Listeria monocytogenes is an important cause of meningoencephalitis associated with high mortality. The treatment of choice for listeriosis is ampicillin alone or in combination with gentamicin or penicillin G. However, only low ampicillin concentrations are recorded in the central nervous system (CNS). In this study, we analysed the effect of subinhibitory concentrations of ampicillin on the morphology, growth and survival of L. monocytogenes. The non-inhibitory concentration (NIC), the minimum inhibitory concentration (MIC) and the MIC/NIC ratio were determined. Gram and Live/Dead staining showed aggregates of L. monocytogenes cells when grown at subinhibitory concentrations of ampicillin, with >90% of viable cells. The L. monocytogenes strains tested showed an intermediate heteroresistance to ampicillin, characterised by a MIC/NIC ratio of 4. Our results seem to indicate that both intermediate heteroresistance and the formation of aggregates could play a role in the clinical failure of ampicillin in the treatment of CNS infections caused by L. monocytogenes. However, more studies are necessary to elucidate this question.
Listeria monocytogenes es una importante causa de meningoencefalitis asociada con una elevada mortalidad. El tratamiento de elección para la listeriosis es ampicilina asociada o no a gentamicina o penicilina G. Sin embargo, en el sistema nervioso central (SNC) solo se alcanzan concentraciones bajas de ampicilina. En este estudio, analizamos el efecto de las concentraciones subinhibitorias de ampicilina sobre la morfología, el crecimiento y la supervivencia de L. monocytogenes. Para ello determinamos la concentración no inhibitoria (CNI), la concentración mínima inhibitoria (CMI) y el cociente CMI/CNI. Además, la tinción de Gram y la de vivos y muertos mostraron agregados de L. monocytogenes cuando crece a concentraciones subinhibitorias de ampicilina, con >90% de las células viables. Las cepas de L. monocytogenes testadas mostraron heterorresistencia intermedia a la ampicilina, caracterizada por un cociente CMI/CNI de 4. Nuestros resultados parecen indicar que tanto la formación de agregados como la heterorresistencia intermedia podrían jugar un papel en el fracaso terapéutico observado en las infecciones del SNC causadas por L. monocytogenes. Sin embargo, se necesitan más estudios para aclarar esta cuestión.
Listeriosis, whose etiologic agent is Listeria monocytogenes, is an important cause of bacteraemia and meningoencephalitis in some high-risk populations such as elderly, immunosuppressed and other individuals with altered cellular immunity.1 The current treatment of listeriosis consists of a combination of high doses of ampicillin with or without intravenous gentamicin,1 or penicillin G.2
Although the treatment is established, listeriosis has a mortality rate of 30% when the Central Nervous System (CNS) is involved.3 This high mortality rate may be due to the relatively low ampicillin concentrations that are reached in the CNS. The penetration of ampicillin in cerebrospinal fluid (CSF) is very variable, reaching extracellular concentrations ranging from 0.42 to 1.2mg/L and from 1.85 to 3.4mg/L under dosage of 200mg/kg and 400mg/kg, respectively.4 Furthermore, the penetration of ampicillin in CNS is really affected by the degree of inflammation of the meninges.5 Thus, it could be considered that the ampicillin concentration along with the dosage interval may reaches sub-MIC values. The objective of this work was to study the effect of sub-MIC concentrations of ampicillin on the morphology, growth and survival of L. monocytogenes.
MethodsBacterial strainsA search of the CNS infections by L. monocytogenes in the period 2003–2018, at the University Hospital Virgen del Rocío (Seville, Spain), was performed. Thirty-eight cases were identified, of which a 70% correspond to the serotype 4b and a 30% to the serotype 1/2b. Based on these data, two clinical isolates of L. monocytogenes, belonging to the serotypes 1/2b and 4b were used as model strains to study the effect of the ampicillin sub-MIC concentrations on the bacterial morphology, growth and survival.
Susceptibility testingThe non-inhibitory concentration (NIC)6 is defined as the highest antibiotic concentration that does not affect bacterial growth at 24h of incubation. It was determined in triplicate by colony forming unit (CFU) counts in Tryptic Soy Broth (TSB) with two ampicillin log2 dilutions ranging from 0.016 to 8mg/L in the first assay, and from 0.02 to 6mg/L in a second assay, to obtain a more precise growth curves. The minimal inhibitory concentration (MIC) of ampicillin was determined in triplicate by naked eye reading of bacterial growth inhibition in TSB at 24h of incubation at 37°C. Furthermore, the MIC/NIC ratio,7 which indicates the heteroresistance level of the population, was calculated. A MIC/NIC ratio>8 defines the population as heteroresistant, a MIC/NIC value between >2 and 8 as intermediate heteroresistant, and a MIC/NIC ratio ≤2 as homogeneous against an antibiotic. A bacterial inoculum adjusted to 0.5 McFarland was used in all determinations and Streptococcus pneumoniae ATCC 49619 as a quality control strain.
