This study sought to study the epidemiological characteristics of the extrapulmonary tuberculosis (EPTB) in relation to pulmonary tuberculosis (PTB) from 2007 to 2012 in Spain, and plot its trend across the same period.
MethodsWe conducted an analytical cross-sectional study in which the following variables were analysed: age; sex; disease site; history of antituberculosis treatment; country of birth; presence of HIV infection; and culture results. Age-related differences were ascertained using the test of comparison of proportions, and crude and adjusted ORs were calculated using linear regression models.
ResultsOf the total of 44,050 cases of tuberculosis reported in the period 2007–2012 and included in the study, 31,508 (71.53%) were pulmonary tuberculosis and 12,542 (28.47%) were EPTB. EPTB rates decreased across all age groups. The main EPTB risk factor was presence of HIV infection (OR 1.39). Persons aged under 65 years had a lower risk of EPTB.
ConclusionsAlthough persons aged over 65 years displayed the highest incidence, there was nevertheless a downward trend across all age groups. Whereas children showed a significant decrease in tuberculous meningitis, this was not so for all extrapulmonary forms. EPTB rates among persons born outside Spain were much higher than those among the Spanish population.
Estudiamos las características epidemiológicas de la tuberculosis extrapulmonar (TBEP) en comparación con la tuberculosis pulmonar del 2007 al 2012 en España, y argumentamos las tendencias durante el mismo período.
MétodosRealizamos un estudio transversal analítico en el cual analizamos las siguientes variables: edad, sexo, lugar de la tuberculosis, historia de tratamiento antituberculosis, país de nacimiento, presencia de infección por VIH y resultados de cultivo. Se realizó el test de comparación de proporciones para determinar los diferentes grupos de edad y calcular las OR brutas y ajustadas usando modelos de regresión lineal.
ResultsDe un total de 44,050 casos de tuberculosis reportados en el período 2007–2012 e incluidos en el estudio, 31,508 (71.53%) fueron tuberculosis pulmonar y 12,542 (28.47%) fueron TBEP. Las tasas de TBEP disminuyeron en todos los grupos de edad. El principal factor de riesgo para TBEP fue la presencia de infección por VIH (OR 1.39). El grupo de edad de menores de 65 años tuvo un menor riesgo de TBEP.
ConclusionesAunque las personas mayores de 65 años mostraron una mayor incidencia, en general en todos los grupos de edad hubo una tendencia al descenso. Mientras en los niños se observó un descenso significativo en la meningitis tuberculosa, dicha tendencia no se observó en el resto de las formas de tuberculosis extrapulmonar. La tasa de TBEP entre las personas nacidas fuera de España fue mucho mayor que entre las personas nacidas en España.
Tuberculosis (TB) remains a global public health problem.1 It is estimated that in 2012 approximately 8.6 million persons developed TB and 1.3 million died from the disease (including 320,000 deaths among HIV-positive persons).2
Although the most frequently affected organ is the lung, all organs can be affected by the bacillus.3 The site of tuberculosis appears to be determined by the virulence of the mycobacterium, the transmission pathway and the host's innate immunity.4 The proportion of patients with EPTB versus PTB may be associated with concomitant diseases, ethnic groups and countries.3 According to the World Health Organisation (WHO), Regional Office for Europe, during the year 2012, 253,769 new cases of TB were reported, 42,489 of which were EPTB (17%).5 Over the period 2002–2011, a total of 167,562 EPTB cases were reported by 30 European Union (EU) Member States, and though PTB decreased, reported EPTB remained stable with slight variations, going from 3.4 per 100,000 population in 2002 to 3.2 per 100,000 population in 2011.6
In developed countries with good prevention and control programs EPTB rates have declined more slowly than have PTB rates and, proportionally speaking, seem to have risen in recent years.3 While this may be due to an improvement in diagnostic techniques and better disease reporting, in these countries, the highest incidence appears to be concentrated on certain social groups, i.e., residents of urban areas, children, the elderly, HIV-co-infected patients.6
Tuberculosis is a statutorily notifiable disease in Spain. Until 2004, the only extrapulmonary site recorded at a national level was tuberculous meningitis. From this year onwards all the remaining extrapulmonary sites were included in the National Epidemiological Surveillance Network (NESN). This information was progressively incorporated by the Autonomous Regions, and as from 2007 nation-wide EPTB data became available.
Accordingly, this study sought to study the epidemiological characteristics of EPTB in relation to PTB from 2007 to 2012 in Spain, and plot its trend across the same period.
MethodsType of studyWe conducted an analytical cross-sectional study.
Characteristics of the study population and variablesThe study covered all cases of tuberculosis reported to the NESN in the period 2007–2012, whether or not laboratory confirmed. In Spain, cases were reported on an individualised basis, with various clinical and epidemiological variables being recorded in line with European guidelines.7 All personal information on cases was removed at a regional level before being reported at the national level, thereby ensuring that the central databases were anonymous and that cases could not be identified. In addition, there was a system in place for eliminating duplicates.
DefinitionsWe used the European case definition of tuberculosis,7 which considers all TB cases reported in the year of interest, bacteriological confirmed or clinically diagnosed case. Pulmonary TB was defined as a case with TB affecting the lung parenchyma, the tracheobronchial tree or the larynx; and extrapulmonary TB was defined as TB with non-pulmonary presentations, and including pleural, intra-thoracic lymphatic, extra-thoracic lymphatic, spine, bone/joint other than spine, meninges, central nervous system other than meninges (CNS), genitourinary, peritoneal/digestive, and disseminated TB, and other extrapulmonary TB. Some site of the EPTB could also be recorded as unknown. Disseminated TB included TB of more than two organ systems, miliary TB and TB in which M. tuberculosis complex had been isolated from the blood. Cases with concurrent pulmonary and extrapulmonary TB were included in the pulmonary TB category.6
AnalysisFor analysis purposes, cases were grouped into PTB and EPTB. Quantitative variables were described by reference to their mean and standard deviation. Age-related differences were ascertained, using the test of comparison of proportions. Crude and adjusted ORs were calculated using a logistic regression model for various risk factors of EPTB vis-a-vis PTB, using PTB as the reference category. For the analysis of logistic regression a stratified analysis was conducted, by calculating the OR of Mantel Haenszel (univariate – crude OR), and afterwards the logistic regression by taking the multivariate regression was conducted, and building this way the model of main effects that was taken into account. The following study variables were analysed: age; sex; disease site; history of anti-tuberculosis treatment; country of birth; presence of HIV infection; and culture results. The goodness of adjustment of the model was verified and in the end the OR was shown in the table (regression OR/adjusted OR).
