To determine the in vitro activity of a polyhexanide–betaine solution against collection strains and multidrug-resistant (MDR) nosocomial isolates, including high-risk clones.
MethodsWe studied of 8 ATCC and 21 MDR clinical strains of Staphylococcus aureus, Enterococcus faecium, Enterococcus faecalis, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, including the multiresistant high-risk clones. The MICs and MBCs of a 0.1% polyhexanide–0.1% betaine solution were determined by microdilution. For each species, strains with the highest MICs were selected for further experiments.
The dilution–neutralization test (PrEN 12054) was performed by incubating bacterial inocula of 106CFU/mL for 1min with undiluted 0.1% polyhexanide–betaine solution. The CFUs were counted after neutralization.
Growth curves and time-kill curves at concentrations of 0.25, 1, 4, and 8×MIC, were performed. MICs of recovered strains were determined when regrowth was observed in time-kill studies after 24h of incubation.
Strains with reduced susceptibility were selected by serial passage on plates with increasing concentrations of polyhexanide–betaine, and MICs were determined.
ResultsPolyhexanide–betaine MIC range was 0.5–8mg/L. MBCs equalled or were 1 dilution higher than MICs. The dilution–neutralization method showed total inoculum clearance of all strains. In time-kill curves, no regrowth was observed at 4×MIC, except for S. aureus (8×MIC). Increased MICs were not observed in time-kill curves, or after serial passages after exposure to polyhexanide–betaine.
ConclusionsPolyhexanide–betaine presented bactericidal activity against all MDR clinical isolates tested, including high-risk clones, at significantly lower concentrations and time of activity than those commercially used.
Determinar la actividad in vitro de una solución de polihexanida-betaína frente a una colección de cepas nosocomiales multirresistentes, incluyendo clones de alto riesgo.
MétodosEstudiamos 8 cepas ATCC y 21 cepas clínicas de Staphylococcus aureus, Enterococcus faecium, Enterococcus faecalis, Escherichia coli, Enterobactercloacae, Klebsiella pneumoniae, Acinetobacter baumannii y Pseudomonas aeruginosa, incluyendo clones de alto riesgo multirresistentes. Determinamos las CMI y las CMB de una solución 0,1% de polihexanida y 0,1% de betaína por microdilución. De cada especie, seleccionamos las cepas con mayores CMIs para los siguientes experimentos.
Realizamos el test de dilución-neutralización (PrEN 12054) incubando 106UFC/ml 1min con solución 0,1% de polihexanida-betaína, calculando las UFCs tras un paso de neutralización.
Realizamos curvas de crecimiento y de tiempo-muerte a concentraciones 0,25, 1, 4 y 8×CMI. Determinamos las CMIs de las cepas recuperadas tras recrecimiento a las 24h.
Seleccionamos cepas con sensibilidad reducida tras pases seriados en placas con concentraciones crecientes de polihexanida-betaína y determinamos sus CMI.
ResultadosEl rango de CMI fue de 0,5–8mg/l. Las CMBs fueron iguales o una dilución mayor. El test de dilución-neutralización presentó aclaramiento total del inóculo en todas las cepas. En las curvas de tiempo-muerte, no se observó recrecimiento a 4×CMI, excepto para S. aureus (8×CMI). No se incrementó la CMI ni aquí ni en los pases seriados con polihexanida-betaína.
ConclusionesPolihexanida-betaína presenta actividad bactericida frente a todas las cepas multirresistentes estudiadas, incluyendo clones de alto riesgo, a concentraciones y tiempos de exposición significativamente menores que los usados comercialmente.
Chronic wounds affect more than 1% of the population and various types of bacteria are normally involved, living in biofilm communities that are highly resistant to cleansing, irrigation and antibiotic treatment. Furthermore, bacterial wound colonization, not just infection, is closely associated with impaired healing; this is quite common in clinical practice, with over 80% of leg ulcers being colonized.1
The microbial flora in wounds change over time, being initially colonized by skin commensals and generally followed by Gram-positive pathogens in chronic wounds, whereas Gram-negative bacteria, and even anaerobic flora are involved in wounds of longer duration. The microbiology of acute wound infections is different, although some microorganisms are more prevalent than others.1
Successful bacterial clones have played a key role in the dissemination of resistance worldwide, since they provide support for preserving and spreading the genes that produce resistance. Gram-positive and Gram-negatives high-risk clones pathogens are cause of nosocomial infections and worldwide outbreaks.2,3 These high-risk clones are also powerful vehicles for disseminating genetic components (genes, integrons, transposons, and plasmids).3 In addition, they confer not only resistance properties but virulence determinants and genome plasticity, which help species adapt to changing conditions in the hospital setting and to achieve the presently successful global spread.2 Fundamental steps in the dissemination of these microorganisms involve colonization, both of humans and of the environment, which leads to difficult-to-control outbreaks with limited therapeutic options.