StainingAfter 24h a Gram's stain and a cell viability assay (LIVE/DEAD® Cell Viability Assays. Thermo Fisher Scientific, Waltham, MA, USA) were performed. The mean number of cells per aggregate was estimated by counting the mean number of cells per aggregate in 50 aggregates, at each ampicillin concentration.
Bacterial survivalIn order to prove whether these aggregates may be resolved in individual cells once the antibiotic is removed, a bacterial survival assay was performed. After 5hours of incubation at 37°C at 0.5xMIC of ampicillin, with the aim of promoting aggregates formation, 1ml of a bacterial suspension adjusted to a 0.5 McFarland was inoculated in 9ml of fresh TSB and incubated at 37°C with agitation. The original strain growing in medium without ampicillin was used as a control. At 0, 1, 2, 3 and 4h, an aliquot of 100μl from serial dilutions was plated, incubated at 37°C during 48h and then, CFU were counted. Simultaneously, a Gram stain was performed in order to see the morphological changes of L. monocytogenes. The number of duplications were calculated using the formula Nf=N02n, where N0 is the number of cells at the starting point, Nf is the number of cells after a period of incubation, and n is the number of cell divisions in the specified period of time.8
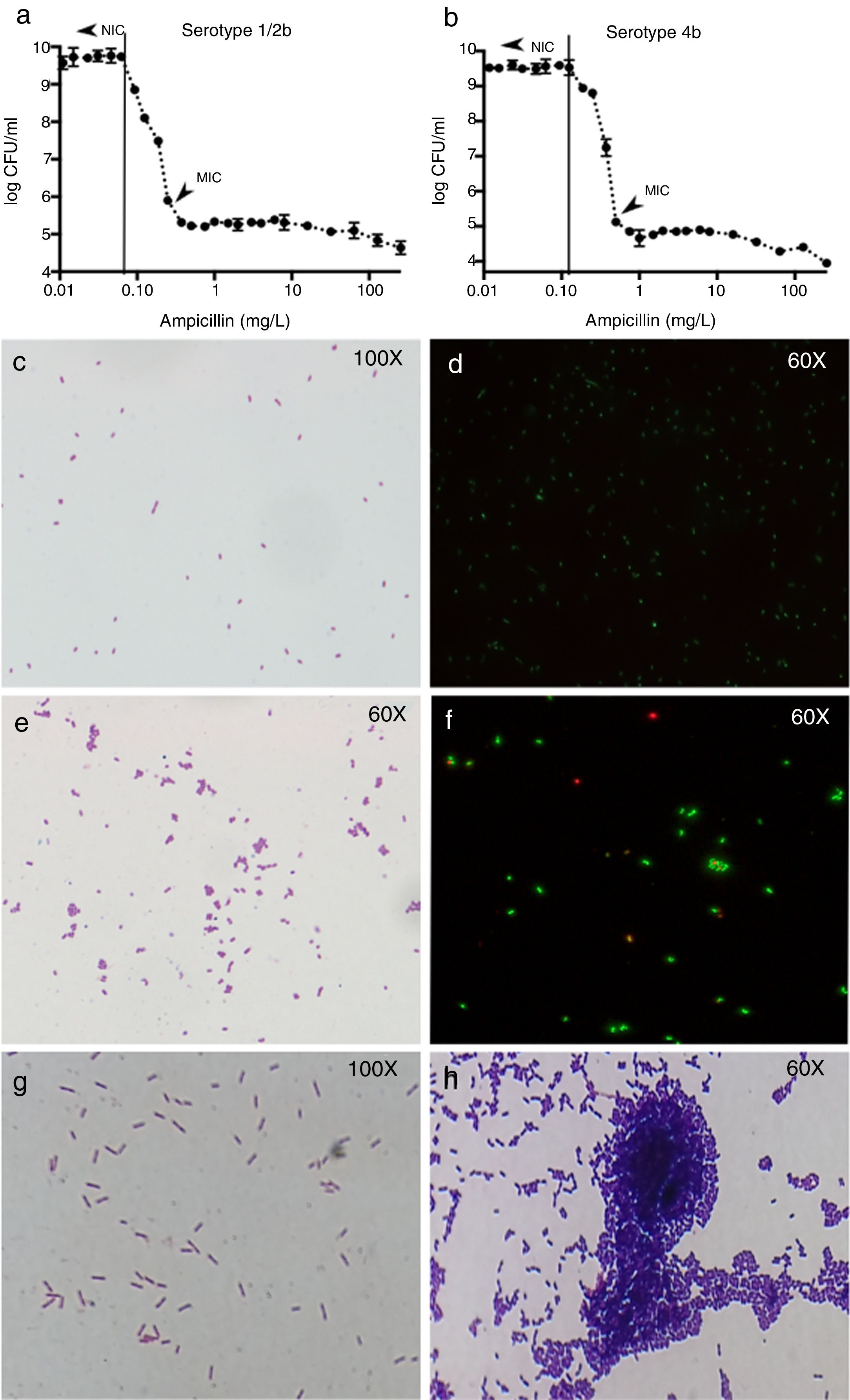
ResultsSusceptibility testingFor the 1/2b and 4b serotype strains, the ampicillin NIC were 0.0625mg/L and 0.125mg/L and the MIC 0.25mg/L and 0.5mg/L, respectively (Fig. 1a and b). The MIC/NIC ratio was 4 in both serotypes showing an intermediate heteroresistance for the two model strains suggesting that the susceptibility of Listeria populations to ampicillin is not homogeneous.