We calculated: the overall incidence rates for Spain using population estimated from the National Statistics Institute (NSI); and the rates for the Spanish- and foreign-born populations using the figures drawn from the NSI's continuous population survey. Linear regression models were used to plot the trend over the period 2007–2012 by utilising the Minimum Squared Method.
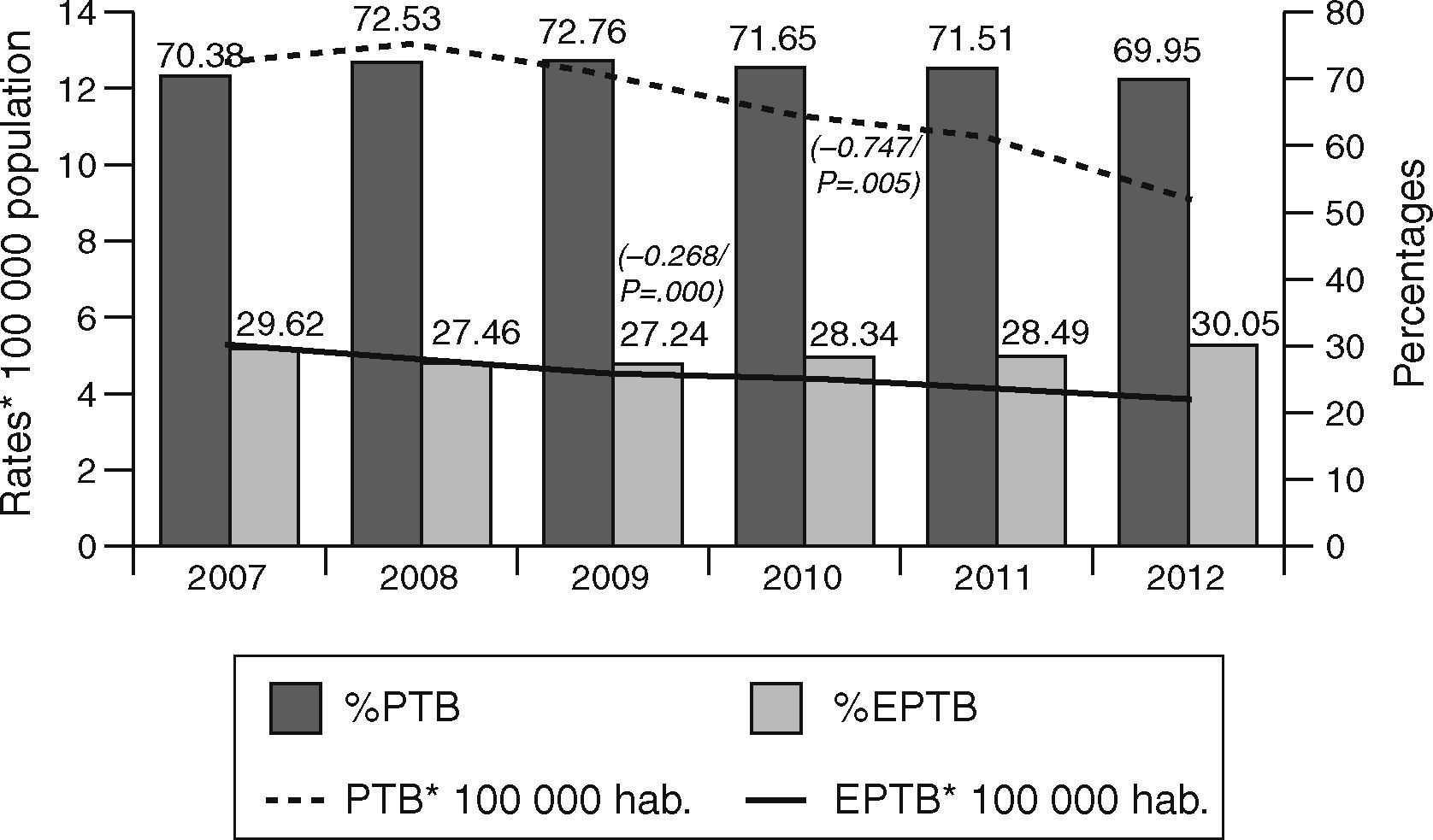
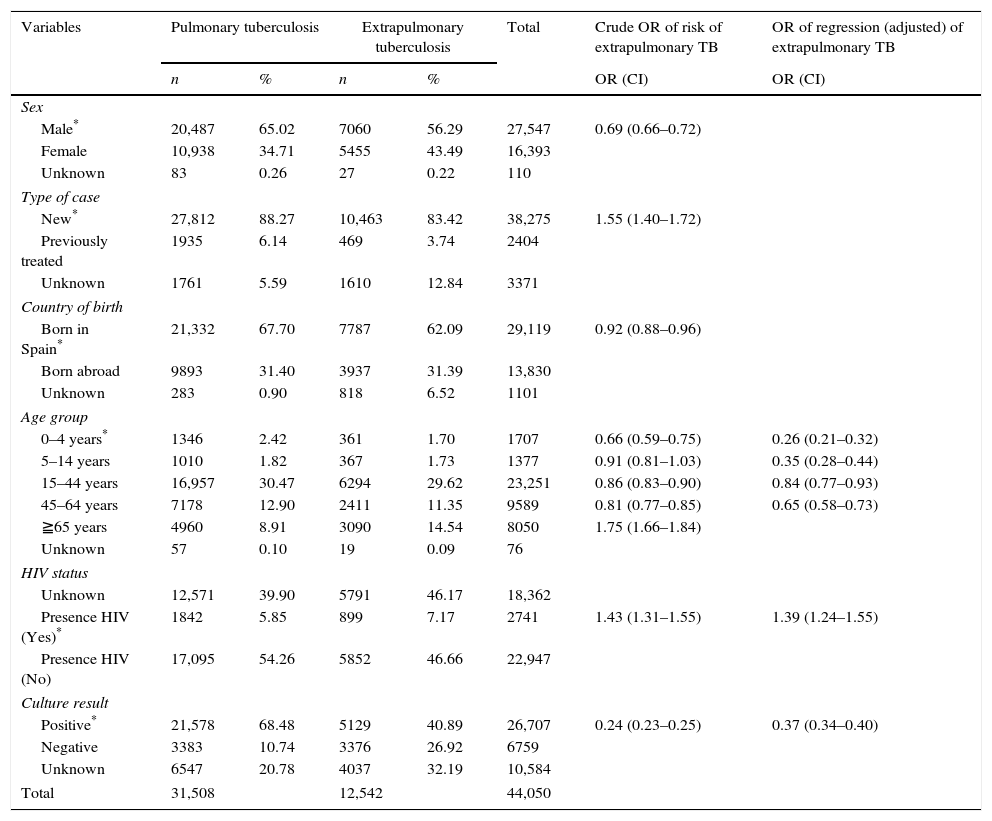
ResultsOf the total of 44,050 cases of tuberculosis reported in the period 2007–2012 and included in the study, 31,508 (71.53%) were PTB and 12,542 (28.47%) were EPTB. During this period, PTB rates decreased from 12.62 cases/100,000 population in 2007 to 9.06 cases/100,000 population in 2012 (β=−0.747, p=0.005). EPTB rates also registered a decline, albeit less pronounced, over the same period (from 5.31 cases/100,000 population in 2007 to 3.89 in 2012, β=−0.268, p=0.000). In parallel with the fall in rates, the percentage of EPTB cases with respect to total cases of tuberculosis decreased until 2009 (from 29.6% in 2007 to 27.4% in 2009) and then steadily increased until 2012 (30.05%) (p=0.04) (Fig. 1).
A breakdown by sex showed that PTB and EPTB rates for men were higher than those for women throughout. PTB rates thus declined from 16.53 to 11.73 among men, and from 8.81 to 6.03 among women over the period 2007–2012: similarly, EPTB rates declined from 6.01 to 4.27 among men, and from 4.63 to 3.34 among women over the same period.
The mean age of cases was 45.47±22 years for EPTB (44.90 years among men and 46.26 years among women) versus 41.49±20 years for PTB (43.78 years among men and 37.11 years among women).
The characteristics of tuberculosis cases included in the study are shown in Table 1. The proportion of women among EPTB cases was higher than that among PTB cases (43.49% of women with EPTB versus 34.71% with PTB). The 15–44-year age group displayed the highest percentage of cases in both groups, i.e., 30.47% in PTB and 29.62% in EPTB.
Characteristics of patients with extrapulmonary tuberculosis: Spain, 2007–2012.
