The patient was a 24-week pregnant woman who came to the Accident and Emergency department due to lumbar pain and pressure in the vaginal area of 4–5 days' duration. She had three full-term deliveries and three miscarriages. The patient was afebrile.
Examination revealed non-specific leucorrhoea, negative bilateral fist percussion and one centimetre of dilation. Suspecting threatened preterm labour, an ultrasound scan showed an 8-mm cervix, amniotic sludge and cervical funnelling. In addition, continuous cardiotocography (CTG) showed uterine irritability.
After two hours of observation, a new ultrasound scan was performed, showing a shortening of the cervix (6&#¿;mm), so it was decided to start treatment with atosiban, corticosteroids for lung maturation and magnesium sulphate for neurological development. At three hours, the patient reported discharge of foul-smelling fluid and pressure in the vaginal area. Examination revealed a stained and foul-smelling discharge of fluid. Laboratory tests showed leukocytosis with a predominance of neutrophils. Suspecting chorioamnionitis, antibiotic therapy with intravenous ampicillin (2&#¿;g/6&#¿;h) and intravenous gentamicin (240&#¿;mg/24&#¿;h) was started. A fever spike of 38.1&#¿;°C was recorded two hours after her waters broke.
Clinical courseA baby girl with an Apgar score of 1/1 was delivered, with a bad smell at birth. Samples of placenta, vaginal and endocervical exudates, urine culture from the mother and blood cultures from both were taken and sent to the Microbiology Department. The girl was started on vancomycin (10&#¿;mg/kg/12&#¿;h) and ceftazidime (50&#¿;mg/kg/12&#¿;h).
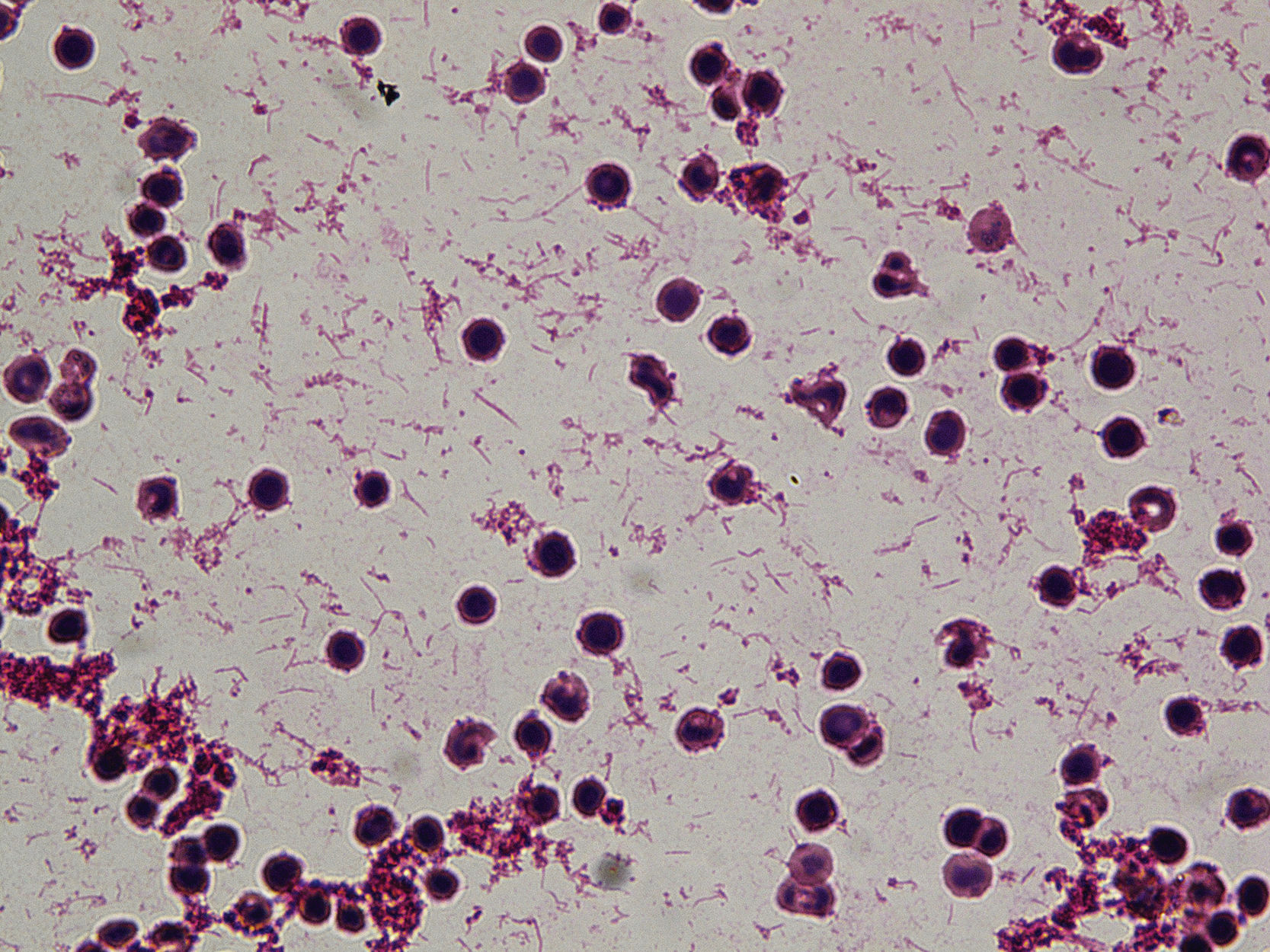
The placenta was cultured on blood agar and chocolate agar incubated at 35&#¿;°C in a 5% CO2 atmosphere and on anaerobic media incubated at 35&#¿;°C in an anaerobic atmosphere. After 48&#¿;h of incubation, both the placenta culture and the girl's blood culture showed growth in pure culture of spindle-shaped, pleomorphic Gram-negative bacilli forming yellow-pink, flat, rough colonies with a halo surrounding the central zone in blood agar (Figs. 1 and 2), which were identified as Capnocytophaga sputigena by MALDI-TOF (Maldi Biotyper, Bruker Daltonics, Germany; BDALv.10) with scores of 2.59 and 2.48, respectively. Genus and species identification of both strains was confirmed by PCR and Sanger sequencing of the 16S rRNA gene. Placenta culture for anaerobic bacteria and all other cultures were negative.
