We developed a survey to obtain information on the monitoring practices of major systemic antifungals for treatment and prevention of serious fungal infection.
MethodsThe survey included questions relating to methodology and practice and was distributed among 137 colleagues of the Study Group of Medical Mycology (GEMICOMED) from July to December 2019.
ResultsMonitoring was routinely carried out by most respondents, mainly for voriconazole, and was more likely used to determine the efficacy of the dose administered and less for minimizing drug toxicity. Most responders did not follow the strategies of voriconazole dosage based on CYP2C19 genotyping. Monitoring of posaconazole, itraconazole, or other azole metabolites was not carried out or scarcely demanded. Most responders rarely used flucytosine in their clinical practice nor did they monitor it. According to the answers given by some responders, monitoring isavuconazole, amphotericin B, caspofungin and fluconazole exposure would be also interesting in daily clinical practice in selected patient populations.
ConclusionsThe survey reveals common practices and attitudes towards antifungal monitoring, sometimes not performed as per best recommendations, offering an opportunity for education and research. Appropriate use of therapeutic drug monitoring may be an objective of antifungal stewardship programmes.
Describimos los resultados de una encuesta diseñada para obtener información sobre prácticas de monitorización de los principales antifúngicos sistémicos utilizados en el tratamiento y la prevención de la infección fúngica grave en España.
MétodosLa encuesta, que incluye preguntas relacionadas tanto con metodología como con su uso práctico, se distribuyó entre 137 compañeros del Grupo de Estudio de Micología Médica (GEMICOMED), de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica.
ResultadosLa mayoría de los encuestados respondieron utilizar la monitorización de antifúngicos de forma rutinaria, principalmente para voriconazol, y en especial para evaluar la eficacia de la dosis administrada y menos para minimizar su toxicidad. La mayoría de ellos no siguieron las estrategias de dosificación de voriconazol basadas en el conocimiento previo del genotipo CYP2C19, relacionado con su metabolización. Por el contrario, la monitorización de posaconazol, itraconazol o de algunos de sus metabolitos no se realizó o apenas se solicitó. La mayoría de los encuestados rara vez usan 5-fluocitosina en su práctica clínica y por tanto tampoco monitorizan su exposición. Un alto porcentaje consideraría de utilidad poder evaluar la exposición a isavuconazol, anfotericina B, caspofungina y fluconazol en determinadas situaciones en la práctica clínica.
ConclusionesLa encuesta revela prácticas y actitudes hacia la monitorización de antifúngicos que en ocasiones no se realizan según las principales recomendaciones, lo que ofrece una oportunidad para la educación y la investigación. Abordar esta formación podría convertirse en objetivo de los programas racionales del uso de antimicrobianos a nivel nacional.
Therapeutic drug monitoring (TDM) of antimicrobial drugs has proven to be useful for a better patient management. It is defined as the use of analytical procedures for determination of drug concentrations (mainly in blood and in the steady-state) to develop safe and effective drug regimens. In medically advanced countries, it is common that hospitals incorporate this assay to the analytical activities available. Concerning antifungal agents, most studies analysing the impact of this procedure on efficacy and safety found it to be beneficial, particularly with certain types of triazoles (mainly voriconazole) and flucytosine, due to their large individual pharmacokinetic variability and their high tendency for drug-drug interactions or toxicity.1–5 However, still important barriers, such as the availability in the hospital of bioanalytical experts and TDM equipments, need to be addressed before routine TDM can be widely employed worldwide. In view of results reported, most clinicians are convinced of antifungal TDM for dose optimization, but significant variation across institutions on how this procedure is applied in terms of antifungals, patient selection, sampling time points, methods for concentration measurement, as well as specific pharmacodynamics targets and approaches to dose modification has been reported.6–8 In an attempt to know contemporary opinions and attitudes of Spanish clinicians regarding the monitoring of commonly prescribed systemic antifungals, we aimed to evaluate TDM service by a simple survey, mainly to identify potential improvements based on recent recommendations.
Material and methodsWe developed a simple questionnaire to obtain information on monitoring practices (TDM) for major systemic antifungals. We sought to survey a representative sample of professionals familiar with antifungal use from Spanish hospitals. The questionnaire included questions relating to methodology and practice, and was distributed among 137 colleagues of the Medical Mycology Study Group (GEMICOMED), from 16 different Spanish regions (24 cities). Questionnaire details are described in Table 1. From July to December 2019, an open invitation to answer the survey was published on the GEMICOMED website (https://seimc.org/grupos-de-estudio/gemicomed/noticias). Participation was voluntary and no compensation was offered (other than the option of being listed as an acknowledgment). The data were exported from the Google Drive into a Microsoft Excel file. The file was anonymized and any personal data removed.