| Variables | Pulmonary tuberculosis | Extrapulmonary tuberculosis | Total | Crude OR of risk of extrapulmonary TB | OR of regression (adjusted) of extrapulmonary TB | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | OR (CI) | OR (CI) | ||
| Sex | |||||||
| Male* | 20,487 | 65.02 | 7060 | 56.29 | 27,547 | 0.69 (0.66–0.72) | |
| Female | 10,938 | 34.71 | 5455 | 43.49 | 16,393 | ||
| Unknown | 83 | 0.26 | 27 | 0.22 | 110 | ||
| Type of case | |||||||
| New* | 27,812 | 88.27 | 10,463 | 83.42 | 38,275 | 1.55 (1.40–1.72) | |
| Previously treated | 1935 | 6.14 | 469 | 3.74 | 2404 | ||
| Unknown | 1761 | 5.59 | 1610 | 12.84 | 3371 | ||
| Country of birth | |||||||
| Born in Spain* | 21,332 | 67.70 | 7787 | 62.09 | 29,119 | 0.92 (0.88–0.96) | |
| Born abroad | 9893 | 31.40 | 3937 | 31.39 | 13,830 | ||
| Unknown | 283 | 0.90 | 818 | 6.52 | 1101 | ||
| Age group | |||||||
| 0–4 years* | 1346 | 2.42 | 361 | 1.70 | 1707 | 0.66 (0.59–0.75) | 0.26 (0.21–0.32) |
| 5–14 years | 1010 | 1.82 | 367 | 1.73 | 1377 | 0.91 (0.81–1.03) | 0.35 (0.28–0.44) |
| 15–44 years | 16,957 | 30.47 | 6294 | 29.62 | 23,251 | 0.86 (0.83–0.90) | 0.84 (0.77–0.93) |
| 45–64 years | 7178 | 12.90 | 2411 | 11.35 | 9589 | 0.81 (0.77–0.85) | 0.65 (0.58–0.73) |
| ≧65 years | 4960 | 8.91 | 3090 | 14.54 | 8050 | 1.75 (1.66–1.84) | |
| Unknown | 57 | 0.10 | 19 | 0.09 | 76 | ||
| HIV status | |||||||
| Unknown | 12,571 | 39.90 | 5791 | 46.17 | 18,362 | ||
| Presence HIV (Yes)* | 1842 | 5.85 | 899 | 7.17 | 2741 | 1.43 (1.31–1.55) | 1.39 (1.24–1.55) |
| Presence HIV (No) | 17,095 | 54.26 | 5852 | 46.66 | 22,947 | ||
| Culture result | |||||||
| Positive* | 21,578 | 68.48 | 5129 | 40.89 | 26,707 | 0.24 (0.23–0.25) | 0.37 (0.34–0.40) |
| Negative | 3383 | 10.74 | 3376 | 26.92 | 6759 | ||
| Unknown | 6547 | 20.78 | 4037 | 32.19 | 10,584 | ||
| Total | 31,508 | 12,542 | 44,050 | ||||
The percentage of cases that had received previous anti-tuberculosis treatment was lower in EPTB (3.74% versus 6.14% in PTB). The percentage of cases born outside Spain was similar for PTB and EPTB, e.g. 31% in both groups. Microbiological confirmation by culture was lower in EPTB cases (41% versus 68% in PTB). The percentage of HIV-co-infected patients was higher in EPTB (7.17% versus 5.85% in PTB).