Primary strategies for treating wound infections include systemic and topical antibiotics. Insufficient accumulation in the soft tissue remains a drawback of systemic antibiotics, which also have to contend with increasing bacterial resistance. In addition to antibiotic therapy, wound cleansing and debridement are essential steps in chronic wound management, where antiseptic solutions and wound irrigating agents are routinely used.4 One compound is polyhexanide (polyhexamethylene biguanide, or PHMB), a broad-spectrum antiseptic with excellent tissue tolerance due to its specific mechanism of action against the acidic lipids within the bacterial membranes, and with minor effects on the neutral lipids of human cells. Several studies have demonstrated the antimicrobial activity of PHMB5–9 with properties that include improved wound healing, no resistance development, biofilm reduction, and an in vitro biocompatibility of >1.9 Prontosan® (B. Braun Medical S.A., Rubi, Spain) is a wound cleansing solution with 0.1% PHMB and 0.1% betaine. Betaine (undecylenamidopropylbetaine, a surfactant with antimicrobial properties developed for use in personal care products that is able to reduce cytotoxicity and increase the antimicrobial activity of PHMB on its own10). Although some studies have recommended the use of Prontosan® on the basis of clinical data and with limited numbers of patients,11,12 there is very little in vitro data to support the use of this irrigation solution and it has been tested only against standard strains.10
The objective of our study was to determine the in vitro activity of a commercial polyhexanide–betaine (PHMB–B) solution against reference (ATCC) and clinical strains including successful MDR high-risk clones belonging to the most common nosocomial pathogens.
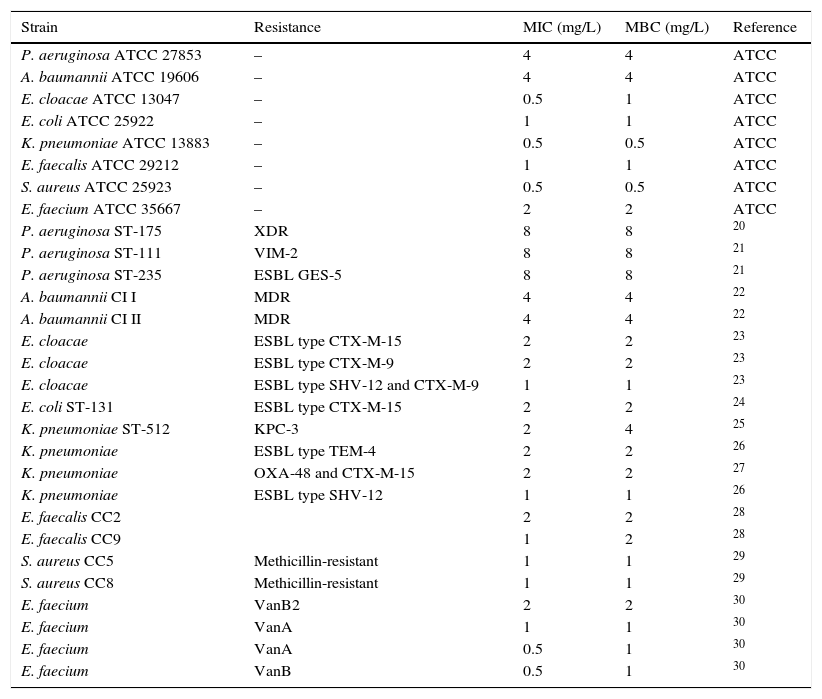
MethodsStrainsEight ATCC strains (Pseudomonas aeruginosa ATCC 27853, Acinetobacter baumannii ATCC 19606, Enterococcus faecium ATCC 35667, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, Enterobacter cloacae ATCC 13047, Staphylococcus aureus ATCC 25923, and Klebsiella pneumoniae ATCC 13883) and twenty-one multidrug-resistant clinical isolates (Table 1) were studied. The clinical strains included high-risk clones with major resistance mechanisms, such as S. aureus CC5 and CC8, both methicillin-resistant; vancomycin-resistant E. faecium; E. faecalis CC2 and CC9; E. coli ST-131 producing ESBL type CTX-M-15; E. cloacae producing ESBL types CTX-M-15, CTX-M-9, and SHV-12 plus CTX-M-9; K. pneumoniae ST-512 producing carbapenemase type KPC-3, carbapenemase type OXA-48 plus ESBL type CTX-M-15, and ESBL type TEM-4; multidrug-resistant A. baumannii CI I and CI II; and extremely drug-resistant P. aeruginosa ST-175 and ST-111 producing the metallo-beta-lactamase VIM-2, and ST-235 producing GES-5-type carbapenemase.
