The use of systemic corticosteroids during Epstein-Barr virus (EBV)-induced infectious mononucleosis is a controversial but widespread practice. We aimed to investigate the frequency of complications in adolescents and adults with infectious mononucleosis in relation to the use of corticosteroids.
MethodsWe reviewed the clinical records of 396 patients admitted to the hospital with infectious mononucleosis (52.0% male; median age, 19 years; range, 15–87 years), with a focus on both short-term (infectious and non-infectious) and long-term (hematological malignancies) complications in relation to corticosteroid use.
ResultsA total of 155 (38.6%) patients received corticosteroids at some point during infectious mononucleosis. Corticosteroid use was significantly (P≤0.002) associated with sore throat, lymphadenopathy, leukocytosis, and with antibiotics use (mainly indicated after suspicion of tonsillar bacterial superinfection). Overall, 139/155 (89.7%) patients who were treated with corticosteroids also received antibiotics either before or during hospitalization, compared with 168/241 (69.7%) patients who did not. The frequency of short-term severe complications, either infectious (peritonsillar–parapharyngeal abscess or bacteremia) or non-infectious (splenic rupture, severe thrombocytopenia, myopericarditis, or lymphocytic meningitis) were similar in patients receiving and not receiving corticosteroids. After a median of 15 years of follow-up, only one Hodgkin's lymphoma was diagnosed, in a patient who was not treated with corticosteroids during infectious mononucleosis.
ConclusionsThe use of systemic corticosteroids during EBV-induced infectious mononucleosis is generally safe, at least with concomitant antibiotic therapy. However, this should not encourage the use of corticosteroids in this context, given that their efficacy has yet to be demonstrated.
El uso de corticoides durante la mononucleosis infecciosa es una práctica controvertida, pero habitual. El objetivo del estudio fue investigar la frecuencia de complicaciones del empleo de corticoides en adolescentes y adultos con mononucleosis.
MétodosSe revisaron las historias de 396 pacientes ingresados en el hospital por mononucleosis (52,0% varones; edad 15-87 años), las complicaciones a corto plazo (infecciosas y no infecciosas) y a largo plazo (neoplasias hematológicas) y su posible relación con el uso de corticoides.
ResultadosUn total de 155 (38,6%) pacientes recibieron corticoides en algún momento de la enfermedad. El uso de corticoides se asoció (p≤0,002) con la presencia de odinofagia, adenopatías, leucocitosis y con el empleo de antibióticos por sospecha de sobreinfección bacteriana. En conjunto, 139/155 (89,7%) pacientes tratados con corticoides recibieron también antibióticos antes o durante el ingreso, comparado con 168/241 (69,7%) pacientes que no fueron tratados con corticoides. La frecuencia de complicaciones graves a corto plazo, fueran infecciosas (absceso periamigdalino/parafaríngeo o bacteriemia) o no infecciosas (rotura esplénica, trombopenia grave, miopericarditis o meningitis linfocitaria) fue similar en los pacientes que recibieron corticoides y en los que no los recibieron. Tras una mediana de 15 años solo se diagnosticó un linfoma de Hodgkin en un paciente no tratado con corticoides durante la mononucleosis.
ConclusionesEl uso de corticoides sistémicos durante la mononucleosis infecciosa es seguro, al menos con el uso concomitante de antibióticos. Sin embargo, esto no debe ser una justificación para su uso en la mononucleosis, pues su eficacia está por demostrar.
Infectious mononucleosis (IM) is the clinical manifestation of primary Epstein-Barr virus (EBV) infection.1 Given that spontaneous resolution is the rule in this entity, symptomatic treatment is typically sufficient and is the standard of therapy.1 The additional use of corticosteroids during IM is controversial. Strictly, corticosteroids are only indicated for certain systemic complications of IM, such as hematological (e.g., thrombocytopenia or immune hemolytic anemia) or neurological, or for local complications such as upper airway obstruction, all of which are rare.1 However, the use of corticosteroids as an adjunct to symptomatic treatment is a widespread practice in patients with general symptoms or severe sore throat.2,3 A systematic review of seven randomized controlled trials with a total of 362 participants aged between 14 and 30 years old concluded that there is insufficient evidence to support the efficacy of steroids for symptom control in IM.4
Moreover, systematic reviews have indicated that there is a lack of research on the short-term and long-term adverse effects of corticosteroid use during IM.4 Much of the controversy on the use of corticosteroids is due to the potential for adverse events in a disease that is largely self-limiting.4 Corticosteroid therapy during IM can reduce blood total lymphocyte counts and subsets.5 Corticosteroids are immunosuppressive drugs that could predispose patients to a secondary infection, such as peritonsillar abscess, as previously suggested.6,7 Moreover, there are theoretical concerns about the unknown long-term effects of using an immunosuppressive drug for a virus that usually establishes intracellular latency and participates in the pathogenesis of hematological malignancies.4,8 The present study was aimed at investigating the frequency of IM complications and their possible association with the use of corticosteroids in adolescent and adults during the acute phase of the disease.
