Spain, which has one of the largest migrant populations in Europe, has committed to eliminating the hepatitis C virus (HCV). The aim of this study was to estimate the prevalence of HCV among migrant groups in Spain, a country of 46 million people, with an estimated HCV-antibody prevalence of 1.7%.
MethodsStudies on HCV and migration in Spain were identified by systematically searching three databases from the first records to 30 November 2017, and consulting experts at the Ministry of Health and in the 17 Spanish autonomous communities. A meta-analysis was conducted to determine pooled HCV prevalence for the general migrant population. Prevalences were also calculated for high-risk migrant populations and populations who had undergone hospital screening, stratified by region of origin.
ResultsOut of 243 studies identified, 26 met the eligibility criteria. The meta-analysis of the general migrant population found HCV antibody prevalence to be 1.6%. Migrants originating from European countries, including those at high or moderate risk for HCV, had the highest pooled prevalence (7.1%). In the general migrant population, prevalence was highest among sub-Saharan African migrants (3.1%) and lowest among Latin American migrants (0.2%).
ConclusionBased on the limited available data, the prevalence among the general migrant population was found to be the same as the general Spanish population. Further research is needed to more accurately determine HCV prevalence for the overall migrant population and specific migrant subpopulations with a higher risk in the country as a whole and in each of Spain's 17 autonomous communities.
España, con una de las mayores poblaciones de inmigrantes en Europa se ha comprometido en la tarea de eliminar el virus de la hepatitis C (VHC). El objetivo de este estudio fue estimar la prevalencia del VHC entre los grupos de migrantes en España, un país de 46 millones de personas, con una prevalencia estimada de anticuerpos contra el VHC del 1,7%.
MétodosSe identificaron los estudios sobre el VHC y la migración en España mediante la búsqueda sistemática de 3 bases de datos desde los primeros registros hasta el 30 de noviembre de 2017. Se consultaron expertos del Ministerio de Salud y de las 17 comunidades autónomas españolas. Se realizó un metaanálisis para determinar la prevalencia combinada del VHC para la población migrante general. También se calcularon las prevalencias para poblaciones migrantes de alto riesgo, y para aquellas poblaciones con cribado realizado a nivel hospitalario, estratificadas por región de origen.
ResultadosDe 243 estudios identificados, 26 cumplieron con los criterios de elegibilidad. El metaanálisis de la población migrante general encontró que la prevalencia de anticuerpos contra el VHC era del 1,6%. Los migrantes provenientes de países europeos, incluidos aquellos con alto o mediano riesgo del VHC, tuvieron la mayor prevalencia combinada (7,1%). En la población migrante general, la prevalencia fue más alta entre los migrantes del África subsahariana (3,1%) y más baja entre los migrantes de América Latina (0,2%).
ConclusiónEn función de los datos disponibles que son limitados, el estudio muestra que la prevalencia entre la población general migrante en España es la misma que la de la población general española. Se necesitan más estudios para determinar con mayor precisión la prevalencia del VHC en la población general de migrantes y las subpoblaciones de población inmigrante con mayor riesgo específicas en el país en su conjunto y en las 17 comunidades autónomas de España.
The International Organization for Migration defines a migrant as “any person who is moving or has moved across an international border or within a State away from his/her habitual place of residence”.1 Migrants are a diverse population, and the mechanisms of migration are complex and multifaceted. Some migrants are drawn to the real or perceived benefits of a new country, while others are forced to flee their countries of origin because of oppressive economic, political or social situations.2,3
As of 1 January 2016, there were 35.1 million voluntary migrants and forcibly displaced persons living in the European Union.4,5 With 13% of its population foreign-born,6 Spain has the largest migrant population in Europe's Mediterranean region. More than half of all migrants in Spain originate from six countries: Romania, Morocco, the United Kingdom, Italy, Ecuador and China, respectively.7
In 2017, there were an estimated 71 million people chronically infected with hepatitis C virus (HCV) worldwide.8,9 However, data on HCV prevalence in migrants remain scarce. Some countries have reported relatively high HCV rates among their migrant populations.10 The surge of migrants to Europe in 2015 raised concerns about their HCV status, given recent global commitments to combat and eliminate the disease.11,12
The WHO Regional Office for Europe has encouraged Member States to prioritise access to health services for refugees and migrants.13 However, a lack of HCV prevalence data in the region limits the ability of health systems to monitor the disease burden and provide quality services to the most affected. National HCV surveillance systems have not been fully implemented in most of the region14 and data are particularly lacking for migrant populations.11,15 Furthermore, many European countries have inadequate national systems for registering migrants. This compounds the challenge of determining the HCV disease burden in migrant populations.16
It has been estimated that 90,000 people infected HCV immigrated to Spain between 1993 and 2008, based on an HCV serological test.17 Although there has been research involving this population,17–19 some of the studies did not specifically target migrants20 or were only carried out in high-risk populations.21
With the advent of direct-acting antivirals in 2013, hepatitis C became readily curable.22 And in 2016, the World Health Organization (WHO) released its first Global health sector strategy on hepatitis 2016–2021, which calls for the elimination of viral hepatitis as a public health threat. In order to reach the strategy's targets, it is crucial to identify which groups are at higher risk for the infection. The further scale-up of HCV testing in these groups can accelerate efforts to link more people to care and treatment, consequently reducing the burden of HCV disease.