Prontosan® MIC and MBC values against clinical isolates.
| Strain | Resistance | MIC (mg/L) | MBC (mg/L) | Reference |
|---|---|---|---|---|
| P. aeruginosa ATCC 27853 | – | 4 | 4 | ATCC |
| A. baumannii ATCC 19606 | – | 4 | 4 | ATCC |
| E. cloacae ATCC 13047 | – | 0.5 | 1 | ATCC |
| E. coli ATCC 25922 | – | 1 | 1 | ATCC |
| K. pneumoniae ATCC 13883 | – | 0.5 | 0.5 | ATCC |
| E. faecalis ATCC 29212 | – | 1 | 1 | ATCC |
| S. aureus ATCC 25923 | – | 0.5 | 0.5 | ATCC |
| E. faecium ATCC 35667 | – | 2 | 2 | ATCC |
| P. aeruginosa ST-175 | XDR | 8 | 8 | 20 |
| P. aeruginosa ST-111 | VIM-2 | 8 | 8 | 21 |
| P. aeruginosa ST-235 | ESBL GES-5 | 8 | 8 | 21 |
| A. baumannii CI I | MDR | 4 | 4 | 22 |
| A. baumannii CI II | MDR | 4 | 4 | 22 |
| E. cloacae | ESBL type CTX-M-15 | 2 | 2 | 23 |
| E. cloacae | ESBL type CTX-M-9 | 2 | 2 | 23 |
| E. cloacae | ESBL type SHV-12 and CTX-M-9 | 1 | 1 | 23 |
| E. coli ST-131 | ESBL type CTX-M-15 | 2 | 2 | 24 |
| K. pneumoniae ST-512 | KPC-3 | 2 | 4 | 25 |
| K. pneumoniae | ESBL type TEM-4 | 2 | 2 | 26 |
| K. pneumoniae | OXA-48 and CTX-M-15 | 2 | 2 | 27 |
| K. pneumoniae | ESBL type SHV-12 | 1 | 1 | 26 |
| E. faecalis CC2 | 2 | 2 | 28 | |
| E. faecalis CC9 | 1 | 2 | 28 | |
| S. aureus CC5 | Methicillin-resistant | 1 | 1 | 29 |
| S. aureus CC8 | Methicillin-resistant | 1 | 1 | 29 |
| E. faecium | VanB2 | 2 | 2 | 30 |
| E. faecium | VanA | 1 | 1 | 30 |
| E. faecium | VanA | 0.5 | 1 | 30 |
| E. faecium | VanB | 0.5 | 1 | 30 |
Minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations (MBC) of Prontosan® (0.1% betaine and 0.1% polyhexanide in aqueous solution, B. Braun Medical S.A., Rubi, Spain) were determined by microdilution following CLSI procedures.13 For each species, the strain with the highest MIC was selected for further experiments.
Dilution–neutralization testThe bactericidal activity of PHMB–B was tested by the dilution–neutralization method, following European Standard PrEN 12054.14 Briefly, an initial bacterial inoculum of 106CFU/mL was incubated for 1min against undiluted product and, following a neutralization step with a neutralizing solution (8% v/v Tween 80, 6% w/v saponin, 0.8% w/v lecithin, 2% w/v sodium dodecyl sulphate, PBS), CFUs were counted by serial dilution plating onto Mueller–Hinton agar.
Growth curvesGrowth curves were recorded by monitoring the turbidity (with OD at 595nm) of the medium using an automated microplate reader (Infinite F200PRO, Tecan Ibérica Instrumentación S.L., Barcelona, Spain). The experiments were performed in the absence and presence of different concentrations of PHMB–B (0.25, 1, 4, and 8×MIC) using a bacterial inoculum of 5×105CFU/mL.
Time-kill curvesThe bactericidal activity of PHMB–B was also tested by time-kill curves, adopting the methodology described in European Standard PrEN 12054.14 Before performing the time-kill curves, it was confirmed that the neutralizing solution did not inhibit bacterial growth at concentrations used in the assay and that a 1:10 dilution of the neutralizing solution was able to neutralize PHMB–B bactericidal activity. The time-kill curves were performed in Mueller–Hinton broth using an initial inoculum of 5×105CFU/mL (obtained making dilutions from an overnight culture) and PHMB–B concentrations equivalent to 0.25, 1, 4, and 8×MIC. At various time points (0, 5′, 10′, 15′, 30′, 1h, 2h, 4h, 8h, and 24h), the aliquots were removed, neutralized, and inoculated onto Mueller–Hinton agar for bacterial counts. PHMB–B was considered to have bactericidal activity when a ≥3logCFU/mL reduction of the initial inoculum was reached at the concentration tested.
After completion of the time-kill curves, for strains where both bactericidal activity at different concentrations of PHMB–B and subsequent regrowth after 24h was observed, the MICs of colonies taken after exposure to PHMB–B were determined in order to test the possibility of selection for mutants or tolerant colonies with decreased susceptibility to PHMB–B.
Reduced in vitro susceptibilityThe selection of strains with reduced susceptibility to PHMB–B was performed by serial passage of the strains on Mueller–Hinton agar plates containing increasing concentrations of PHMB–B. Starting at 0.5×MIC of each strain, a colony was taken from the previous plate and streaked with an inoculation loop in plates with higher PHMB–B concentrations, increasing by log2. The MICs of colonies that grew in the plate with the highest concentration of PHMB–B were determined.
All experiments were performed in triplicate on 3 different days.
ResultsSusceptibility testingTable 1 shows the MIC and MBC values obtained for the ATCC strains (0.5–4mg/L) and the clinical isolates (0.5–8mg/L); for each species, the isolate with the highest MIC was selected for further experiments.
