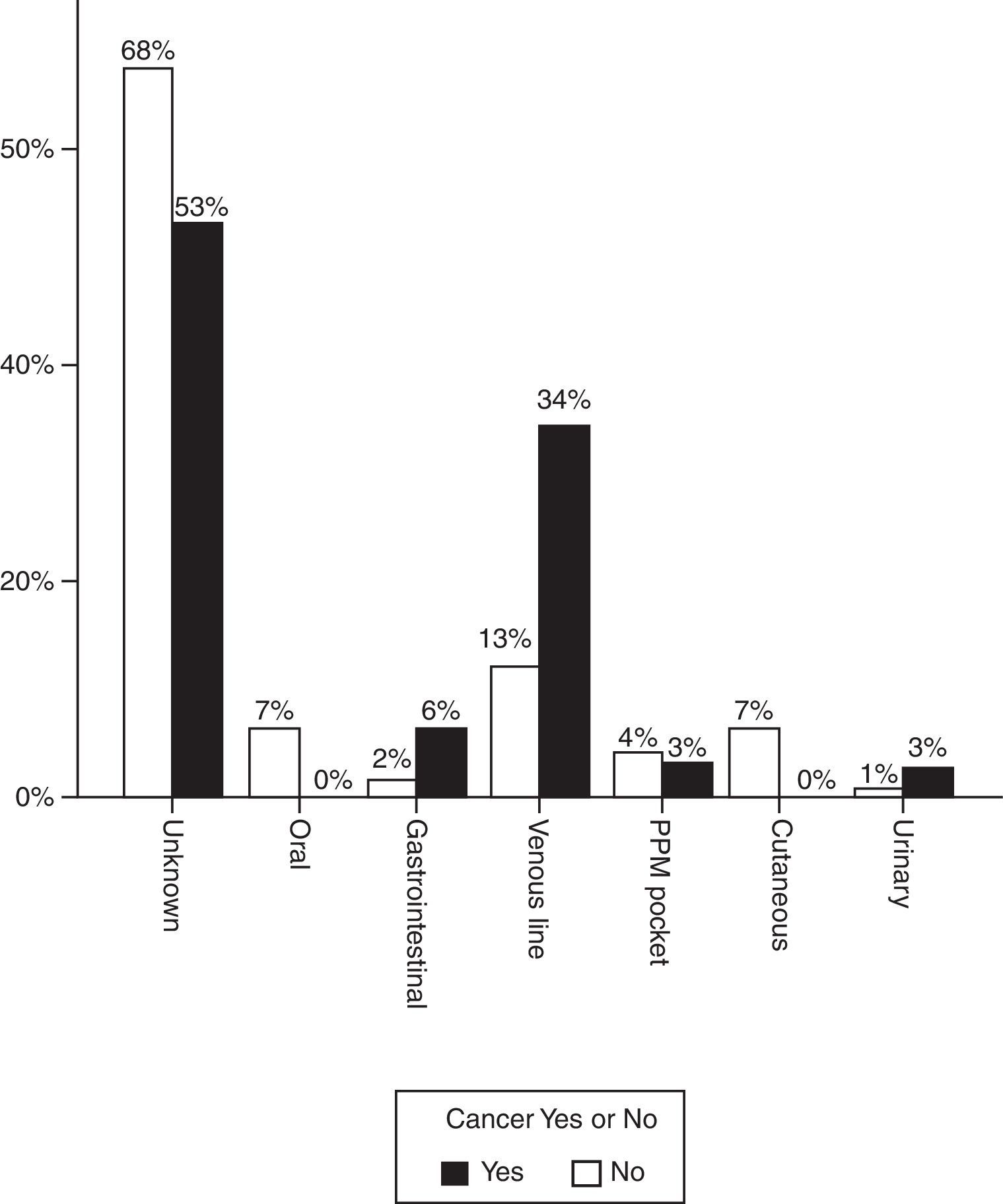
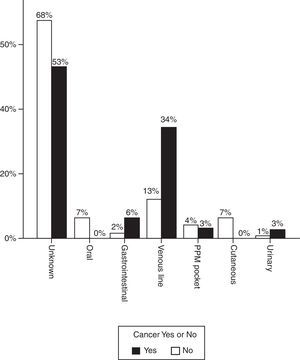
Fig. 1 shows the most likely origin of the infection. In most cases the source of the infection was not identified but when it was, catheter infection predominated in patients with oncological disease. In 7 cases (21.9%), the infection was acquired in the immediate postoperative period of cancer surgery.