In 2015, Spain approved an ambitious national hepatitis C strategic plan that recognises the public health importance of caring and treating migrant groups.23 The aim of this review and meta-analysis is to estimate the prevalence of HCV-antibodies among the migrant population in Spain, thereby contributing to informed decision-making around Spanish efforts to achieve HCV elimination.
MethodsThe methodology was structured following the Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE) guidelines.24
Three bibliographic databases were systematically searched for studies on HCV and migration in Spain: MEDLINE (via Ovid), Embase (via Ovid) and CINAHL (via EBSCOhost). Each was searched from inception to 30 November 2017. Searches were conducted by combining the following three groups of terms: (1) hepatitis C, hepacivirus, hep C, HCV; (2) migration, migrants, transients, emigrants, migrants, refugees, displaced persons; and (3) Spain, Spanish (Annex 1, supplementary material). The search string was compared to a longer one that included the names of all autonomous communities of Spain and was found to yield the same results. Authors of recently published abstracts and manuscripts in press were contacted to retrieve full articles.
This search was supplemented by reviewing online sources from the Spanish Ministry of Health and regional health agencies/ministries in each of the 17 autonomous communities of Spain. Finally, national public health officers and the 17 regional health agencies/ministries were contacted twice (December 2016 and January 2017) to obtain additional documents reporting HCV prevalence in migrants.
Results were limited to include only findings involving humans. No restriction was applied to study design or language of publication. After removal of duplicates, the abstracts of the remaining records were screened to identify potentially relevant studies. Those studies then were manually screened to identify studies for inclusion in the final analysis.
Studies were eligible for inclusion if they met all of the following five criteria: (1) they reported the prevalence of HCV, defined as the presence of HCV antibodies; (2) they focused on Spain or at least one of its autonomous communities; (3) they reported on migrant populations; (4) they were primary sources of quantitative data; and (5) they were published after 1 June 2004. (Commentaries, editorials, letters and narrative reviews were excluded.)