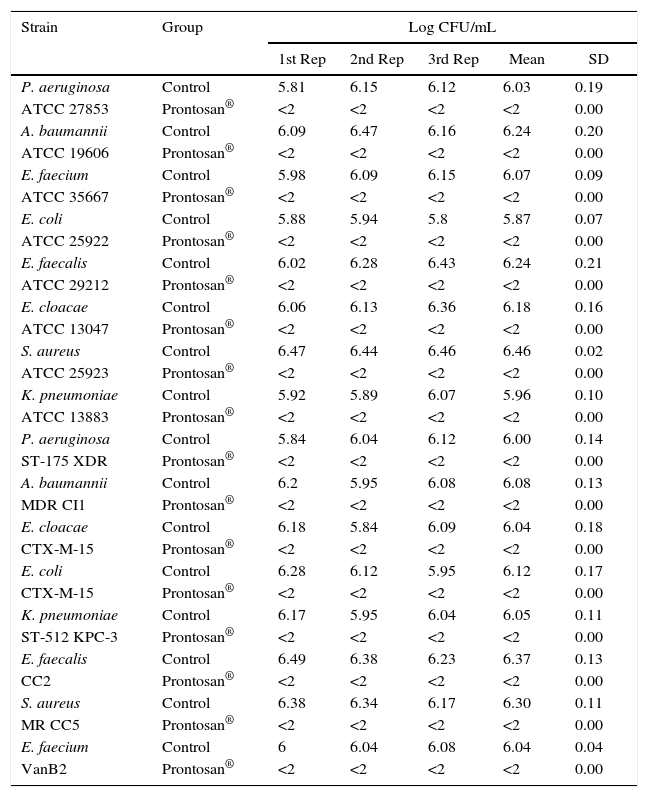
Dilution–neutralization testBefore the experiment was performed, the dilution–neutralization test was validated in accordance with European Standard PrEN 12054 and no remarkable bactericidal activity was noted due to neutralization. Furthermore, it was also proven that neutralization inhibited Prontosan® bactericidal activity.
The test results showed that Prontosan® had bactericidal activity under the conditions described in European Standard PrEN 12054 (test temperature: 20±1°C; product test concentration: undiluted; contact time: 1min; counting procedure: spread plate; temperature of incubation: 37±1°C) and showed complete bactericidal activity after 1min of contact for each microorganism tested (Table 2).
Dilution–neutralization test of Prontosan® against standard and clinical strains.
| Strain | Group | Log CFU/mL | ||||
|---|---|---|---|---|---|---|
| 1st Rep | 2nd Rep | 3rd Rep | Mean | SD | ||
| P. aeruginosa | Control | 5.81 | 6.15 | 6.12 | 6.03 | 0.19 |
| ATCC 27853 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| A. baumannii | Control | 6.09 | 6.47 | 6.16 | 6.24 | 0.20 |
| ATCC 19606 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. faecium | Control | 5.98 | 6.09 | 6.15 | 6.07 | 0.09 |
| ATCC 35667 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. coli | Control | 5.88 | 5.94 | 5.8 | 5.87 | 0.07 |
| ATCC 25922 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. faecalis | Control | 6.02 | 6.28 | 6.43 | 6.24 | 0.21 |
| ATCC 29212 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. cloacae | Control | 6.06 | 6.13 | 6.36 | 6.18 | 0.16 |