MethodsDesign and settingIn this retrospective cohort study, we reviewed the clinical records of adolescent and adult (aged 15 years or older) patients with IM who were admitted to the Santiago de Compostela (Spain) University Hospital between 1995 and 2018, as reported elsewhere.9–11 The hospital is the reference center for an area with approximately 400,000 inhabitants. The main reasons for hospital admission were severe signs of systemic inflammatory response, difficulty with oral intake, and the presence of complications.
Inclusion criteria and study populationA definitive diagnosis of IM was considered when a compatible clinical syndrome was accompanied by positive immunoglobulin-M antibodies against the viral capsid antigen of EBV and/or a positive heterophile antibody result, as revealed by either a classic Paul-Bunnell test or a commercial rapid test.1 A total of 402 hospitalized patients met the diagnostic criteria. A total of 320 patients (of the 339 tested) had positive immunoglobulin IgM antibodies against the viral capsid antigen, 339 (of the 381 tested) had a positive heterophile antibody result, and 257 patients had both tests positive. Complete information about corticosteroid therapy was unavailable for six cases. The study therefore included 396 patients (52.0% male; median age, 19 years [range, 15–87 years]).
Main determinationsMedical records were reviewed, with special interest in the use of corticosteroids before admission, during hospitalization and immediately after discharge. The corticosteroid dose was analyzed as mg/day of methylprednisolone or equivalent. We also focused on complications (infectious and non-infectious) during the acute phase of infection. Simple tonsillar bacterial coinfection, mild thrombocytopenia,9 liver disease,10 and skin rash11 have been reported elsewhere and were not considered for this study. In addition, the records were reviewed until April 2023 to explore the possibility of late (hematological malignancies) complications related to EBV infection and corticosteroid treatment. The follow-up period was calculated from the day the patients were discharged alive from the hospital (n=393) until death or until the last reliable contact with the health care system, to account for potential misclassification owed to change of residence.
Statistical analysesWe employed the Mann–Whitney test to compare numerical data between groups. We employed the chi-squared test (with continuity correction, when appropriate) to compare proportions. Cox regression was used for multivariate analyses of factors associated with mortality. All tests were two-tailed.
Ethical approvalThe study was reviewed and approved by the institutional Ethics Committee (code 2017/578) who waived the requirement for informed consent from the study participants, in agreement with Spanish regulations for retrospective studies of clinical records.