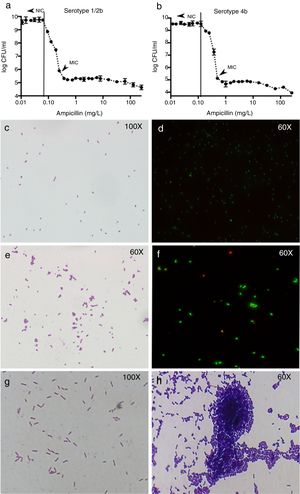
Results of colony counts, Gram and Live/Dead stains of L. monocytogenes. (a and b) Show the results of colony counts in serotype 1/2b and 4b, respectively. (c and d) Show L. monocytogenes growing without antibiotic. (e and f) Show L. monocytogenes serotype 4b growing at subinhibitory concentrations of ampicillin. (g and h) Show L. monocytogenes serotype 4b growth at 5h after the exposure to 0.5xMIC of ampicillin (1h) and control without treatment (1g).
At ampicillin concentrations at NIC or below, L. monocytogenes appeared as Gram-positive bacilli without any morphological change (Fig. 1c and d), but when the ampicillin pass the NIC, the cells appeared rounded and arranged in aggregates (Fig. 1e). The mean number of cells in the aggregates was from 3±1 to 7±2 cells when ampicillin concentration is closer to the MIC and higher in serotype 1/2b than 4b. The Live/Dead stain showed that around 90% of the cells from each aggregate were viable (Fig. 1f). However, once the MIC is reached, no morphologic changes were found in either of the two serotypes (not shown).
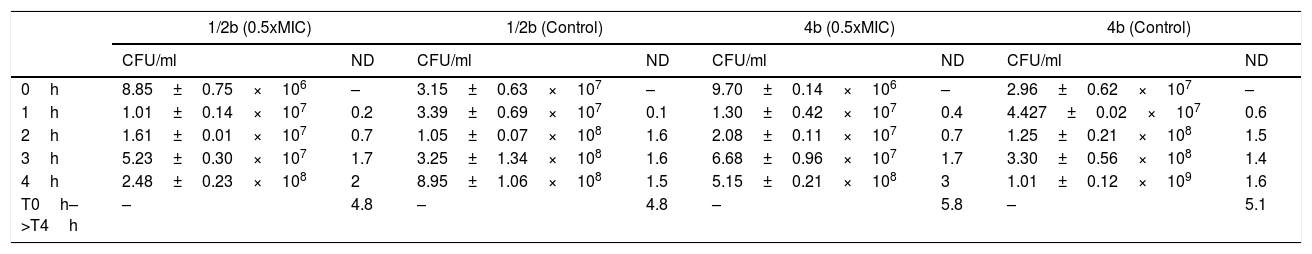
Bacterial survivalThe number of duplications along the entire interval was 4.8 in both the exposed and control strains (without previous ampicillin exposure) for serotype 1/2b, and 5.8 and 5.1 duplications in the exposed and control strains for serotype 4b, respectively (Table 1). Regarding the duplications per hour (d/h), in the exposed strains the number of duplications were increased from 0.2 in the first hour to 2 d/h in the fourth hour, in the serotype 1/2b; and from 0.4 d/h in the first hour to 3 d/h in the fourth hour in the serotype 4b. However, in control strains the number of duplications in the first hour was 0.1 in serotype 1/2b and 0.6 d/h in serotype 4b, but remained constant from here to the fourth hour at approximately 1.5 d/h in both serotypes (Table 1). This indicates that, although the global number of duplications is similar in both groups, it exists an increment in the number of duplications per hour in the exposed strains; while in the control strains, the number of duplications remains constant at 1.5 d/h. This difference suggests that aggregates may be resolved in single cells.