| ATCC 13047 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| S. aureus | Control | 6.47 | 6.44 | 6.46 | 6.46 | 0.02 |
| ATCC 25923 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| K. pneumoniae | Control | 5.92 | 5.89 | 6.07 | 5.96 | 0.10 |
| ATCC 13883 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| P. aeruginosa | Control | 5.84 | 6.04 | 6.12 | 6.00 | 0.14 |
| ST-175 XDR | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| A. baumannii | Control | 6.2 | 5.95 | 6.08 | 6.08 | 0.13 |
| MDR CI1 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. cloacae | Control | 6.18 | 5.84 | 6.09 | 6.04 | 0.18 |
| CTX-M-15 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. coli | Control | 6.28 | 6.12 | 5.95 | 6.12 | 0.17 |
| CTX-M-15 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| K. pneumoniae | Control | 6.17 | 5.95 | 6.04 | 6.05 | 0.11 |
| ST-512 KPC-3 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. faecalis | Control | 6.49 | 6.38 | 6.23 | 6.37 | 0.13 |
| CC2 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| S. aureus | Control | 6.38 | 6.34 | 6.17 | 6.30 | 0.11 |
| MR CC5 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
| E. faecium | Control | 6 | 6.04 | 6.08 | 6.04 | 0.04 |
| VanB2 | Prontosan® | <2 | <2 | <2 | <2 | 0.00 |
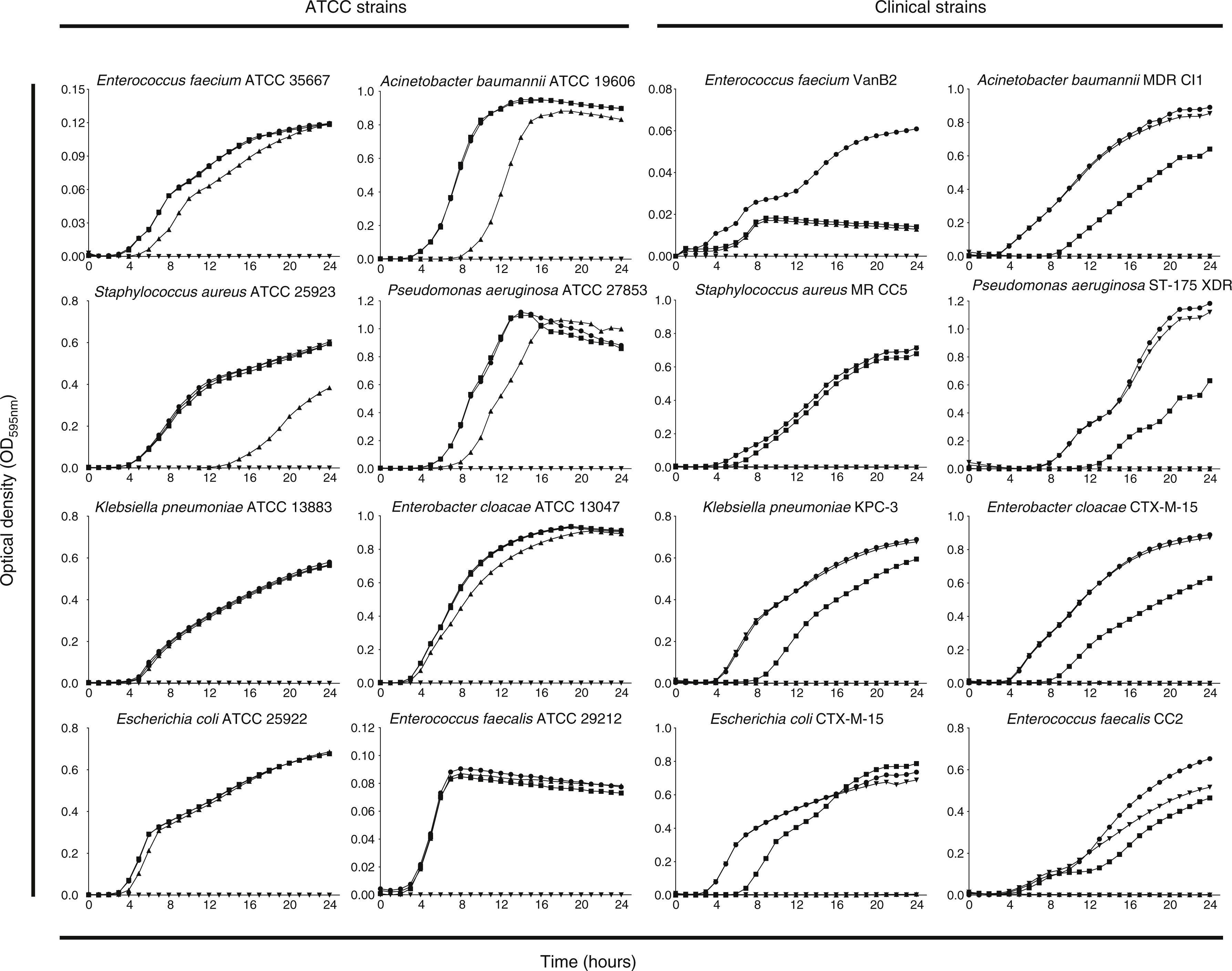
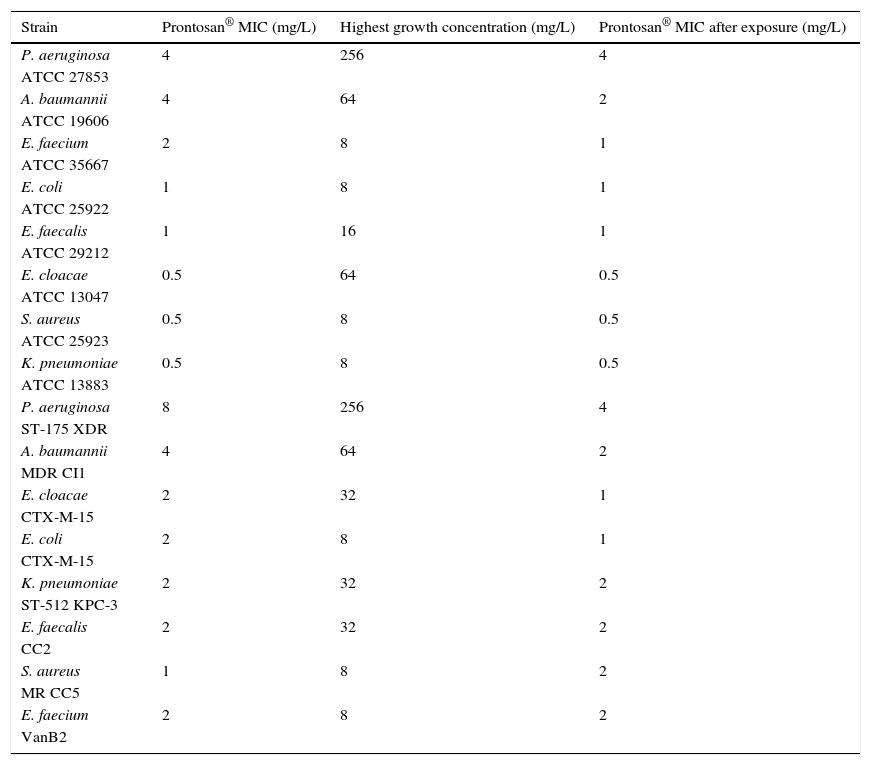
Fig. 1 shows the growth curves of strains exposed to different concentrations of PHMB–B (0.25, 1, 4, and 8×MIC). The concentration at which growth inhibition occurred was equivalent to 4×MIC for all strains studied, except for S. aureus ATCC 25923 and E. cloacae ATCC 13047, where it was at 8×MIC. The highest concentration tested was 32mg/L.