ResultsGeneral characteristics of the patients treated with corticosteroidsA total of 155 (38.6%) patients received systemic corticosteroids at some point (before admission, during admission, or continued after admission) (Fig. 1). The exact duration and dose of corticosteroid therapy prior to hospitalization was not recorded. Corticosteroid dose during hospitalization was intermediate-high in most cases (the median initial dose was 60mg/day and the interquartile range [IQR] was 50–75mg/day of methylprednisolone or equivalent). Only 4.2% of corticosteroid-treated patients received low (equal or lower than 20mg/day of methylprednisolone or equivalent) initial doses. The median length of corticosteroid therapy was 4 (IQR 3–9) days. A total of 33 patients also received corticosteroids after hospital discharge (Fig. 1), in tapering doses, for a median of 8 (IQR 6–12) days. Antivirals (acyclovir) were administered to a single patient with clonal B lymphocytosis and severe hepatitis.10
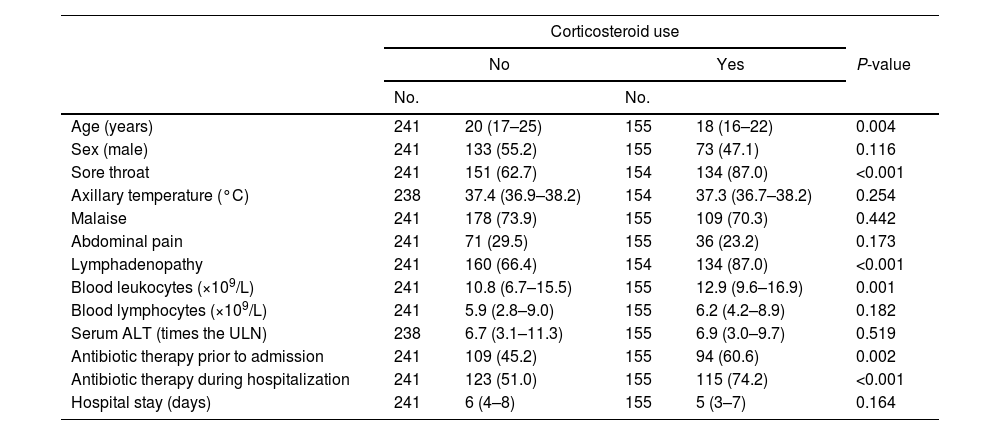
Table 1 represents a comparison of patients who received corticosteroids during IM and those who did not. Patients who received corticosteroids were younger (Table 1). They also presented more frequently with prominent sore throat and lymphadenopathy. Accordingly, corticosteroid-treated patients received antibiotics more frequently than patients who were not (Table 1). A total of 203 patients received antibiotics prior to hospital admission (164 received beta-lactams [amoxicillin with or without clavulanic acid in 118 of these cases], 24 received macrolides, 8 received quinolones, and miscellaneous antibiotics were administered to the remaining 7 patients). A total of 238 patients received antibiotics during hospitalization (macrolides in 124 cases, beta-lactams in 55 cases [amoxicillin with or without clavulanic acid in 11 of these cases], clindamycin in 45 cases, and quinolones in 14 cases). As a whole, 139/155 (89.7%) patients who were treated with corticosteroids received antibiotics either before or during hospitalization, compared with 168/241 (69.7%) of patients who were not (P<0.001). Total leukocyte counts were also higher in patients who received corticosteroids than in those who did not (Table 1). The hospital stay was similar in patients who received corticosteroids and those who did not (Table 1).
Demographic and clinical characteristics of patients with infectious mononucleosis, stratified by the use of corticosteroids (either prior to admission or during hospitalization).
| Corticosteroid use | |||||
|---|---|---|---|---|---|
| No | Yes | P-value | |||
| No. | No. | ||||
| Age (years) | 241 | 20 (17–25) | 155 | 18 (16–22) | 0.004 |
| Sex (male) | 241 | 133 (55.2) | 155 | 73 (47.1) | 0.116 |
| Sore throat | 241 | 151 (62.7) | 154 | 134 (87.0) | <0.001 |
| Axillary temperature (°C) | 238 | 37.4 (36.9–38.2) | 154 | 37.3 (36.7–38.2) | 0.254 |
| Malaise | 241 | 178 (73.9) | 155 | 109 (70.3) | 0.442 |
| Abdominal pain | 241 | 71 (29.5) | 155 | 36 (23.2) | 0.173 |
| Lymphadenopathy | 241 | 160 (66.4) | 154 | 134 (87.0) | <0.001 |
| Blood leukocytes (×109/L) | 241 | 10.8 (6.7–15.5) | 155 | 12.9 (9.6–16.9) | 0.001 |
| Blood lymphocytes (×109/L) | 241 | 5.9 (2.8–9.0) | 155 | 6.2 (4.2–8.9) | 0.182 |
| Serum ALT (times the ULN) | 238 | 6.7 (3.1–11.3) | 155 | 6.9 (3.0–9.7) | 0.519 |
| Antibiotic therapy prior to admission | 241 | 109 (45.2) | 155 | 94 (60.6) | 0.002 |
| Antibiotic therapy during hospitalization | 241 | 123 (51.0) | 155 | 115 (74.2) | <0.001 |
| Hospital stay (days) | 241 | 6 (4–8) | 155 | 5 (3–7) | 0.164 |
Data are medians and interquartile ranges (within parentheses) or absolute numbers and percentages (within parentheses). No., number of patients with available determination. Clinical and laboratory characteristics were registered on admission. P-values were obtained with the chi-squared test for categorical variables and the Mann–Whitney test for numerical variables. ALT, alanine aminotransferase; ULN, upper limit of normal.