Results of colony count (CFU/ml) and number of duplications (ND) of the two strains growing in antibiotic-free medium after the exposure at subinhibitory concentration of ampicillin and the control strain without previous antibiotic exposure.
| 1/2b (0.5xMIC) | 1/2b (Control) | 4b (0.5xMIC) | 4b (Control) | |||||
|---|---|---|---|---|---|---|---|---|
| CFU/ml | ND | CFU/ml | ND | CFU/ml | ND | CFU/ml | ND | |
| 0h | 8.85±0.75×106 | – | 3.15±0.63×107 | – | 9.70±0.14×106 | – | 2.96±0.62×107 | – |
| 1h | 1.01±0.14×107 | 0.2 | 3.39±0.69×107 | 0.1 | 1.30±0.42×107 | 0.4 | 4.427±0.02×107 | 0.6 |
| 2h | 1.61±0.01×107 | 0.7 | 1.05±0.07×108 | 1.6 | 2.08±0.11×107 | 0.7 | 1.25±0.21×108 | 1.5 |
| 3h | 5.23±0.30×107 | 1.7 | 3.25±1.34×108 | 1.6 | 6.68±0.96×107 | 1.7 | 3.30±0.56×108 | 1.4 |
| 4h | 2.48±0.23×108 | 2 | 8.95±1.06×108 | 1.5 | 5.15±0.21×108 | 3 | 1.01±0.12×109 | 1.6 |
| T0h–>T4h | – | 4.8 | – | 4.8 | – | 5.8 | – | 5.1 |
The Gram stain showed large differences between exposed and control strains of both serotypes. In the control strain, no morphological changes were found at any time point (Fig. 1g). However, in the exposed strain, despite antibiotic removal, Gram stain showed aggregates of L. monocytogenes cells, most of them containing more than 80 cells per aggregate after 4h of incubation in fresh medium (Fig. 1h).
A slight increase in the MIC values was found when the aggregates obtained after 5h at 0.5xMIC of ampicillin were used as inoculum. The MIC increased from 0.25mg/L to 0.5mg/L with serotype 1/2b, but it was not affected with the serotype 4b (0.5mg/L). The reason of the lack of change in the MIC value in serotype 4b should be due to the fact that the increase in the MIC did not reach the next log2 dilution from 0.5mg/L (1mg/ml). On the other hand, the larger increase observed in the serotype 1/2b strain MIC is correlated with the larger size of the aggregates formed.
DiscussionAntimicrobial activity usually does not produce an ‘all or none’ effect on a bacterial population, and the role of the antibiotic concentration on clinical outcomes is sometimes poorly understood. Here, we have studied the in vitro activity of sub-MIC ampicillin concentrations on two clinical isolates of L. monocytogenes.
Under sub-MIC concentrations, ampicillin, which acts as bacteriostatic drug against L. monocytogenes,9 induces the formation of cellular aggregates with an increasing number of cells per aggregate, affecting to the cell structure and growth but not to the bacterial viability. The explanation for these morphologic changes remains unclear, but it may be related with the interaction between ampicillin and PBPs. Krawczyk-Balska et al.10 have reported that the morphologic alterations in L. monocytogenes cells are due to the overexpression of PBP3. However, Guinane et al.11 have reported that lack of PBP5 induce irregular forms in L. monocytogenes similar those observed in this study. On the other hand, Travier et al.12 reported that ActA factor promotes L. monocytogenes aggregation. This bacterial aggregation leads to increase L. monocytogenes persistence in the intestine, prolong faecal shedding and consequently facilitates transmission.12 Therefore, the mechanism under these morphologic changes should be investigated in future works.
On the other hand, L. monocytogenes shows an intermediate heteroresistance to ampicillin, which may be linked with the aggregates formation.
In conclusion, the fact that concentrations of ampicillin in the CNS (at dosages of 2g/4h) do not reach the MIC during the entire treatment interval could lead to the bacterial aggregates formation in L. monocytogenes. This could explain, at least in part, the high rate of treatment failure in CNS infections caused by L. monocytogenes, especially relevant when the immune system is compromised. More studies are necessary to clarify this question.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.