All studies were assessed for quality using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies from the United States National Institutes for Health.25 The following data elements were extracted by two reviewers from the final set of studies: bibliographic details, Spanish autonomous community where the study took place, study year, methodology, target population, median age of study participants, serological test used to determine HCV status, HCV antibody prevalence, sample size of study population and continent of origin of study participants. When continent-of-origin data were not available for everyone tested in a study, the prevalence data were designated “continent of origin not reported”. Disagreements between the two reviewers were resolved through discussion or, when necessary, consultation with a third author.
Data were extracted and HCV antibody prevalence was stratified, when possible, by continent of origin, the autonomous community where the study was conducted and risk level of the study population. Risk level was defined using the following criteria: study participants tested in the community, primary care settings, maternity wards or blood banks were categorised as “general migrant population”. Those tested in hospitals or tropical medicine clinics were categorised as “hospital-based studies” and participants in studies that focused specifically on people who inject drugs, people living with HIV, sex workers and prisoners were categorised as “high-risk”.
Hospital-based studies and studies undertaken in high-risk populations were not excluded during data extraction. However, the pooled prevalence was calculated using only data from studies of general migrant populations. HCV prevalence in high-risk groups was reported in order to make comparisons to high-risk groups among the non-migrant population.
To conduct the meta-analysis, the Freeman-Turkey double arcsine transformation was used to calculate pooled-prevalence estimates, with corresponding 95% confidence intervals (CI).26 A DerSimonian and Laird random-effects model was utilised and a Cochran's Q statistic was adopted to estimate heterogeneity between studies.27,28 The I2 index was also reported, indicating the variation between studies attributed to heterogeneity rather than chance. Where heterogeneity was found not to be statistically significant, pooled prevalence estimates were calculated based on inverse variance from a fixed-effect model. Point prevalence and 95% CI from individual studies as well as pooled prevalence estimates and 95% CI for all strata are presented in the forest plot. Two-tailed tests were used for all analyses. Stata 15 was used for all analyses.29
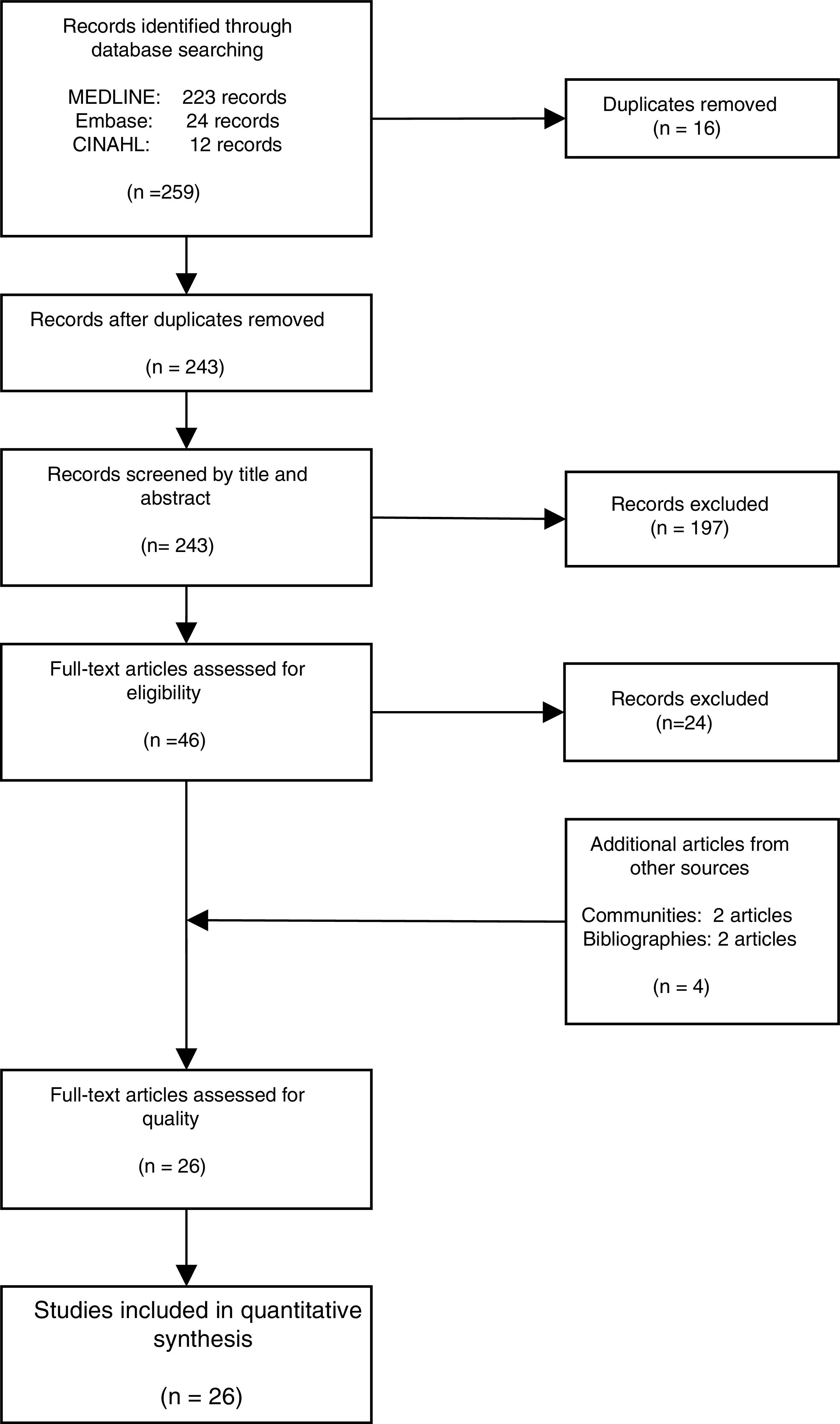
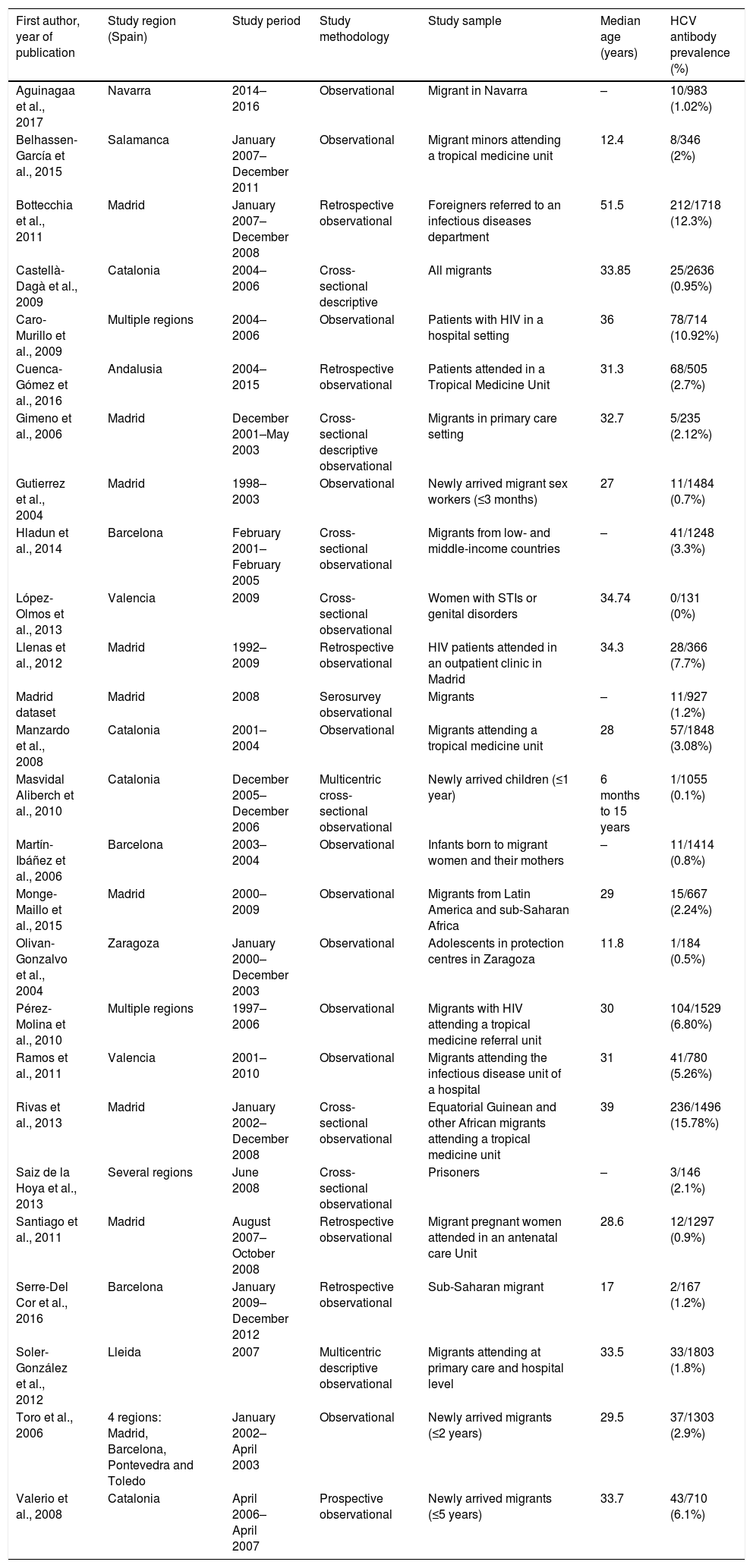
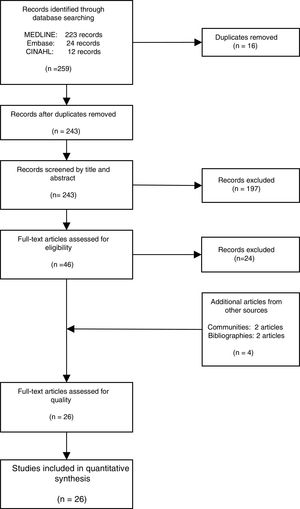