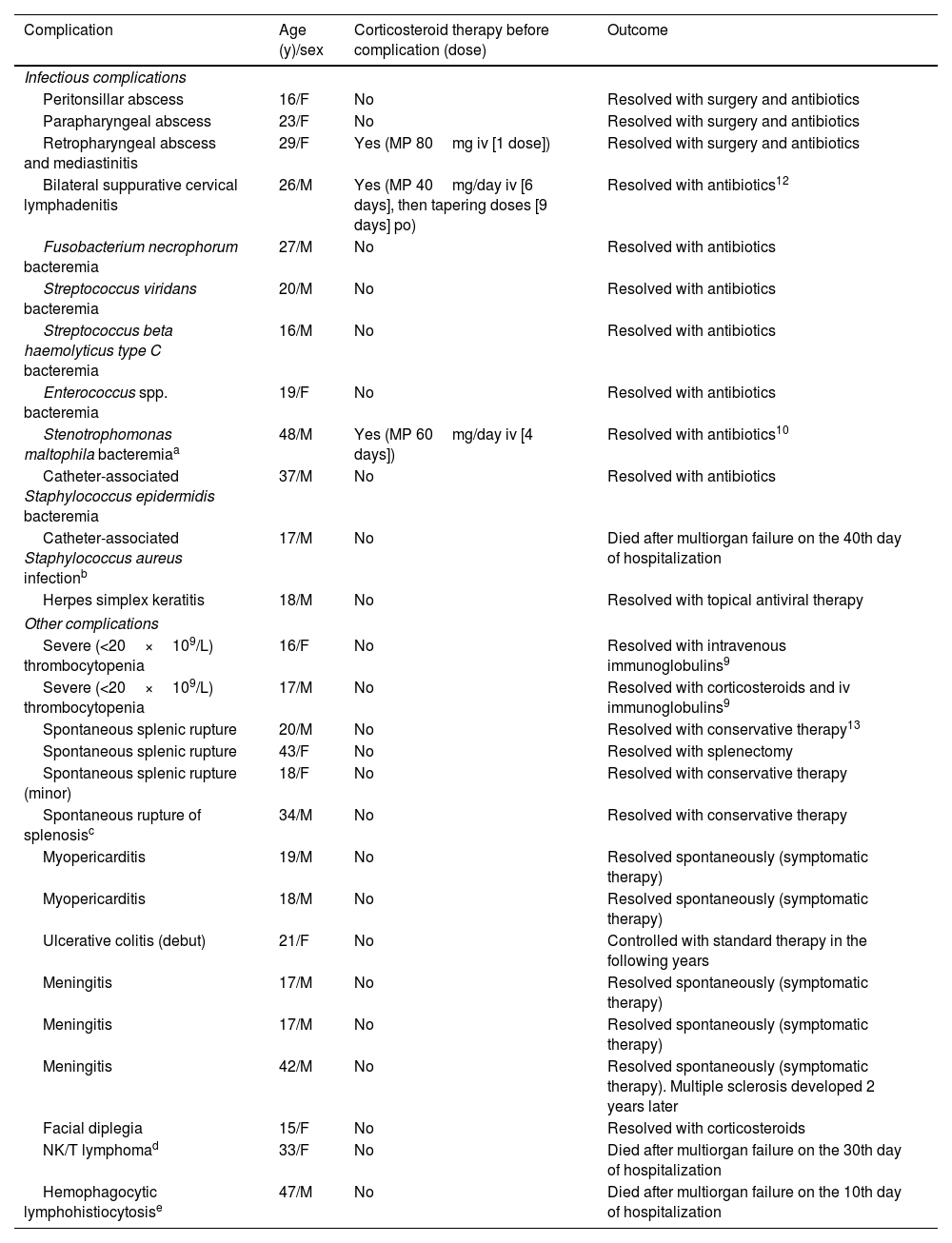
Table 2 shows the main individual characteristics of the patients with significant complications in the series and their possible association with corticosteroid administration. Four (1.0%) patients developed suppurative complications contiguous to the oropharyngeal infection (peritonsillar abscess, parapharyngeal abscess, retropharyngeal abscess with mediastinitis, and bilateral suppurative cervical lymphadenitis in one case each) (Table 2). There were two (1.3%) cases among 155 patients receiving corticosteroids, although one of the patients received a single dose (Table 2). That patient had previously been treated with azithromycin and the other did not receive prior antibiotic therapy. There were also two cases among 241 patients not receiving corticosteroids (0.9%; P=0.654). One of these patients had previously been treated with a beta-lactam antibiotic and the other did not receive prior antibiotic therapy. All cases evolved favorably with antibiotic therapy, with or without surgery (Table 2).