Growth curves of ATCC and clinical strains exposed to different concentrations of Prontosan®.
Growth curves of ATCC and clinical strains exposed to concentrations of Prontosan® below, equal and under the MIC of the strains. Circle, control without biocide; square, 0.25×MIC; triangle, 1×MIC; inverted triangle, 4×MIC; diamond, 8×MIC.
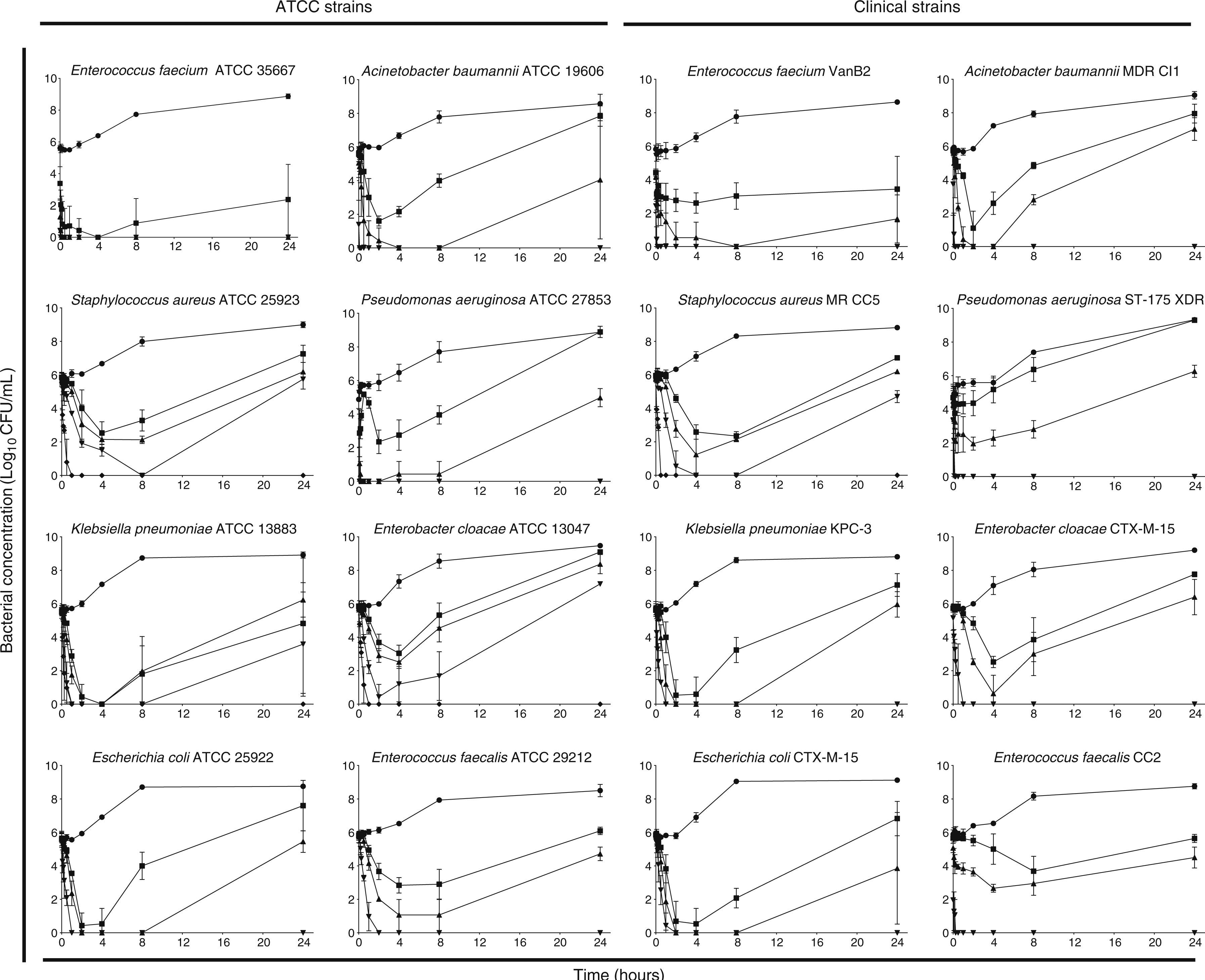
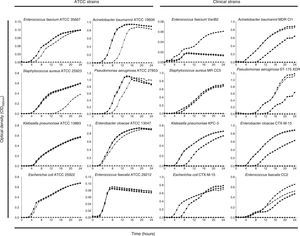
PHMB–B showed concentration-dependent bactericidal activity against the strains studied (Fig. 2). At low concentrations, all strains showed bacterial regrowth after 24h of incubation. Complete eradication of the bacterial inoculum (with no subsequent regrowth), however, was observed at variable concentrations of PHMB–B (ATCC strains: 1×MIC for E. faecium, 4×MIC for E. coli, A. baumannii, P. aeruginosa, and E. faecalis, and 8×MIC for S. aureus, K. pneumoniae, and E. cloacae; clinical isolates: 4×MIC for all isolates except for S. aureus, which was at 8×MIC).