The incidence of tricuspid IE was 3 times higher in patients with cancer (18.8% vs 6.2%). Three of the cancer patients with tricuspid endocarditis had a subcutaneous venous reservoir and in the other cases the infection was acquired from a peripheral line. Staphylococcus aureus was identified as a causative agent in half of these episodes. Prosthetic valve endocarditis was significantly less frequent (6.2% vs 26.1%, p=0.01).
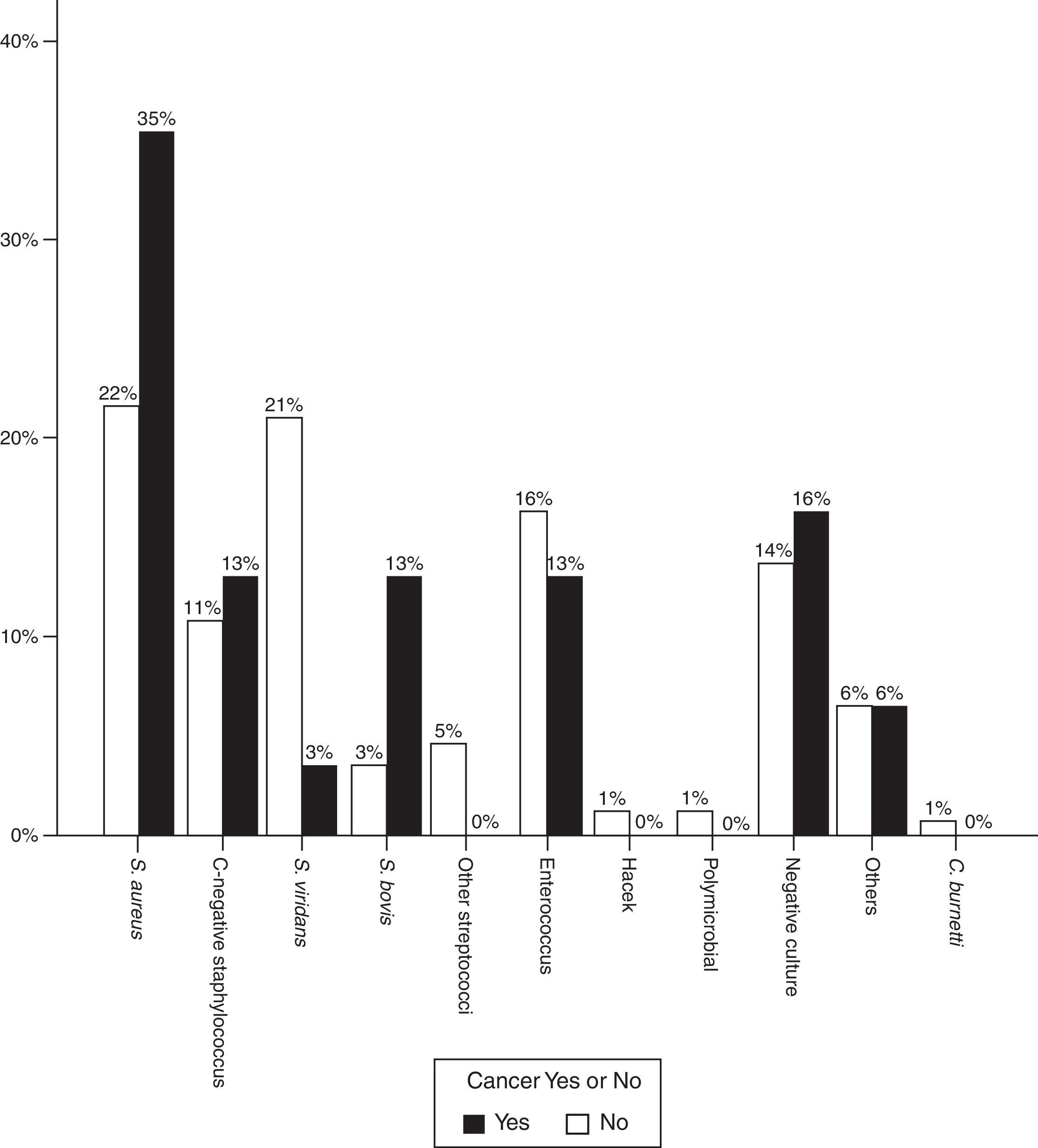
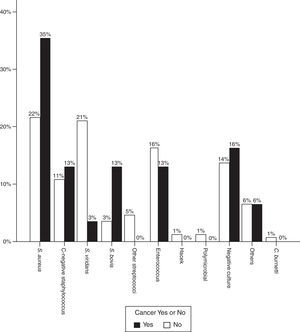
Regarding the infectious agent, a significantly higher proportion of S. aureus was found in patients with IE and oncological disease. This was the bacterium isolated in most cases (37.5% vs 21%, p=0.02). Streptococcus viridans was less common (15.6% vs 29.5%, p=0.04).