Main complications of infectious mononucleosis and previous corticosteroid therapy.
| Complication | Age (y)/sex | Corticosteroid therapy before complication (dose) | Outcome |
|---|---|---|---|
| Infectious complications | |||
| Peritonsillar abscess | 16/F | No | Resolved with surgery and antibiotics |
| Parapharyngeal abscess | 23/F | No | Resolved with surgery and antibiotics |
| Retropharyngeal abscess and mediastinitis | 29/F | Yes (MP 80mg iv [1 dose]) | Resolved with surgery and antibiotics |
| Bilateral suppurative cervical lymphadenitis | 26/M | Yes (MP 40mg/day iv [6 days], then tapering doses [9 days] po) | Resolved with antibiotics12 |
| Fusobacterium necrophorum bacteremia | 27/M | No | Resolved with antibiotics |
| Streptococcus viridans bacteremia | 20/M | No | Resolved with antibiotics |
| Streptococcus beta haemolyticus type C bacteremia | 16/M | No | Resolved with antibiotics |
| Enterococcus spp. bacteremia | 19/F | No | Resolved with antibiotics |
| Stenotrophomonas maltophila bacteremiaa | 48/M | Yes (MP 60mg/day iv [4 days]) | Resolved with antibiotics10 |
| Catheter-associated Staphylococcus epidermidis bacteremia | 37/M | No | Resolved with antibiotics |
| Catheter-associated Staphylococcus aureus infectionb | 17/M | No | Died after multiorgan failure on the 40th day of hospitalization |
| Herpes simplex keratitis | 18/M | No | Resolved with topical antiviral therapy |
| Other complications | |||
| Severe (<20×109/L) thrombocytopenia | 16/F | No | Resolved with intravenous immunoglobulins9 |
| Severe (<20×109/L) thrombocytopenia | 17/M | No | Resolved with corticosteroids and iv immunoglobulins9 |
| Spontaneous splenic rupture | 20/M | No | Resolved with conservative therapy13 |
| Spontaneous splenic rupture | 43/F | No | Resolved with splenectomy |
| Spontaneous splenic rupture (minor) | 18/F | No | Resolved with conservative therapy |
| Spontaneous rupture of splenosisc | 34/M | No | Resolved with conservative therapy |
| Myopericarditis | 19/M | No | Resolved spontaneously (symptomatic therapy) |
| Myopericarditis | 18/M | No | Resolved spontaneously (symptomatic therapy) |
| Ulcerative colitis (debut) | 21/F | No | Controlled with standard therapy in the following years |
| Meningitis | 17/M | No | Resolved spontaneously (symptomatic therapy) |
| Meningitis | 17/M | No | Resolved spontaneously (symptomatic therapy) |
| Meningitis | 42/M | No | Resolved spontaneously (symptomatic therapy). Multiple sclerosis developed 2 years later |
| Facial diplegia | 15/F | No | Resolved with corticosteroids |
| NK/T lymphomad | 33/F | No | Died after multiorgan failure on the 30th day of hospitalization |
| Hemophagocytic lymphohistiocytosise | 47/M | No | Died after multiorgan failure on the 10th day of hospitalization |
MP, methylprednisolone.