Time-kill curves of ATCC and clinical strains exposed to different Prontosan® concentrations.
Time-kill curves of ATCC and clinical strains exposed to concentrations of Prontosan® below, equal and under the MIC of the strains. Circle, control without biocide; square, 0.25×MIC; triangle, 1×MIC; inverted triangle, 4×MIC; diamond, 8×MIC.
In strains where both bactericidal activity with PHMB–B and regrowth after 24h were observed, the MICs of colonies determined before and after exposure to PHMB–B were similar. Colonies with persistent reduced susceptibility to PHMB–B were not therefore detected.
Reduced in vitro susceptibilityIn the serial passage experiment on plates with increasing concentrations of PHMB–B, we observed that all strains grew on plates at concentrations that were higher than their MICs (from 3 to 7 dilutions higher, Table 3), although the MICs of strains recovered at these concentrations remained equal to the MICs before exposure to PHMB–B.
Growth on plates with increasing concentrations of Prontosan®.
| Strain | Prontosan® MIC (mg/L) | Highest growth concentration (mg/L) | Prontosan® MIC after exposure (mg/L) |
|---|---|---|---|
| P. aeruginosa | 4 | 256 | 4 |
| ATCC 27853 | |||
| A. baumannii | 4 | 64 | 2 |
| ATCC 19606 | |||
| E. faecium | 2 | 8 | 1 |
| ATCC 35667 | |||
| E. coli | 1 | 8 | 1 |
| ATCC 25922 | |||
| E. faecalis | 1 | 16 | 1 |
| ATCC 29212 | |||
| E. cloacae | 0.5 | 64 | 0.5 |
| ATCC 13047 | |||
| S. aureus | 0.5 | 8 | 0.5 |
| ATCC 25923 | |||
| K. pneumoniae | 0.5 | 8 | 0.5 |
| ATCC 13883 | |||
| P. aeruginosa | 8 | 256 | 4 |
| ST-175 XDR | |||
| A. baumannii | 4 | 64 | 2 |
| MDR CI1 | |||
| E. cloacae | 2 | 32 | 1 |
| CTX-M-15 | |||
| E. coli | 2 | 8 | 1 |
| CTX-M-15 | |||
| K. pneumoniae | 2 | 32 | 2 |
| ST-512 KPC-3 | |||
| E. faecalis | 2 | 32 | 2 |
| CC2 | |||
| S. aureus | 1 | 8 | 2 |
| MR CC5 | |||
| E. faecium | 2 | 8 | 2 |
| VanB2 |
This study showed the in vitro activity of a commercial polyhexanide–betaine wound cleansing solution (Prontosan®) against standard and MDR clinical isolates, including those belonging to successful international high-risk clones. There is no clear consensus regarding when and how a wound should be treated, and experts continue debating whether antimicrobial agents should be used for treatment, which agents should be used, or whether they should be administered topically or systemically. Topical antimicrobial agents include both antiseptics and antibiotics, which increases the options available, so posing an added dilemma for clinicians.4 Some of these commercially available options include PHMB and betaine solutions, although there is hardly any literature about their activity and the few data available do not include tests against difficult-to-treat MDR clinical isolates, despite the fact that several studies have recommended PHMB,7 and they are extensively used for cleansing wounds.