ResultsThe literature search yielded 243 potential articles after duplicates were removed. After screening by title and abstract, 46 articles remained (Fig. 1). An additional 24 articles were removed following full-article screening, resulting in 22 records. The main reasons for exclusion were case reports or case series, studies not reporting data on HCV, studies not reporting prevalence data, data not reported from Spain or data not reported on migrant population. While experts representing all 17 autonomous communities were contacted to obtain HCV prevalence data among the study population, only Madrid and Navarra were able to provide data appropriate for the analysis (one dataset and one article, respectively). The authors identified two additional studies through reviewing the references of articles included from the original search string, yielding a total of 26 data sources – 25 articles and one dataset (Table 1).18,30–53
Characteristics of included studies (n=26).
| First author, year of publication | Study region (Spain) | Study period | Study methodology | Study sample | Median age (years) | HCV antibody prevalence (%) |
|---|---|---|---|---|---|---|
| Aguinagaa et al., 2017 | Navarra | 2014–2016 | Observational | Migrant in Navarra | – | 10/983 (1.02%) |
| Belhassen-García et al., 2015 | Salamanca | January 2007–December 2011 | Observational | Migrant minors attending a tropical medicine unit | 12.4 | 8/346 (2%) |
| Bottecchia et al., 2011 | Madrid | January 2007–December 2008 | Retrospective observational | Foreigners referred to an infectious diseases department | 51.5 | 212/1718 (12.3%) |
| Castellà-Dagà et al., 2009 | Catalonia | 2004–2006 | Cross-sectional descriptive | All migrants | 33.85 | 25/2636 (0.95%) |
| Caro-Murillo et al., 2009 | Multiple regions | 2004–2006 | Observational | Patients with HIV in a hospital setting | 36 | 78/714 (10.92%) |
| Cuenca-Gómez et al., 2016 | Andalusia | 2004–2015 | Retrospective observational | Patients attended in a Tropical Medicine Unit | 31.3 | 68/505 (2.7%) |
| Gimeno et al., 2006 | Madrid | December 2001–May 2003 | Cross-sectional descriptive observational | Migrants in primary care setting | 32.7 | 5/235 (2.12%) |
| Gutierrez et al., 2004 | Madrid | 1998–2003 | Observational | Newly arrived migrant sex workers (≤3 months) | 27 | 11/1484 (0.7%) |
| Hladun et al., 2014 | Barcelona | February 2001–February 2005 | Cross-sectional observational | Migrants from low- and middle-income countries | – | 41/1248 (3.3%) |
| López-Olmos et al., 2013 | Valencia | 2009 | Cross-sectional observational | Women with STIs or genital disorders | 34.74 | 0/131 (0%) |
| Llenas et al., 2012 | Madrid | 1992–2009 | Retrospective observational | HIV patients attended in an outpatient clinic in Madrid | 34.3 | 28/366 (7.7%) |
| Madrid dataset | Madrid | 2008 | Serosurvey observational | Migrants | – | 11/927 (1.2%) |
| Manzardo et al., 2008 | Catalonia | 2001–2004 | Observational | Migrants attending a tropical medicine unit | 28 | 57/1848 (3.08%) |
| Masvidal Aliberch et al., 2010 | Catalonia | December 2005–December 2006 | Multicentric cross-sectional observational | Newly arrived children (≤1 year) | 6 months to 15 years | 1/1055 (0.1%) |