Bacteremia was detected in 8 (4.2%) of 187 patients who underwent blood cultures. Patients in whom blood cultures were performed had a higher axillary temperature on admission than patients without them (median 37.6°C and IQR 37.0–38.4°C, versus median 37.2°C and IQR 36.7–38.0°C, respectively, P<0.001). Three patients had severe lymphoproliferative disorders, namely pre-existing clonal B lymphocytosis, EBV-related hemophagocytic lymphohistiocytosis and EBV-related NK/T lymphoma (Table 2). Bacteremia occurred late after admission in these three immunosuppressed cases, all of whom had received corticosteroids prior to the bacteremia episode (Table 2). Two of these patients ultimately died after multiorgan failure in the intensive care unit due to disease progression (Table 2). One patient without corticosteroid therapy developed Staphylococcus epidermidis bacteremia after catheter-associated phlebitis several days after admission (Table 2). In the rest of the patients with bacteremia, blood cultures had been taken at the time of admission, without corticosteroid therapy. Streptococcus spp. was the most frequently encountered group in patients with bacteremia (Table 2). Fusobacterium necrophorum bacteremia was detected in one patient. The patient had not received corticosteroids and did not show the jugular phlebitis or septic embolisms of full-blown Lemièrre syndrome (Table 2). Bacteremia evolved favorably with antibiotics in all cases, except in two patients with severe EBV-associated severe lymphoproliferative disorders (Table 2). An additional patient with severe disability died of staphylococcal sepsis, with negative blood cultures after prolonged hospitalization (Table 2). Apart from one patient with herpetic keratitis, who was treated topically with acyclovir, no other significant infectious complications were recorded (Table 2).
Overall, significant infectious complications (cervical abscess, bacteremia, severe bacterial sepsis, or herpetic keratitis) were observed in 7/155 (4.5%) patients who received corticosteroids at some point during the course of the disease and in 7/241 (2.9%) of those who did not receive them (P=0.396), although it must be taken into account that only in 3 of the 7 patients who were treated with corticosteroids the complication occurred after their introduction into therapy (Table 2). For this reason, the potential effect of corticosteroid treatment before hospital admission was analyzed separately. Among patients who did not receive antibiotics before admission (n=193), the rate of infectious complications was 0/11 in those who had received corticosteroids, and 9/182 (4.9%) in those who had not (P=0.450). Among patients who received antibiotics before admission (n=203), the rate of infectious complications was 1/15 (6.6%) in those who had received corticosteroids, and 4/188 (2.1%) in those who had not (P=0.275).
Acute non-infectious complications and their association with corticosteroidsSpontaneous rupture of the spleen (or splenosis foci) occurred in four (1.0%) cases, none of which was related to the use of corticosteroids. All resolved well with surgery or with conservative measures (Table 2).
Severe thrombocytopenia occurred in two (0.5%) patients, as previously reported,9 and were not related to the use of corticosteroids. This complication was treated with corticosteroids according to the usual standards (Table 2).
Acute myopericarditis occurred in two (0.5%) patients; these cases were unrelated to the use of corticosteroids and resolved spontaneously (Table 2).
Four (1.0%) patients presented neurological complications, none of which were related to prior corticosteroid therapy. Three of these patients presented lymphocytic meningitis that resolved spontaneously; one of these patients developed multiple sclerosis 2 years later. The remaining patient presented facial diplegia that resolved with corticosteroids (Table 2).
The two patients with EBV-related fatal lymphoproliferative disorders (hemophagocytic lymphohistiocytosis and NK/T lymphoma, as mentioned above) had not received corticosteroids before diagnosis of neoplastic disorder (Table 2).
Long-term disorders potentially related to EBV and their association with corticosteroidsMedian follow-up of the 393 patients discharged from hospital was 180 months (IQR 114–234 months; range 0–332 months). A total of 7 patients died during follow-up. Overall mortality was similar between patients who had received corticosteroids during the IM episode (1/153, 0.6%) and those who had not (6/240, 2.5%; P=0.177). On a multivariate analysis (Cox regression) that included age and sex, corticosteroid therapy during the IM episode was not significantly associated with mortality (hazard ratio [HR] 1.11, 95% CI 0.10–11.8; P=0.927). Age (in years) was the only factor significantly associated with mortality (HR 1.095, 95% CI 1.053–1.139; P<0.001).
Three patients developed neoplastic hematologic disorders during long-term follow-up. A 30-year-old woman developed a stage IIA Hodgkin lymphoma 6 years after the diagnosis of IM, during which she had not received corticosteroids. Eleven years after the lymphoma diagnosis, she is in complete remission. A 34-year-old woman developed a multiple myeloma 6 years after IM diagnosis. The patient had received methylprednisolone (dose not recorded) only prior to admission for IM. Ten years after the myeloma diagnosis, she is in remission, undergoing treatment. A 53-year-old man developed a multiple myeloma 4 years after IM diagnosis. The patient was chronically receiving low doses of methylprednisolone for chronic inflammatory arthropathy, which were increased transiently during hospitalization for IM. Prior to the diagnosis of myeloma, the patient already had a monoclonal gammopathy of uncertain significance. Eight years later, the myeloma is in remission, under treatment.