With respect to the MIC results, we obtained concentrations against ATCC strains of 0.5–4mg/L, similar to those obtained by Koburger et al.,8 who found that polyhexanide MICs were lower than or equal (0.5–4mg/L) to the MICs of other antiseptics (triclosan, PVP–iodine, octenidine dihydrochloride, and chlorhexidine digluconate). MIC results against MDR clinical isolates belonging to successful high-risk clones were similar to those obtained with standard strains (0.5–8mg/L), pointing to the excellent antimicrobial activity of the solution regardless of the resistance profile or clonality of the isolates. This is also corroborated by Fabry et al., with MICs of PHMB from 1 to 32mg/L against Gram-negative clinical isolates,6 and from 0.5 to 2mg/L against MSSA and MRSA.5 The MICs obtained in our study against P. aeruginosa (8mg/L) and MRSA (1mg/L) were lower than those of Fabry et al. (32 and 2mg/L, respectively), which could partly be explained by the added antimicrobial activity of the betaine. Furthermore, the MBCs and MICs were similar, indicating that PHMB–B exhibits high bactericidal activity even against MDR isolates belonging to high-risk clones.
Growth curves and time-kill curve results supported the high bactericidal activity of PHMB–B against reference strains and MDR clinical isolates, as inhibition of growth in the first experiments and bactericidal activity (complete reduction of the initial inoculum) in the second were reached at concentrations of up to 32mg/L. These concentrations should easily be reached at the site of infection, since the commercial product contains concentrations of 1000mg/L of both polyhexanide and betaine. It is important to note that at several concentrations, regrowth was observed in the time-kill curves, although at concentrations that were lower than for complete bacterial eradication. We hypothesize that this is due to the loss of activity of PHMB–B, not to the development of tolerance or resistance to the product. This hypothesis is supported by the observation that the MICs of PHMB–B determined before and after exposure to the product were the same, independently of the strain tested. In favor of this hypothesis are the results obtained on plates with increasing concentrations of PHMB–B, where colonies recovered from plates with concentrations above MIC presented a similar MIC (±1log2) to those before exposure, suggesting a transitional tolerance to the disinfectant but not resistance development. At any rate, the concentrations at which strains grew (up to 256mg/L) were less than commercial ones (1000mg/L), so that this tolerance should be difficult to observe in clinical practice, which is in accordance with the literature.15
In the dilution–neutralization test, undiluted product was tested at inoculum densities of around 6logCFU/mL. For all the strains studied, the entire inoculum was removed (≥4logCFU/mL reduction) after 1min of contact, until the detection limits of the assay (<2logCFU/mL), presenting bactericidal activity under the conditions described in the European Standard PrEN 12054.14 These results suggest faster bactericidal activity (1min) than the length of time of application recommended by the manufacturer (15min), independently of the species, resistance profile or high-risk clonality of the strain, which allowed us perform further in vivo experiments to elucidate the potential use of Prontosan®. Our results are in concordance with those showed by Koburger et al., who tested PHMB against P. aeruginosa and S. aureus.8 In that study, the authors compared the activity of several antiseptics and concluded that PHMB was the most efficacious agent when prolonged contact was feasible and that it appeared to be preferable for chronic wounds due to its higher tolerability.8
One drawback of our study might be that the experiments were carried out in accordance with standard methods but without interfering substances such as proteins or organic material that might have hindered the antimicrobial activity of the compounds, and so did not mimic clinical conditions exactly. The role of interfering material is not clear, since some authors have found reduced antimicrobial activity of disinfectants (PHMB included) in the presence of organic material (up to 3% bovine serum albumin),16,17 while others have demonstrated that bovine serum albumin did not influence bactericidal activity,6 and others that the antimicrobial activity of PHMB is reached at concentrations below cytotoxicity level in conditions similar to the composition of artificial wound fluid (10% fetal bovine serum),9 presenting an index of biocompatibility of >1. The good in vitro index of biocompatibility9 shown by PHMB is reflected in the high safety profile of the disinfectant, with low toxicity against cellular cultures and good tolerability in human beings being demonstrated in several studies.11,12,18 Although some allergic reactions have been recorded, and clinical cases of severe anaphylaxis have been mentioned, this disinfectant remains safer than other commercially available antiseptics.19
On the other hand, there are few in vitro studies available showing the activity of PHMB–B, and most cases have tested susceptible standard isolates5,6,8–10; hence, the strength of our work lies in the use of successful high-risk clones. KPC-producing K. pneumonia ST 258, ESBL-producing E. coli ST 131 and carbapenem-resistant P. aeruginosa ST178 are among the most prevalent and successful MDR clones causing both nosocomial and healthcare-associated infections.3 These difficult-to-treat pathogens have played a major role in the global dissemination of resistance and are a real problem in healthcare, so that the evaluation of agents with activity against them has become mandatory in order to reduce colonization and subsequent dissemination of these bacteria.
In conclusion, our results show that Prontosan® has high bactericidal activity against the studied successful multidrug-resistant pathogens at concentrations significantly lower than those commercially used. Furthermore, this bactericidal activity occurs rapidly (1min), within a much shorter period of time than that recommended by the manufacturer (15min). Finally, the in vitro results suggest that selection of strains with decreased susceptibility to Prontosan® should not be a problem in vivo.
FundingThis study was partially supported by a grant from B. Braun laboratories.
Conflicts of interestNone declared.
Ethical approvalNot required.
We would like to thank Drs. Pilar Villalón, Carmen Torres, Antonio Oliver, and Rafael Cantón for kindly providing us with some of the strains used in this study.