The results of the logistic regression analysis showed that the main risk factor for EPTB was the presence of HIV infection (OR 1.39). Persons aged less than 65 years had a lower risk of EPTB. EPTB cases were also observed to have a lower likelihood of being confirmed by culture than did PTB cases (Table 1).
Information on the disease site was available in 6454 of the 12,542 EPTB cases (51.4%). Lymphatic, unspecified EPTB and pleural were the predominant extrapulmonary forms [1894 (29%) cases of lymphatic TB; 1401 (22%) cases of unspecified EPTB; and 1348 (21%) cases of pleural TB]. The fourth leading site was tuberculous meningitis (613 cases, 9%). Other kinds of EPTB were: 401 (6%) cases of osteoarticular TB, 365 (6%) genitourinary TB, 304 (5%) miliary TB, 120 (2%) digestive cases and 8 (0%) cases of CNS (except for tuberculous meningitis).
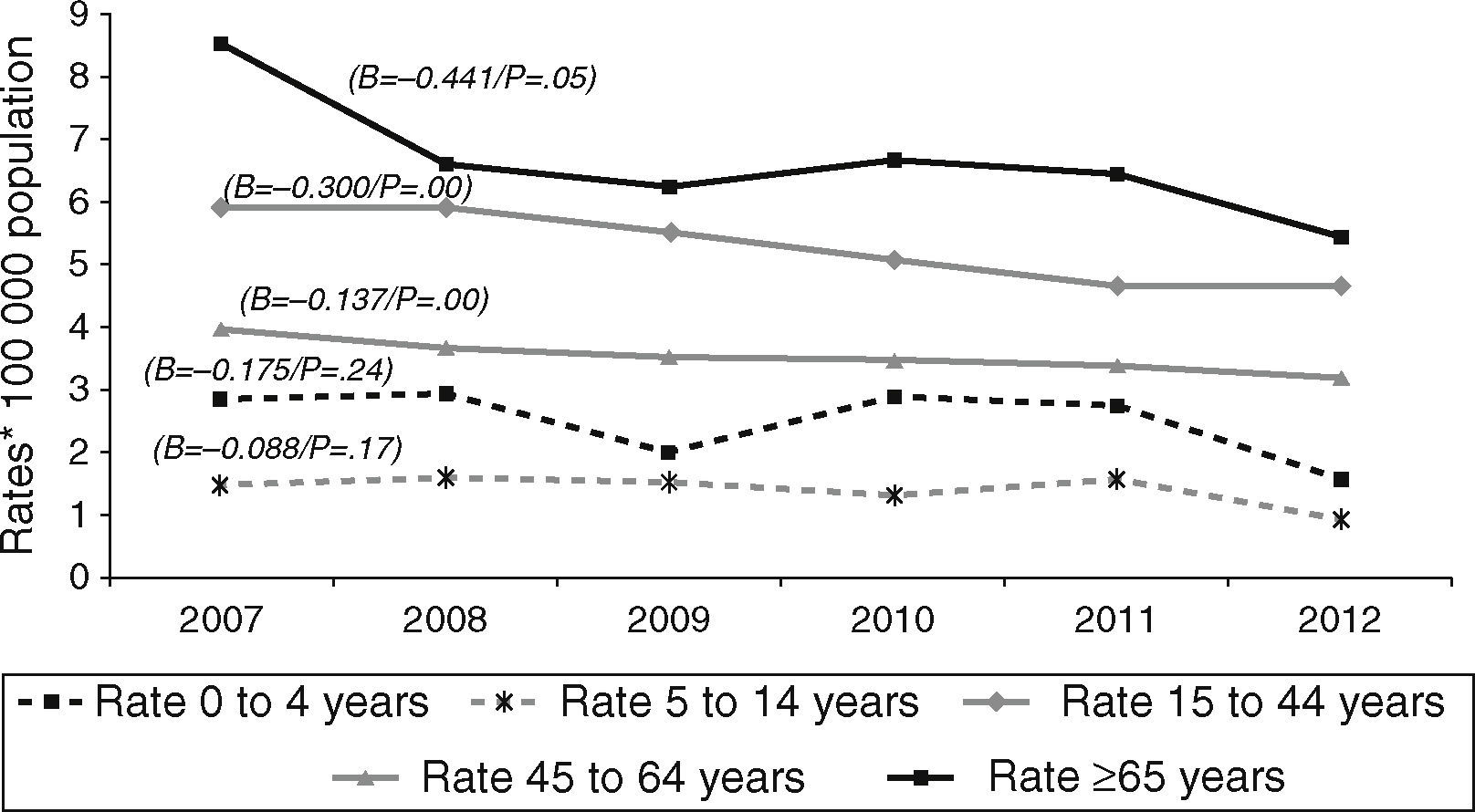
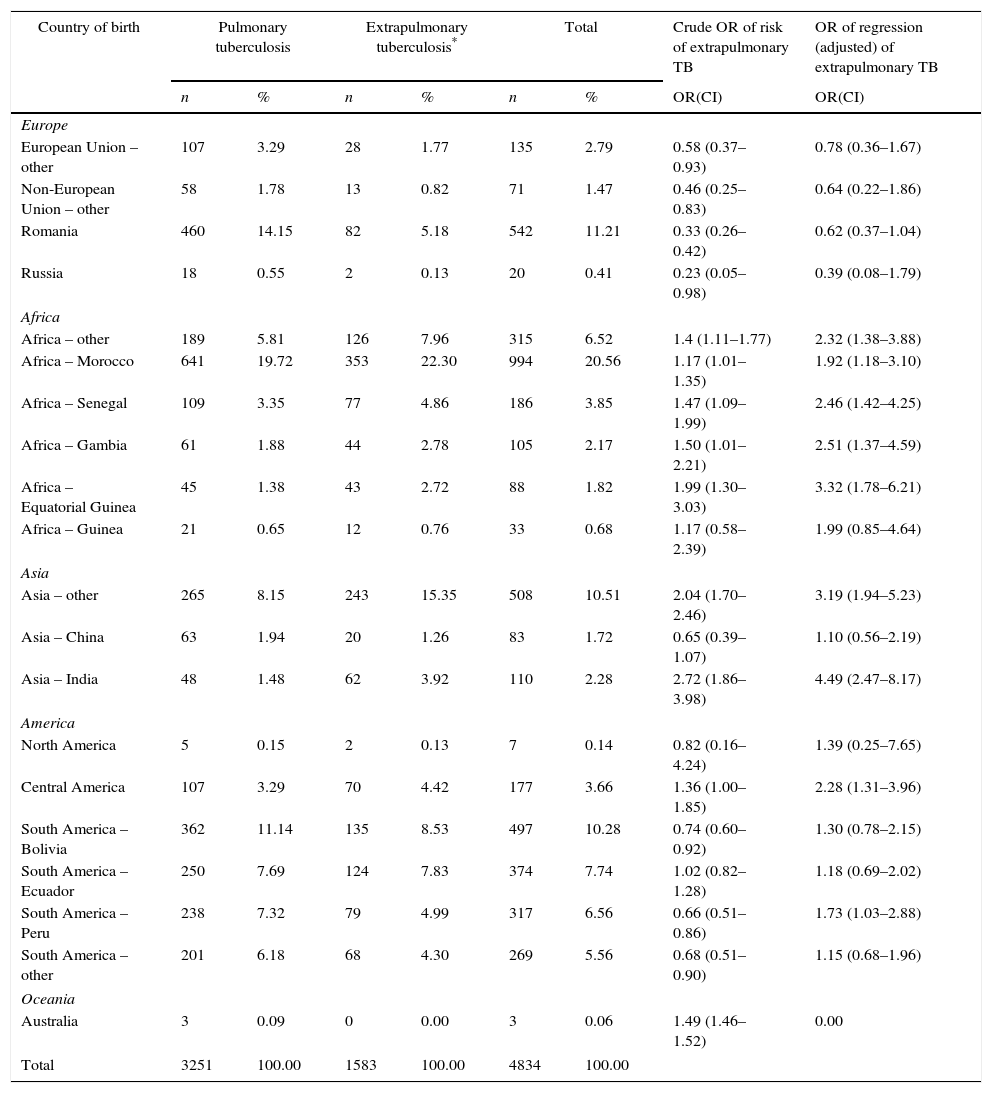
Over the course of the study period, EPTB rates decreased across all age groups (Fig. 2), though this decrease was only significant among adults (≥15 years). This affects the following