DiscussionThe present study shows that the use of systemic corticosteroids during EBV-related IM in adolescents and adults is generally safe. Short-term infectious complications, mainly of a bacterial nature, are rare and did not show a clear association with the use of corticosteroids. Short-term non-infectious complications, most of an immune nature, were not related to the prior use of corticosteroids; moreover, these complications frequently had to be treated with corticosteroids. In patients treated with corticosteroids during the IM episode, no significant increase in hematological neoplasms that could be related to EBV infection was found after a median of 15 years’ follow-up.
Our data show that the use of corticosteroids during IM is common. More than one-third of the patients with IM received corticosteroids at some point during the episode, which is a figure similar to that of other series.2,3 Similar to other retrospective series, it is not possible to define the precise reason for the indication of corticosteroids in most cases, with the exception of those indicated for the treatment of some immune complications related to EBV, as mentioned above. Likewise, Thomson et al. reported that poor oral intake, severe dysphagia, persistent symptoms, repeated visits, or reasons not elucidated on the medical records were the probable reasons for corticosteroid indication in the vast majority of cases in a series of similar age distribution.2 Although not consistently found in systematic reviews,4 some clinical trials have shown a slight symptomatic benefit for pharyngitis symptoms through the use of corticosteroids, alone5,14 or in combination with antivirals.15 In our experience, prior treatment with corticosteroids was associated with a trend toward lower levels of serum bilirubin and higher blood platelet counts on admission.9,10 However, treatment with corticosteroids during hospitalization was not associated with a more rapid decrease in hyperbilirubinemia or a more rapid increase in platelet count in patients with mild thrombopenia.9,10 The use of corticosteroids was associated with prominent sore throat and lymphadenopathy as well as the use of antibiotics, which were generally indicated because of suspected tonsillar bacterial superinfection. Tonsillar superinfection is frequent, and treatment with metronidazole can shorten the hospital stay of patients admitted with IM.1,16 It should be noted that most patients who received corticosteroids in this series also received antibiotics. It can therefore be speculated that the concomitant use of antibiotics could have hidden part of the infectious (bacterial) complications potentially associated with the use of corticosteroids.
Peritonsillar abscess can be a complication of IM.6,7,17,18 Gram-positive cocci and anaerobic bacteria are the predominant flora on the tonsillar surface during IM19; bacterial (mainly streptococcal) pharyngitis can coexist with acute EBV infection,20 which can favor bacterial penetration in the tonsils.21 Although early reports had noted that the risk of peritonsillar abscess could be higher in patients with IM receiving corticosteroids,6,7 no obvious evidence supports it,22 and the occurrence of these abscesses in IM is not strongly associated with corticosteroid treatment in reviews.23 In a series of 206 patients with IM, no significant differences were identified between those who received corticosteroids and those who did not in rates of hospital admission, length of stay, and complication incidence.2 However, 4 of the 8 infectious IM complications in the series (three peritonsillar abscesses and one mediastinal abscess) appeared in the relatively small group of 17 patients who received corticosteroids but no antibiotics at initial presentation.2 In our series, the frequency of peritonsillar abscess and similar suppurative locoregional complications (parapharyngeal abscess, retropharyngeal abscess with mediastinitis, and suppurative lymphadenitis) was low (1.0%) and similar in patients who received corticosteroids and in those who did not. Likewise, the patients who had been treated with corticosteroids did not have a higher rate of bacterial superinfection in an additional series of 292 children and young adults.3 The low number of complications observed here and the almost systematic use of antibiotics in the patients who received corticosteroids do not allow us to analyze to what extent concomitant treatment with antibiotics could prevent these bacterial complications.