The proportion of Streptococcus gallolyticus (formerly called Streptococcus bovis) was also clearly higher (12.9% vs 3.4%) and in 3 out of 4 cases this bacterium was isolated in patients with colon cancer. In other words, of the 8 cases of colorectal cancer, the infectious agents was S. bovis in 3 cases, S. aureus in 1 case, Enterococcus faecalis in 1 case, Staphylococcus epidermidis in 1 case and blood cultures were negative in 2 patients. Fig. 2 shows the distribution of infectious agents in both groups of patients.
Although the infectious agent was not identified in a relatively high proportion of patients (due to the use of antibiotics prior to the extraction of blood samples), the clinical and ultrasound findings in these patients met the criteria for definitive endocarditis.
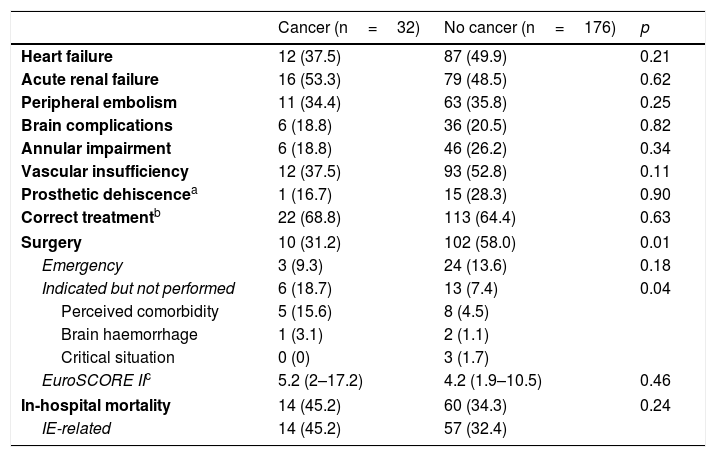
Evolution and survivalAlthough IE-related complications were not significantly more common in patients with cancer (Table 3), in-hospital mortality in this group was 45.2% compared to 34.3% in patients without cancer and in most cases (47.1%) the cause of death was severe sepsis or septic shock.
In-hospital evolution of study patients.
| Cancer (n=32) | No cancer (n=176) | p | |
|---|---|---|---|
| Heart failure | 12 (37.5) | 87 (49.9) | 0.21 |
| Acute renal failure | 16 (53.3) | 79 (48.5) | 0.62 |
| Peripheral embolism | 11 (34.4) | 63 (35.8) | 0.25 |
| Brain complications | 6 (18.8) | 36 (20.5) | 0.82 |
| Annular impairment | 6 (18.8) | 46 (26.2) | 0.34 |
| Vascular insufficiency | 12 (37.5) | 93 (52.8) | 0.11 |
| Prosthetic dehiscencea | 1 (16.7) | 15 (28.3) | 0.90 |
| Correct treatmentb | 22 (68.8) | 113 (64.4) | 0.63 |
| Surgery | 10 (31.2) | 102 (58.0) | 0.01 |
| Emergency | 3 (9.3) | 24 (13.6) | 0.18 |
| Indicated but not performed | 6 (18.7) | 13 (7.4) | 0.04 |
| Perceived comorbidity | 5 (15.6) | 8 (4.5) | |
| Brain haemorrhage | 1 (3.1) | 2 (1.1) | |
| Critical situation | 0 (0) | 3 (1.7) | |
| EuroSCORE IIc | 5.2 (2–17.2) | 4.2 (1.9–10.5) | 0.46 |
| In-hospital mortality | 14 (45.2) | 60 (34.3) | 0.24 |
| IE-related | 14 (45.2) | 57 (32.4) | |
Data are expressed as n (%) or median (interquartile range).