groups: 15–44 years (β=−0.300, p<0.05); 45–64 years (β=−0.137, p<0.05); and over 65 years (β=−0.441, p=0.05). In 2012, the age group with the highest incidence of EPTB was the over-65 segment with 5.45 cases/100,000 population, followed by the 15–44 group with 4.68 cases/100,000 population.
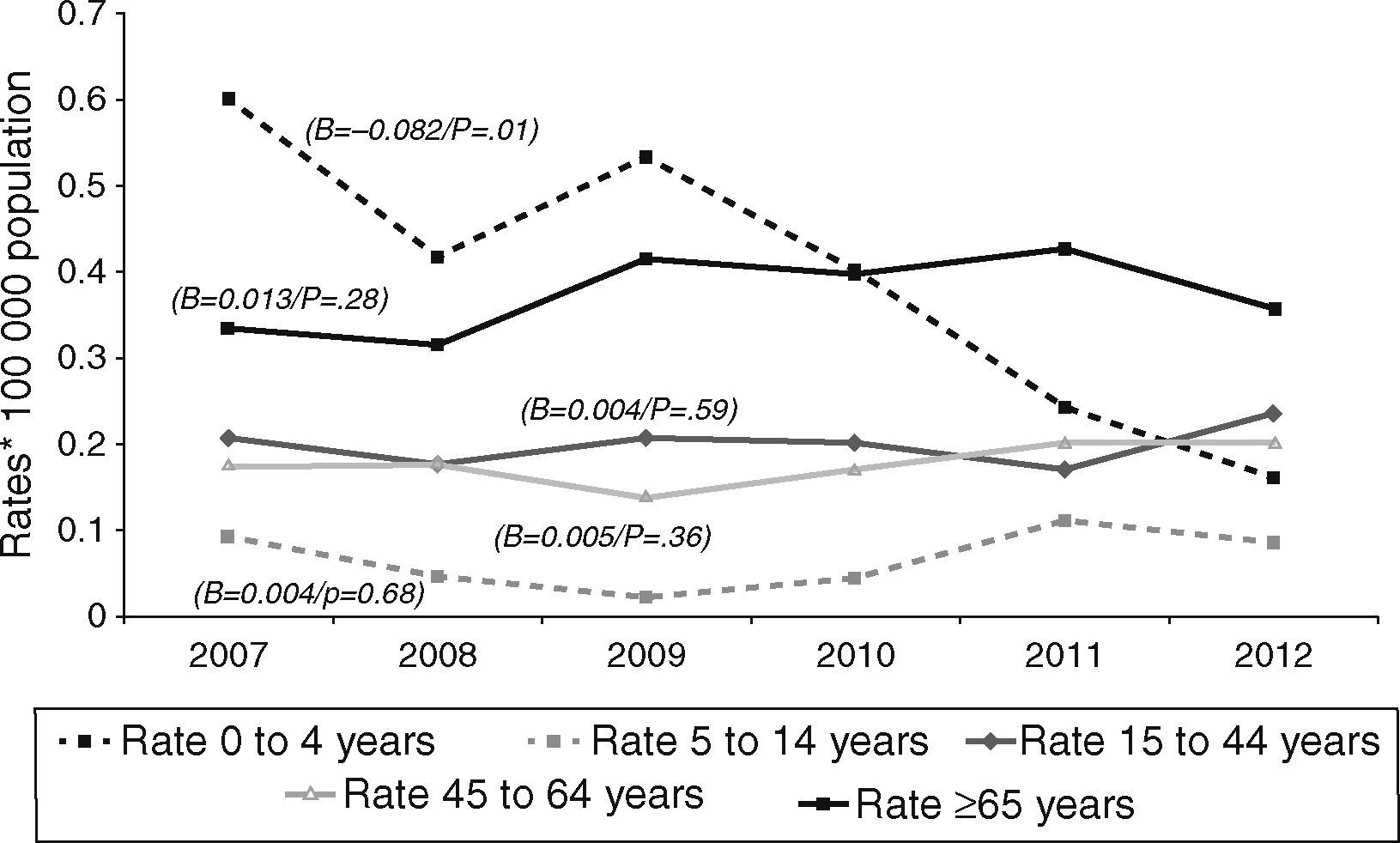
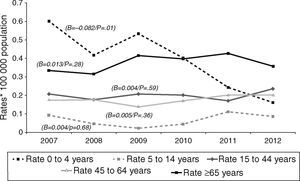
Tuberculous meningitis rates were calculated separately by age group. At this site, incidence displayed a different behaviour pattern, since the only age group to display a decrease (and one, moreover, which was statistically significant) was the 0–4 year group (β=−0.082, p=0.01), whereas the trend in the remaining groups was towards stabilisation and even a slight increase, though without statistical significance, namely, 5–14 years (β=0.004, p=0.68), 15–44 years (β=0.004, p=0.59), 45–64 years (β=0.005, p=0.36), and over-65 years (β=0.013, p=0.28). As in the case of EPTB, the age groups with the highest incidences in 2012 were the over-65 and the 15–44 years old, with 0.36 and 0.24 cases/100,000 population respectively (Fig. 3).