| Martín-Ibáñez et al., 2006 | Barcelona | 2003–2004 | Observational | Infants born to migrant women and their mothers | – | 11/1414 (0.8%) |
| Monge-Maillo et al., 2015 | Madrid | 2000–2009 | Observational | Migrants from Latin America and sub-Saharan Africa | 29 | 15/667 (2.24%) |
| Olivan-Gonzalvo et al., 2004 | Zaragoza | January 2000–December 2003 | Observational | Adolescents in protection centres in Zaragoza | 11.8 | 1/184 (0.5%) |
| Pérez-Molina et al., 2010 | Multiple regions | 1997–2006 | Observational | Migrants with HIV attending a tropical medicine referral unit | 30 | 104/1529 (6.80%) |
| Ramos et al., 2011 | Valencia | 2001–2010 | Observational | Migrants attending the infectious disease unit of a hospital | 31 | 41/780 (5.26%) |
| Rivas et al., 2013 | Madrid | January 2002–December 2008 | Cross-sectional observational | Equatorial Guinean and other African migrants attending a tropical medicine unit | 39 | 236/1496 (15.78%) |
| Saiz de la Hoya et al., 2013 | Several regions | June 2008 | Cross-sectional observational | Prisoners | – | 3/146 (2.1%) |
| Santiago et al., 2011 | Madrid | August 2007–October 2008 | Retrospective observational | Migrant pregnant women attended in an antenatal care Unit | 28.6 | 12/1297 (0.9%) |
| Serre-Del Cor et al., 2016 | Barcelona | January 2009–December 2012 | Retrospective observational | Sub-Saharan migrant | 17 | 2/167 (1.2%) |
| Soler-González et al., 2012 | Lleida | 2007 | Multicentric descriptive observational | Migrants attending at primary care and hospital level | 33.5 | 33/1803 (1.8%) |
| Toro et al., 2006 | 4 regions: Madrid, Barcelona, Pontevedra and Toledo | January 2002–April 2003 | Observational | Newly arrived migrants (≤2 years) | 29.5 | 37/1303 (2.9%) |
| Valerio et al., 2008 | Catalonia | April 2006–April 2007 | Prospective observational | Newly arrived migrants (≤5 years) | 33.7 | 43/710 (6.1%) |
Eleven of the 26 studies were conducted on general migrant populations (primary care centres, maternity wards, community-level facilities, blood banks), 9 were in the hospital-based category and 6 were conducted in high-risk population groups.
The literature included data from 8 of the 17 Spanish autonomous communities (Andalusia, Aragon, Castille-La Mancha, Castille-Leon, Catalonia, Galicia, Madrid and Valencia). Fifteen of the 20 data sources based in specific communities were from Catalonia or Madrid, and the majority of HCV screenings were conducted in Catalonia (11,288), Madrid (8173) and Valencia (911).
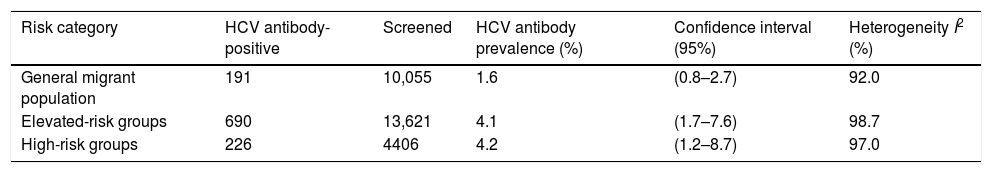
HCV prevalence among migrants in the general migrant population was 1.6% (95% CI: 0.8–2.7, I2=92.0%) and 4.1% (95% CI: 1.7–7.6, I2=98.7%) in the hospital-based category. The prevalence among high-risk populations was 4.2% (95% CI: 1.2–8.7; I2=97.0%) (Table 2).