The proportion of operated patients was significantly lower in this group (31.2% vs 58%, p=0.01), and in 18.7% of cases surgery was not performed even though it was indicated. In patients without cancer, only 7.4% did not undergo surgery (p=0.04).
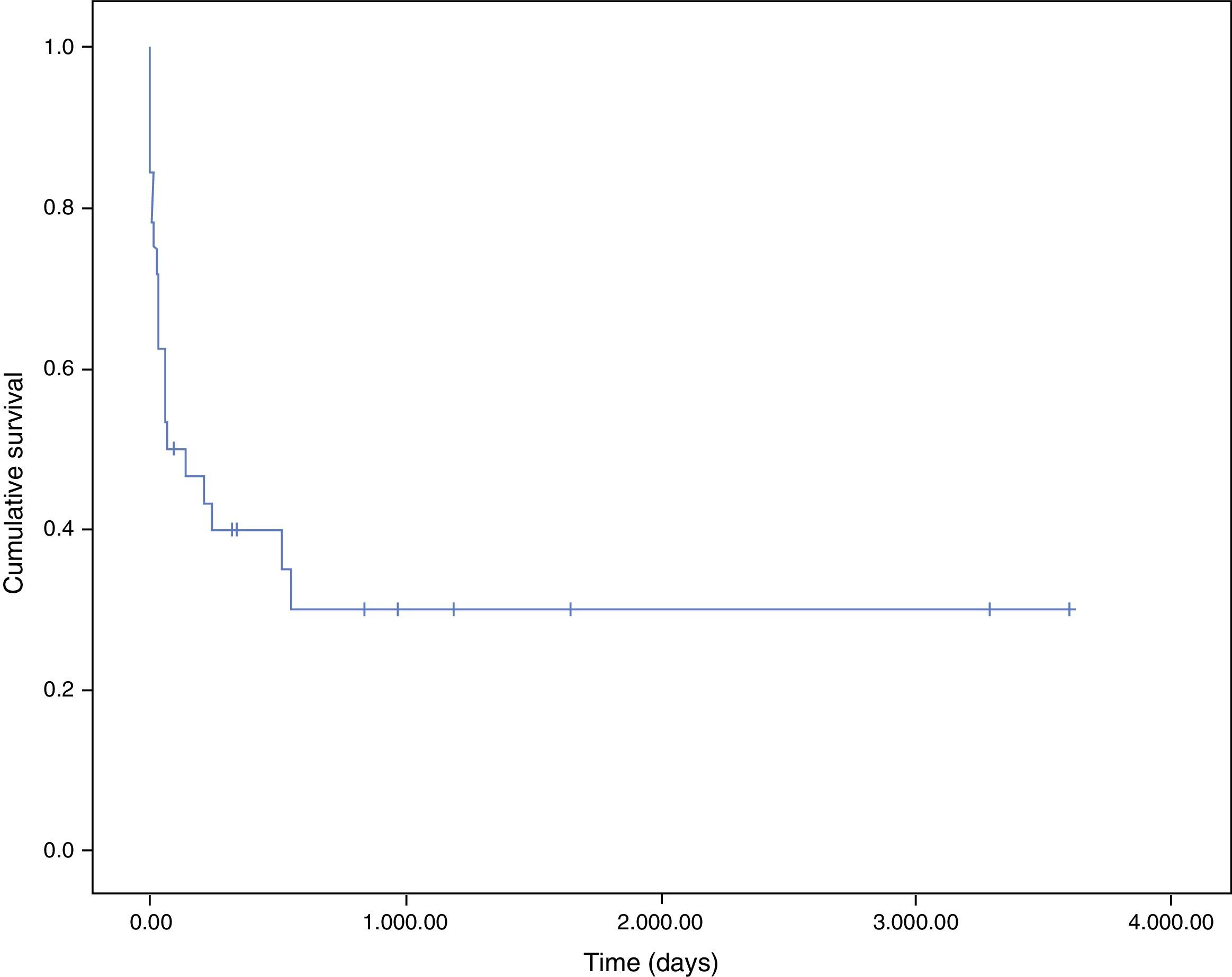
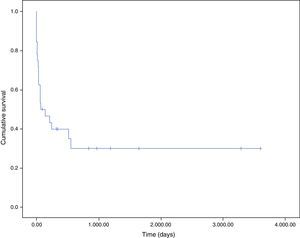
Fig. 3 shows the survival curves of patients with endocarditis and cancer. Most patients died in hospital (100% directly due to endocarditis). One-month survival probability was 62% and one-year survival was 40%. The main cause of death after discharge was the malignant process (5 of 6 patients, 83.3%).