The antibiotic sensitivity study was performed using Etest® (bioMérieux, Marcy-l'Étoile, France) on MHF medium (Mueller-Hinton agar with 5% horse blood and 20&#¿;mg/l of β-NAD, Biomérieux) with incubation at 35&#¿;°C with 5% CO2. The mean inhibitory concentrations (MICs) obtained for the different antimicrobials were: penicillin&#¿;=&#¿;16&#¿;mg/l; amoxicillin-clavulanate&#¿;=&#¿;0.25&#¿;mg/l (amoxicillin); piperacillin-tazobactam&#¿;=&#¿;0.064&#¿;mg/l (piperacillin); cefotaxime&#¿;=&#¿;0.38&#¿;mg/l; ceftazidime&#¿;=&#¿;0.064&#¿;mg/l; meropenem&#¿;=&#¿;0.012&#¿;mg/l and ciprofloxacin&#¿;=&#¿;0.016&#¿;mg/l.
The girl died at 21 days of age due to complications associated with her prematurity.
Closing remarksC. sputigena belongs to a genus that includes spindle-shaped, facultative anaerobic, slow-growing, nutritionally demanding Gram-negative bacilli. It grows on enriched media such as blood agar or chocolate agar and requires 5%–10% CO2 for growth, which gives the genus its name.1,2
It is part of the microbiota of the human oral cavity. It causes bloodstream infection in immunosuppressed patients, such as those undergoing haematopoietic stem cell transplantation, patients with diabetes mellitus or patients with gastric cancer.3 Cases of post-surgical intra-abdominal abscess,4 bacteraemia in neutropenic patients5 and sepsis in patients with severe aplastic anaemia6 have also been reported.
This species has occasionally been isolated from the female genital tract. Perinatal infection, sepsis, chorioamnionitis and preterm birth have been reported.1,7 Orogenital contact may be a route of entry of this bacterium to the uterus, as some literature reviews have found that in some cases of chorioamnionitis, Capnocytophaga spp. was isolated from the oropharynx of the partner with periodontal disease.8–10
In our case, the patient was questioned and denied having periodontal disease.
Antibiotic sensitivity is difficult to interpret, as neither EUCAST (European Committee on Antimicrobial Susceptibility Testing) nor CLSI (Clinical and Laboratory Standards Institute) have established cut-off points for this microorganism, and it is also a slow-growing bacterium. Most are sensitive to ampicillin, amoxicillin-clavulanic acid, piperacillin-tazobactam and cephalosporins.1
C. sputigena causes chorioamnionitis and neonatal sepsis in an unusual way, but it is an organism that should be taken into consideration, as its incidence might be underestimated due to its slow growth and nutritional requirements.
Conflicts of interestNone.
We would like to thank Clara Lejarraga Cañas for confirming the identification of the strains by PCR and 16S rRNA sequencing.