Bacteremia of oropharyngeal origin during IM has been reported anecdotally in isolated case reports and small series.24 To our knowledge, there were no studies reporting the actual frequency of bacteremia during IM, which was present in 4.2% of patients with blood cultures in the present series. Isolates in blood cultures are consistent with typical flora of tonsillar exudate in patients with IM, which is mainly composed of streptococci and anaerobes, including Fusobacterium spp.19 Moreover, previous studies had reported concomitant IM in a sizeable proportion of patients with Lemièrre syndrome, a rare complication of F. necrophorum infection, with extension into the lateral pharyngeal spaces, septic thrombophlebitis of the jugular vein, and potential septic embolisms.24 In our experience, the frequency of F. necrophorum bacteremia in patients with IM is low (only one case, 0.5%), and the patient had no full-blown Lemièrre syndrome. The bacteremias in our study did not appear to be related to the previous use of corticosteroids. Of the eight patients with bacteremia detected, three had received corticosteroids before the episode; however, they were patients with severe hematological diseases, prolonged hospitalization, and fatal outcome in two cases with EBV-related hemophagocytic lymphohistiocytosis and NK/T lymphoma. The role of corticosteroids for bacteremia in these cases could be minor and cannot be extrapolated to the rest of patients without hematological disorders. Except in patients with severe underlying EBV-associated disease, the prognosis for bacteremia during IM was good.
Of the non-infectious complications observed in the short term, none were related to the previous use of corticosteroids. Spontaneous spleen rupture (present in 1% of patients), severe thrombocytopenia (0.5%), myopericarditis (0.5%), and neurological disorders (lymphocytic meningitis and facial diplegia, totalling 1% of the cases), which are well-known complications of IM,8,9,25–27 were not preceded by treatment with corticosteroids in our series. Early studies described isolated cases of neurological complications in patients with IM treated with corticosteroids,27 which were not confirmed in the present study. Furthermore, a patient with facial diplegia required treatment with corticosteroids. One patient with lymphocytic meningitis, who was not treated with corticosteroids, developed multiple sclerosis 2 years later. The association between EBV infection and multiple sclerosis is causally plausible.26
To the best of our knowledge, there have been no previous studies of the possible long-term development of hematological malignancies in patients with EBV-induced IM who had received corticosteroids during the acute phase of the disease, a speculative concern that had been raised by some authors.8 EBV infects B lymphocytes, T lymphocytes, and natural killer cells and can remain in the host for life.28 The long latency and reactivation of EBV can favor malignant transformation, particularly in immunocompromised hosts.28 However, only one case of Hodgkin lymphoma was documented in the present series after a median of 15 years’ follow-up. The patient had not received corticosteroids in the acute phase of IM. Two more patients developed multiple myeloma at young ages. Although both had received corticosteroids during the acute phase of the disease, multiple myeloma is not considered among the hematological malignancies associated with EBV.28 More studies would be needed to ascertain the possible significance of this finding.
The study has limitations that are inherent to its retrospective design. The information was obtained after a review of records that were not originally designed to collect data for research, and some information could therefore be missing. Similar to previous studies,2,3 a main limitation of the study is the lack of details on the precise indication for corticosteroid therapy and the timing relative to the onset of IM-related symptoms. Likewise, precise data on the potential symptomatic benefit in patients treated with corticosteroids are lacking. Potential selection biases also hamper the interpretation of retrospective studies. It must be considered that all the patients in our series were admitted to the hospital, i.e., they represent a selected sample of severe IM cases. Unfortunately, the total number and characteristics of infectious mononucleosis outpatients in the area during the entire period is unknown. They were adolescents and adults, who had clinical manifestations that are generally more severe than those of children with IM.29 Consequently, young adults with IM are frequently hospitalized and are treated more frequently with systemic corticosteroids.3 Of note, primary EBV infection is acquired increasingly later in life in developed countries, therefore involving a growing number of adults.30 Hence, the conclusions of the present study can only be applicable to similar patient populations of young adolescents and adults who are admitted to the hospital. Finally, the sample size is relatively high, but given the rarity of some complications, the study may not have sufficient power to show differences between the treatment groups. On the other hand, the study has the strength of analyzing patients in real life, which contributes to closing the gap in terms of information on the possible adverse effects of corticosteroids with low frequency or long latency, which would be difficult to address in prospective studies.
In summary, the data suggest that the use of corticosteroids during EBV-induced IM is safe procedure. Prospective studies would be needed to accurately determine the safety of corticosteroids in IM, with and without the concomitant use of antibiotics. In any case, the results should not encourage the use of corticosteroids in this setting, given that their efficacy has yet to be demonstrated.
Conflicts of interestNone to declare.