Pooled HCV antibody prevalence, heterogeneity and variation measurements for migrants in Spain, by risk category.a
| Risk category | HCV antibody-positive | Screened | HCV antibody prevalence (%) | Confidence interval (95%) | Heterogeneity I2 (%) |
|---|---|---|---|---|---|
| General migrant population | 191 | 10,055 | 1.6 | (0.8–2.7) | 92.0 |
| Elevated-risk groups | 690 | 13,621 | 4.1 | (1.7–7.6) | 98.7 |
| High-risk groups | 226 | 4406 | 4.2 | (1.2–8.7) | 97.0 |
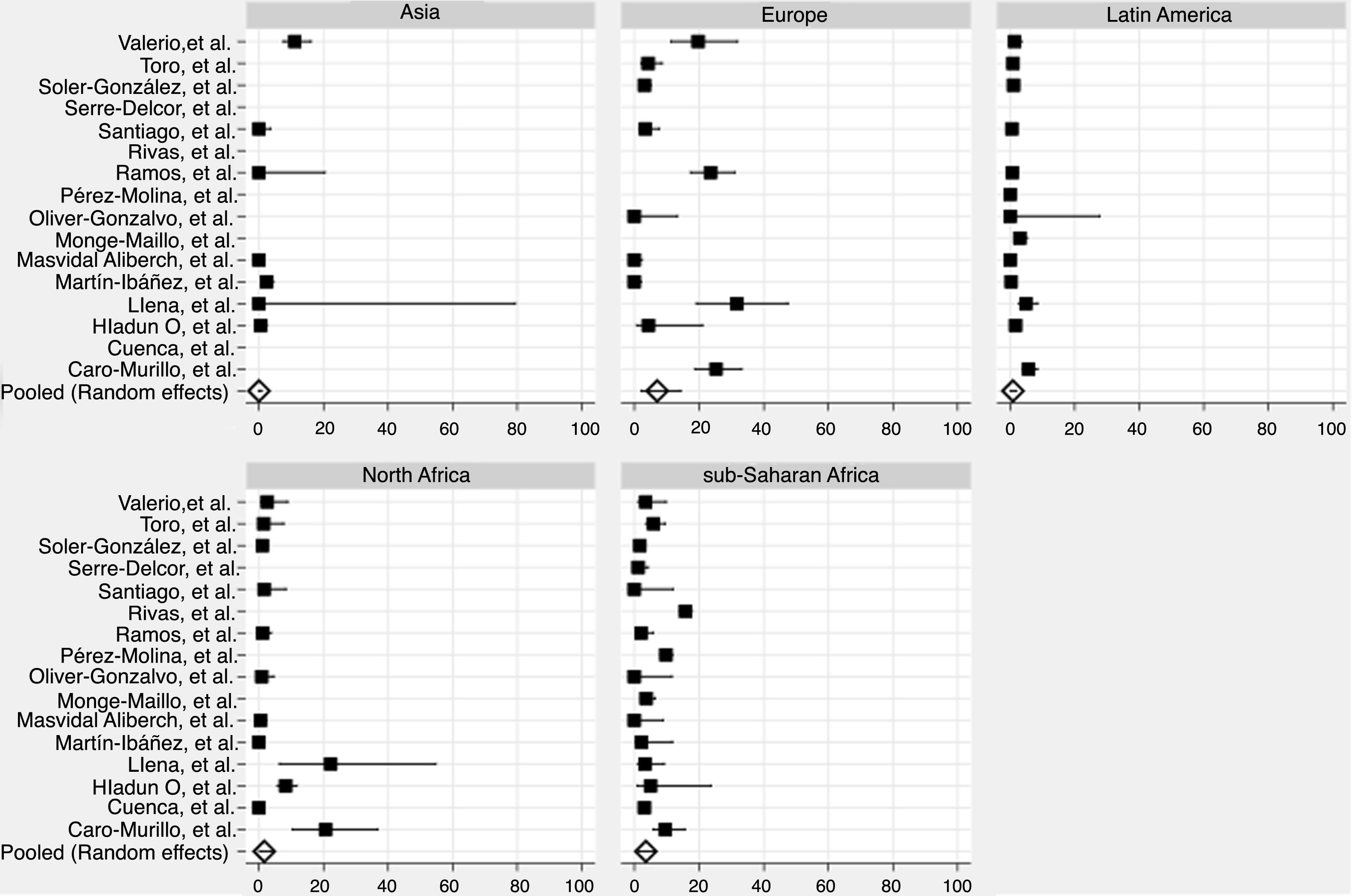
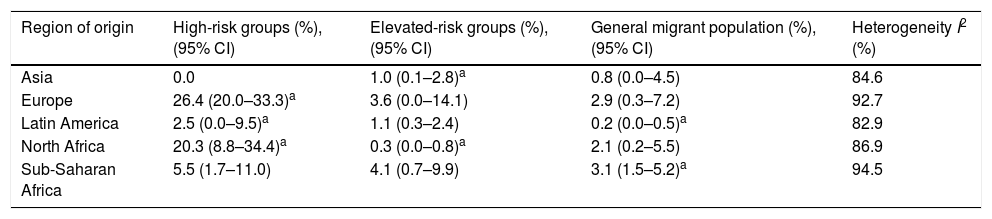
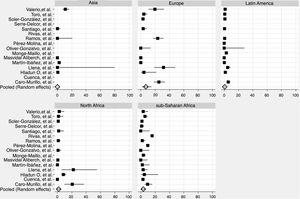
We derived prevalence for HCV infection in migrants originating from the following regions: Asia (0.0%; 95% CI: 0–0.8), Europe (7.1%; 95% CI: 2.2–14.2), Latin America (0.9%; 95% CI: 0.2–1.8), North Africa (1.6%; 95% CI: 0.2–4.0) and sub-Saharan Africa (3.6%; 95% CI: 1.5–6.4) (Fig. 2). Pooled prevalences are reported for all regions-of-origin data because of the high heterogeneity among studies.
In the high-risk group, European migrants had the highest prevalence (26.4%; 95% CI: 20.0–33.3). In the hospital-based group, sub-Saharan African (4.1%; 95% CI: 0.7–9.9) and European migrants (3.6%; 95% CI: 0.0–14.1) had the highest prevalences, respectively. The same pattern was observed in studies conducted among the general migrant population, where the highest prevalences were found among sub-Saharan African (3.1%; 95% CI: 1.5–5.2), European (2.9%; 95% CI: 0.3–7.2) and North African (2.1%; CI: 0.2–5.5) migrants (Table 3).