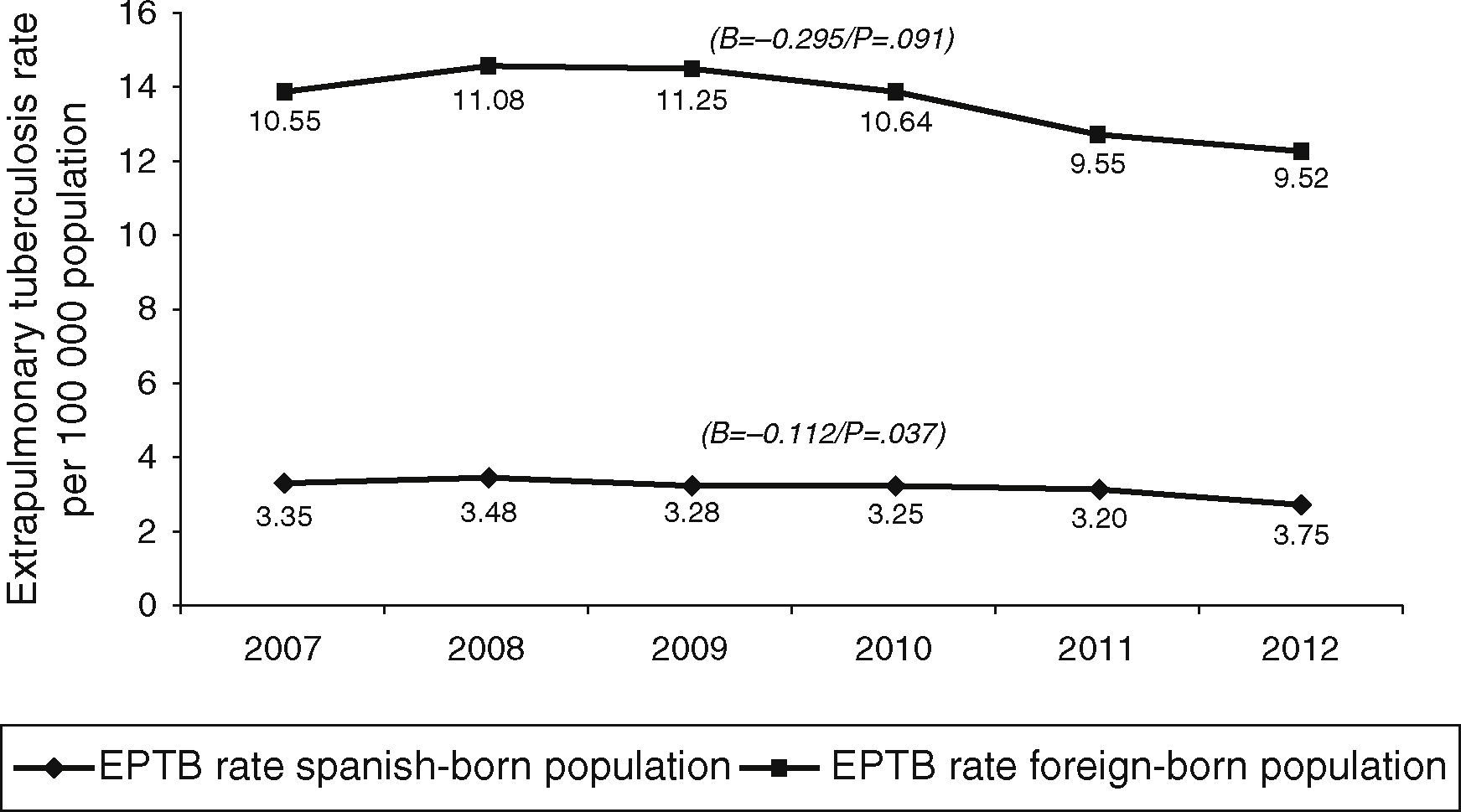
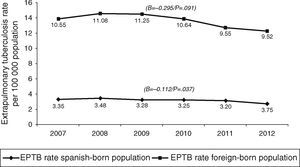
In terms of case origin, EPTB rates were higher among the foreign- than among the Spanish-born population; the rates declined across the study period in both populations, though non-significantly among those born abroad. Among Spanish subjects, EPTB rates went from 3.35 cases/100,000 population in 2007 to 2.75 in 2012 (β=−0.112, p=0.04), while among foreigners they went from 10.55 to 9.53 cases/100,000 population in the same period (β=-0.295, p=0.09) (Fig. 4).