DiscussionLittle is known about the magnitude of the association between IE and cancer, or about the natural history of cancer patients with concomitant diagnosis of infective endocarditis. According to a recent report by García-Albéniz et al., the peri-diagnostic incidence of IE is almost 10 times higher in patients with colorectal cancer and twice as high in patients with lung cancer, and is associated with poorer survival.14
In our setting, 15% of patients with IE have a history of oncological disease, mostly solid abdominal tumours. Very few of these were receiving chemotherapy or presented neutropaenia at the time of IE diagnosis. The vast majority do not have advanced disease. Except for the cancer itself, these patients do not present greater comorbidity but the episodes are more severe.
Endocarditis can be a marker of occult cancer, particularly gastrointestinal cancer15; however, in our centre most infections are healthcare-related, mainly due to central venous catheter bacteraemia and in many cases the infection was acquired in the immediate postoperative period of oncological surgery. This explains why the majority of episodes are caused by staphylococci and why the incidence of tricuspid valve involvement is 3 times higher in cancer patients than in those without cancer.
IE-related mortality is particularly high in our centre and was the subject of a direct comparative study with another Spanish hospital renowned for its work in the study and treatment of this disease. We suspect that the origin of these differences lies in the fact that we treat particularly high-risk patients.16 Whatever the reason, endocarditis clearly worsens the prognosis of cancer patients: almost half die during their hospital stay due to the infection itself and although the antibiotic treatment given was correct and similar to that administered to patients without cancer, fewer oncological patients are treated surgically, even when it is indicated. This explains, at least in part, the high mortality rate: the group of patients with the worst prognosis in our hospital do not undergo surgery even though it is needed.17 This limitation of therapeutic effort, partly due to a misguided perception of comorbidity or surgical risk, is particularly striking in view of the fact that it is the health service itself that is responsible for most episodes of IE. This should compel us to take the following into consideration: first, measures to prevent bacteraemia in these patients need to be stepped up. Second, the onset of fever is a warning sign and indicates the need to actively evaluate the possibility of endocarditis when there is no other clear alternative diagnosis. In fact, in some patients with severe sepsis and septic shock a definitive diagnosis is either never found or found too late, thus decreasing the chances of survival. Finally, we believe that the patient's risk and comorbidity should be objectively evaluated before limiting therapeutic effort. A diagnosis of cancer should not in itself justify adopting a therapeutic strategy that further worsens the prognosis of patients with IE.
Our study has several limitations that must be considered. In the first place, being a single-centre study it does not necessarily reflect the situation in other settings. The sample size is small and includes a significant proportion of patients in whom the origin of endocarditis is not known. A systematic search for the portal of entry would perhaps give a clearer picture of the mechanism of infection.
To conclude, in our setting, cancer patients with IE constitute a special risk group and invasive therapies are responsible for a large number of cases. Short-term mortality rates are high in this patient group and in many cases surgical treatment is ruled out without first objectively considering the patient's comorbidity or risk.
Conflicts of interestNone of the authors has any conflict of interest.
Please cite this article as: Mesa del Castillo-Payá C, Rodríguez-Esteban M, Quijada-Fumero A, Carballo-Arzola L, Farrais-Villalba M, Afonso R, et al. Endocarditis infecciosa en pacientes con enfermedades oncológicas. Enferm Infecc Microbiol Clin. 2018;36:72–77.