Pooled HCV antibody prevalence, heterogeneity and variation measures for migrants in Spain, by region of origin and risk category.
| Region of origin | High-risk groups (%), (95% CI) | Elevated-risk groups (%), (95% CI) | General migrant population (%), (95% CI) | Heterogeneity I2 (%) |
|---|---|---|---|---|
| Asia | 0.0 | 1.0 (0.1–2.8)a | 0.8 (0.0–4.5) | 84.6 |
| Europe | 26.4 (20.0–33.3)a | 3.6 (0.0–14.1) | 2.9 (0.3–7.2) | 92.7 |
| Latin America | 2.5 (0.0–9.5)a | 1.1 (0.3–2.4) | 0.2 (0.0–0.5)a | 82.9 |
| North Africa | 20.3 (8.8–34.4)a | 0.3 (0.0–0.8)a | 2.1 (0.2–5.5) | 86.9 |
| Sub-Saharan Africa | 5.5 (1.7–11.0) | 4.1 (0.7–9.9) | 3.1 (1.5–5.2)a | 94.5 |
This is the first meta-analysis of HCV prevalence in migrant populations in Spain. The findings indicate that 1.6% of migrants from the general migrant population living in Spain are HCV-antibody positive. In our study, the highest prevalence of HCV was found among migrants from countries in Europe (7.1%) and from sub-Saharan Africa (3.6%). The HCV antibody prevalence of the general migrant population was consistent with previous findings11,54 and was found to be same as the estimated prevalence of the general population in Spain.23,55 We report an approximate 1.6% anti-HCV prevalence for the general Spanish population on the basis of evidence published by the European Centre for Disease Control and Prevention23 and similar prevalences reported in Hope et al.’s 2014 article.55 However, the abstract of a yet-unpublished cross-sectional study conducted in Santander, Madrid and Valencia has reported the anti-HCV prevalence among the general Spanish population to be 1.1%, substantially lower than previous findings.56 Moreover, some studies conducted in subnational areas (e.g., Madrid20 and Navarra32) also observed lower prevalence rates among the native population than the foreign-born population. Due to conflicting evidence, it is difficult to accurately estimate HCV prevalence in the general population in Spain, and therefore difficult to determine whether all or some migrant populations are at heightened risk for HCV. We have only found one other systematic review estimating the prevalence of HCV in Spain in migrant populations. However, that study did not perform a meta-analysis, and could not estimate the HCV prevalence among migrants in Spain.19
One study observed that older native-born Spaniards are at greater risk for HCV infection than younger ones.57 Because migrants to Spain tend to be younger than the native population, further research investigating differences in HCV prevalence should take age into account.58 As has been suggested elsewhere, prevalence estimates should be ideally segregated by age group or birth cohort.59
We have chosen to regard the prevalence for the general migrant population as the overall pooled prevalence for all migrants in Spain. Hospital-based studies (e.g., those migrants tested in hospitals and tropical medicine clinics) as well as migrants from high-risk groups may overestimate the HCV prevalence in the studies we included. Hospitalised patients tend to have different characteristics compared with general population.
Our study found that the overall HCV antibody prevalence among migrants tested at hospital level (4.1%) was similar to prevalence in the high-risk migrant group (4.2%). Other studies of native-born high-risk groups in Spain have observed HCV prevalence rates as high as 60% to 80%60 – substantially greater than the high-risk group prevalence this study reports for migrants. However, most of those studies were undertaken in people who inject drugs, while none of the studies included in this review specifically sought to enrol migrants who inject drugs. Instead, the high-risk groups in these studies are sex workers,39 prisoners and people living with HIV.35,49 Studies conducted among sex workers and HIV-positive sex workers in Spain have found similar, though slightly higher, HCV prevalences. The HCV prevalence among the general prison population in Spain is also similar to the prevalence we found for high-risk migrant populations.61 The amount of available data on these groups is quite small, however, and the existing studies are outdated.61,62 Future Spanish studies should focus on groups at high risk for HCV, such as people who inject drugs, sex workers, people living with HIV and prisoners.
We found that migrants from certain regions were underrepresented in our pooled sample. Strikingly, migrants from Europe constituted only 5.6% of the sample, yet they comprise 35% of Spain's migrant population.7 Although it was not possible to determine the HCV prevalence for Eastern and Western European migrants separately in most risk categories, European migrants in our study had the highest HCV antibody prevalence of all migrant groups. That was especially true of high-risk groups, where Europeans co-infected with HIV had the highest prevalence (26.4%). This prevalence was calculated using only two studies with limited sample size,30,35 so its statistical power is limited. Moreover, high-risk groups might be overrepresented and this data might not be representative of the whole European migrant population in Spain. Unfortunately, we could not find data about HCV prevalence in those European countries where migrants most frequently come from.
A study by the European Centre for Disease Prevention and Control likewise found sub-Saharan African and Eastern Europeans migrants to have higher prevalences of HCV than any other migrant group in Spain.63 Finally, the aforementioned higher HCV prevalence among Eastern Europeans co-infected with HIV should be considered in the light of findings from the WHO Global Hepatitis Report, which presents evidence of higher past or present HCV infection among people living with HIV in Eastern Europe than those in any other region.9
Studies elsewhere in Europe have also found that European migrants are at risk for HCV.64 However, some European studies systematically exclude migrants originating from other European countries,65 making the true prevalence of HCV among European migrants more difficult to estimate accurately.