Information on country of birth was available in 4834 of the 13,830 foreign cases (35%): of these, 3251 were PTB and 1583 were EPTB. Among the PTB cases, the greatest percentage of foreign cases corresponded to Morocco (641 cases, 20%), followed by Romania (460 cases, 14%). Among the foreign EPTB cases, Moroccan origin again predominated (353 cases, 22%), with those of Asian origin coming second, particularly India and other Asian countries (mainly Pakistan), which, between the two, accounted for 19% of all EPTB cases (305 cases) (Table 2). The logistic regression analysis showed that the countries with greatest risk of EPTB were: Equatorial Guinea, Gambia, Senegal and Morocco, in the case of Africa (with adjusted ORs of 3.32, 2.51, 2.46 and 1.92 respectively); India, in the case of Asia (OR 4.49); and Central America countries (OR 2.28) and Peru (OR 1.73), in the case of the Americas (Table 2).
Cases of extrapulmonary tuberculosis among foreign nationals by country of birth and TB site: Spain, 2007–2012.
| Country of birth | Pulmonary tuberculosis | Extrapulmonary tuberculosis* | Total | Crude OR of risk of extrapulmonary TB | OR of regression (adjusted) of extrapulmonary TB | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | OR(CI) | OR(CI) | |
| Europe | ||||||||
| European Union – other | 107 | 3.29 | 28 | 1.77 | 135 | 2.79 | 0.58 (0.37–0.93) | 0.78 (0.36–1.67) |
| Non-European Union – other | 58 | 1.78 | 13 | 0.82 | 71 | 1.47 | 0.46 (0.25–0.83) | 0.64 (0.22–1.86) |
| Romania | 460 | 14.15 | 82 | 5.18 | 542 | 11.21 | 0.33 (0.26–0.42) | 0.62 (0.37–1.04) |
| Russia | 18 | 0.55 | 2 | 0.13 | 20 | 0.41 | 0.23 (0.05–0.98) | 0.39 (0.08–1.79) |
| Africa | ||||||||
| Africa – other | 189 | 5.81 | 126 | 7.96 | 315 | 6.52 | 1.4 (1.11–1.77) | 2.32 (1.38–3.88) |
| Africa – Morocco | 641 | 19.72 | 353 | 22.30 | 994 | 20.56 | 1.17 (1.01–1.35) | 1.92 (1.18–3.10) |
| Africa – Senegal | 109 | 3.35 | 77 | 4.86 | 186 | 3.85 | 1.47 (1.09–1.99) | 2.46 (1.42–4.25) |
| Africa – Gambia | 61 | 1.88 | 44 | 2.78 | 105 | 2.17 | 1.50 (1.01–2.21) | 2.51 (1.37–4.59) |
| Africa – Equatorial Guinea | 45 | 1.38 | 43 | 2.72 | 88 | 1.82 | 1.99 (1.30–3.03) | 3.32 (1.78–6.21) |
| Africa – Guinea | 21 | 0.65 | 12 | 0.76 | 33 | 0.68 | 1.17 (0.58–2.39) | 1.99 (0.85–4.64) |
| Asia | ||||||||
| Asia – other | 265 | 8.15 | 243 | 15.35 | 508 | 10.51 | 2.04 (1.70–2.46) | 3.19 (1.94–5.23) |
| Asia – China | 63 | 1.94 | 20 | 1.26 | 83 | 1.72 | 0.65 (0.39–1.07) | 1.10 (0.56–2.19) |
| Asia – India | 48 | 1.48 | 62 | 3.92 | 110 | 2.28 | 2.72 (1.86–3.98) | 4.49 (2.47–8.17) |
| America | ||||||||
| North America | 5 | 0.15 | 2 | 0.13 | 7 | 0.14 | 0.82 (0.16–4.24) | 1.39 (0.25–7.65) |
| Central America | 107 | 3.29 | 70 | 4.42 | 177 | 3.66 | 1.36 (1.00–1.85) | 2.28 (1.31–3.96) |
| South America – Bolivia | 362 | 11.14 | 135 | 8.53 | 497 | 10.28 | 0.74 (0.60–0.92) | 1.30 (0.78–2.15) |
| South America – Ecuador | 250 | 7.69 | 124 | 7.83 | 374 | 7.74 | 1.02 (0.82–1.28) | 1.18 (0.69–2.02) |
| South America – Peru | 238 | 7.32 | 79 | 4.99 | 317 | 6.56 | 0.66 (0.51–0.86) | 1.73 (1.03–2.88) |
| South America – other | 201 | 6.18 | 68 | 4.30 | 269 | 5.56 | 0.68 (0.51–0.90) | 1.15 (0.68–1.96) |
| Oceania | ||||||||
| Australia | 3 | 0.09 | 0 | 0.00 | 3 | 0.06 | 1.49 (1.46–1.52) | 0.00 |
| Total | 3251 | 100.00 | 1583 | 100.00 | 4834 | 100.00 | ||
This is the first nationwide study to analyse the trend and characteristics of EPTB cases over a period of six years, using data furnished by the NESN. During the study period, TB rates in Spain fell significantly, with this decrease being more pronounced in pulmonary forms and less so in extrapulmonary forms. In recent years, there has been observed an increase in the proportion of EPTB cases with respect to the total, a finding in line with publications which report that, where the epidemiological status of general TB improves, the prevalence of EPTB increases.3 Similarly, it has also been reported that a higher incidence of PTB in a society tends to be accompanied by a lower percentage of EPTB and, inversely, a lower incidence of PTB tends to be accompanied by a relatively higher proportion of EPTB,8 as in the case of Spain and other low-TB incidence countries.8 This trend has been observed in Spain,4 in low-incidence countries9 and even in European Union/European Economic Area (EU/EEA) countries, something that suggests difficulties in controlling the disease.6
Case analysis by sex showed that, while the overall rates were higher in men throughout, the percentage of women was higher among EPTB than among PTB cases, a finding that coincides with studies undertaken in the European Union6 and elsewhere.10 While the reason for this has not been elucidated.11
The age groups with the highest risk of presenting with EPTB were those over the age of 65 years, followed by the 15–44 bracket. Some studies argue that young persons are more affected by EPTB12,13 than are elderly persons,10 and even that EPTB is more frequent in children than in adults, presumably due to the immaturity of the former's immune systems.14 In contrast, however, other studies report no association between age and risk of EPTB.3
The percentage of patients that had received previous treatment was lower among patients with EPTB than among those with PTB. This is likely due to the higher risk of under-reporting and under-registration of EPTB cases, which has been documented in the case of the EU,15 and to the delay in diagnosis of EPTB cases as a result of the varied symptomatology of EPTB,16 which often emerges after a long follow-up period. An EU study has shown that the proportion of previously treated EPTB patients is very low (5.1%);6 indeed, in our study this proportion was 3.4%, which is even lower than the EU average.
Microbiological confirmation by culture was lower among EPTB (41%) than among PTB cases (68%), a finding that is in line with some studies, such as that conducted in Estonia.17 In the case of the European Union, percentage confirmation by culture stands at only 33.7%,6 which might be attributable to the difficulty of obtaining an appropriate sample.18
The results of the logistic regression analysis showed presence of HIV infection as being the main EPTB risk factor (OR 1.39), a finding that agrees with various studies.9 Despite the fact that the previous history of HIV was only reported with a percentage of 40% of the patients evaluated, we believe that in the event that the percentage of reported cases would increase, the OR would probably also increase or the IC would be better adjusted due to the fact that it has been claimed that the risk faced by an HIV-infected person might be as much as 5 times higher than that faced by an uninfected person.3 From the mid-1980s onwards, HIV has been shown to be the principal cause of manifestations of EPTB.19
In line with other studies,20 our study showed the leading form of EPTB to be lymphatic. Tuberculous meningitis ranked fourth among the forms of EPTB and was studied separately, in view of the fact that its related fatality ranged from 20% to 69%20 and that its prognosis was heavily influenced by early diagnosis and appropriate treatment.21 Although the decrease in the tuberculous meningitis incidence rate proved significant in the 0–4 age group, the trend in the remaining age groups was not downward. In other countries, such as Germany and Canada,22 the most affected groups have been children under the age of five years. In recent years however, an increase in the number of cases has been observed among children over the age of five years.23 While the percentage of foreign cases was the same in both pulmonary and extrapulmonary forms, EPTB rates in the foreign were higher than those in the national population. These findings agree with those reported by different developed countries, which suggest24 that many immigrants with EPTB come from regions with high EPTB prevalence.12,25 In this respect, some studies state that, in general, risk of tuberculosis is greatest at immigrants’ date of entry into the host country,26 whereas other authors feel that the risk of tuberculosis is maintained for a long post-immigration period,27 e.g. a report from Canada showed that 40% of TB cases were diagnosed at five years after entry into the host country and half of the cases were diagnosed at seven years after immigration.
We had information on country of birth in only 35% of these cases and no information on time of residence in Spain or time elapsed between arrival in the country and diagnosis of TB. The results of the logistic regression showed that, with the single exception of Guinea, persons born on the African continent presented with a higher risk of developing EPTB, as did those born in Asia and specifically in India, a finding in line with other studies which report that patients from Africa and Asia have a higher likelihood of presenting with EPTB.28 In the case of Asian patients, it has been proposed that these might carry a genetic variation which increases their susceptibility to present with forms of EPTB.13,29 Notwithstanding the fact that the greatest proportion of patients of African origin came from Morocco, the risk was higher if the patient came from Senegal, Gambia or Equatorial Guinea. Some studies, such as that undertaken in Holland, have observed that it is patients from Somalia, Asia and Morocco who have the highest risk of presenting with forms of EPTB.30 This study explored the relationship beween type of EPTB nationality an analysis which we were unable to perform because our surveillance system only records patient's country of birth. Lastly, a higher risk of EPTB has been observed among patients from Central America and Peru, a finding in line with studies which have shown that patients from the Americas can generally display a higher risk of EPTB and, moreover, that this risk is further increased if such patients present with HIV co-infection. Unfortunately, the lack of comprehensive data meant that risk of HIV co-infection could not be verified in our study.
Our study has limitations including, among others, the reduction in the number of cases for certain variables, such as country of birth, and the lack of information for variables, such as foreigners’ time of residence or date of entry into the country, due to the fact that these variables have not been considered as mandatory within the monitoring system. Nevertheless, we know the exact number of Spaniards diagnosed with EPTB, and the people born outside Spain, because the above mentioned variables are a compulsory field in the Spanish monitoring system. In our analysis we observed a high percentage of EPTB cases in which the history of previous treatments is unknown. As a matter of fact this may also influence our results, but this happens, because the diagnosis of EPTB has not been considered very often in the initial differential diagnosis. We have also identified a lack of information when exact location of EPTB has been described. The reason is that diagnosis confirming EPTB can vary greatly depending on the affected location, which will be reflected in the monitoring system. Moreover, until a few years ago, it was not mandatory to notify the location in EPTB cases. EPTB diagnosis was just accepted as valid, a fact that also could affect the analysis presented. We believe that these limitations of the monitoring system for tuberculosis could represent a challenge, in order to enhance the quality of the information received, and later improve the types of analysis proposed.
In conclusion, EPTB in Spain could be said to display an epidemiological behaviour pattern similar to that of other countries in the region. The decline in the EPTB rates has been significant, though not as pronounced as in PTB, and the proportion of EPTB cases has increased with respect to the total number of TB cases. Although persons over the age of 65 years registered the highest incidence, there has nevertheless been a downward trend across all age groups. Attention must be paid to children, who displayed a significant decline in tuberculous meningitis but not in all extrapulmonary forms. Persons born outside Spain are a vulnerable group, since EPTB rates are much higher among this segment than among the Spanish-born population, and while these rates have declined, they have not done so significantly. Stress should thus be laid on early diagnosis and treatment of EPTB, by identifying vulnerable groups.
FundingNone declared.
Authors contributionAll authors have worked and reviewed all versions of this manuscript.
Conflict of interestThe authors declare no conflict of interest.
The authors would like to thank to Department of Preventive Medicine and Public Health Autonomous University of Madrid.
The authors would like to thank to Isabel Echevarria Prengel for his help in the translation of previous versions of this manuscript.