There are also few data on Asian migrants (4% of the sample). Therefore, the low prevalence obtained should be interpreted with caution.
Our study is limited by large gaps in the data on migrants including many older studies; additional research is needed, particularly at the subnational level. We found relevant studies for only eight of the autonomous communities in Spain, and we recognise that our results are not necessarily representative of the Spanish migrant population. The autonomous community of Galicia, for example, has a higher proportion of Brazilian migrants than the national average,66 and this population appears to be underrepresented in our meta-analysis.
Anti-HCV testing does not differentiate between current and past HCV infections, and studies using data from anti-HCV tests therefore overestimate the prevalence of chronic HCV.67 This review only used data from anti-HCV tests, because only three of the included studies reported HCV RNA results along with HCV antibody prevalence.18,33,52 As HCV can be cured by antiviral treatment or the body's own immune response, our sample likely greatly overestimated the prevalence of extant HCV disease among migrants in Spain. This overestimation is another limitation of this study and future studies should include RNA testing results, if possible.
Eleven of our 26 data sources were not disaggregated by region of origin, and those that were generally were not further separated by country of origin. This prevented us from analysing country of origin as a risk factor for HCV, which is particularly important in the Asian region due to large difference of prevalence across countries. Furthermore, pooled estimates do not capture the heterogeneity that exists within regions. Migrants from different countries in the same region can have large variation in HCV prevalence, as can national populations within a region.68
All these limitations are due to the limited data available, and therefore results should be interpreted with caution.
The lack of high-quality data on migrants and health remains a widespread challenge globally as well. At the 69th World Health Assembly in 2016, the WHO director general characterised the need for better data on the health needs of migrants, refugees and displaced persons as “urgent”. Effective priority-setting to achieve the 2030 Sustainable Development Goals will require countries to have adequate health information systems with robust epidemiological data on migration.69 Currently, health information systems at the Spanish subnational level lack the capacity to analyse data based on migration status and/or country of origin. Incongruent migration categorisations, the use of different variables as proxies for migration status (such as nationality, ethnicity and origin), and a frequent lack of data on country of birth all make it extremely difficult to compare migrant health data across different communities. In its European action plan on hepatitis, WHO calls for the linking and integration of viral hepatitis strategic information systems with broader health information systems, and suggests that countries expand the cross-border sharing of information to ensure service continuity for refugees, migrants and other mobile populations.70
One important recommendation suggested by our findings concerns HCV testing. As some migrant populations are at higher risk for HCV, testing is a crucial step in linking them to early treatment and care.17,23 In 2014, WHO recommended that HCV testing be offered to any high-risk group71 and two years later the European Association for the Study of the Liver recommended that all at-risk populations, as determined by local epidemiology, be screened for HCV.24 Although screening migrants for hepatitis has been demonstrated to be a cost-effective strategy for controlling the hepatitis B virus (HBV) in some countries,72 Spain does not have any national HCV screening recommendations for migrants according to the national HCV strategic plan.23 At the subnational level, some autonomous communities such as the Canary Islands and Galicia recommend HCV screening for migrants originating from high-prevalence countries,73,74 while others do not.75–79 WHO further recommends that where migrants may have difficulties accessing sexual and reproductive health services, the prevention of the sexual transmission of viral hepatitis can be better ensured through dedicated services for this population,70 and this is another opportunity to provide testing services.
ConclusionsThis systematic review found that the estimated HCV antibody prevalence among migrants in Spain is similar to the prevalence among the native-born population. When segregated by region of origin, HCV prevalence among migrants of European and African origins was higher than the general population. Data were scarce and highly heterogeneous and therefore the results should be interpreted with caution. In spite of this, our meta-analysis provides HCV prevalence estimates that can be used to better understand the burden of HCV in Spain and support the scaling up of HCV testing, linkage to care and treatment in line with the WHO viral hepatitis strategy, which Spain has adopted. Providing Spanish policy-makers and health authorities with better data on HCV prevalence among migrants, including region and country of origin, would help them design effective, targeted interventions in their efforts to eliminate the disease, as set out in the Spanish hepatitis C strategy.
Availability of data and materialThe datasets used and analysed during the current study are available from the corresponding author on reasonable request.
FundingThis study was partially funded by Gilead Sciences with a grant to ISGlobal, Hospital Clínic, University of Barcelona, Spain. The team is also supported by the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) (2017 SGR 924) and by the Tropical Disease Cooperative Research Network (RICET) (RD12/0018/0010). ISGlobal is a member of the Centres de Reserca de Catalunya (CERCA) Programme, Government of Catalonia (Spain).
Authors’ contributionJVL conceptualised, designed and coordinated the study. DJB drafted the manuscript and prepared the final manuscript, with input from ARM, JVL, JDA, JGS and JLC. ON designed the search strings and conducted the database search. DJB and ARM performed the systematic review and data extraction. AC performed meta-analysis. All authors read and approved the final manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge Kristina Louise Hetherington and Kelly Safreed-Harmon for their copy-editing.