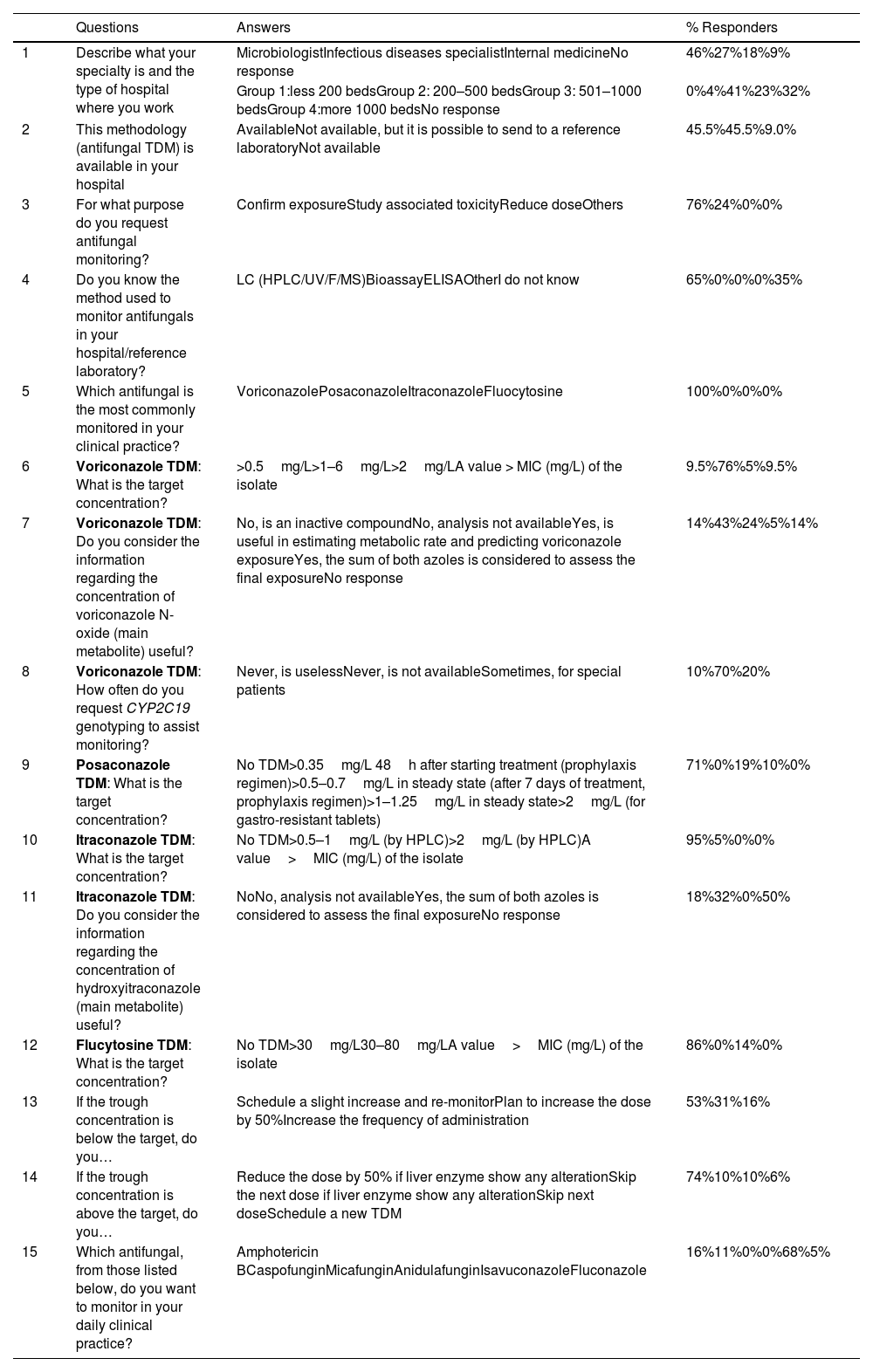
Full text of the questions, possible answers and results.
| Questions | Answers | % Responders | |
|---|---|---|---|
| 1 | Describe what your specialty is and the type of hospital where you work | MicrobiologistInfectious diseases specialistInternal medicineNo response | 46%27%18%9% |
| Group 1:less 200 bedsGroup 2: 200–500 bedsGroup 3: 501–1000 bedsGroup 4:more 1000 bedsNo response | 0%4%41%23%32% | ||
| 2 | This methodology (antifungal TDM) is available in your hospital | AvailableNot available, but it is possible to send to a reference laboratoryNot available | 45.5%45.5%9.0% |
| 3 | For what purpose do you request antifungal monitoring? | Confirm exposureStudy associated toxicityReduce doseOthers | 76%24%0%0% |
| 4 | Do you know the method used to monitor antifungals in your hospital/reference laboratory? | LC (HPLC/UV/F/MS)BioassayELISAOtherI do not know | 65%0%0%0%35% |
| 5 | Which antifungal is the most commonly monitored in your clinical practice? | VoriconazolePosaconazoleItraconazoleFluocytosine | 100%0%0%0% |
| 6 | Voriconazole TDM: What is the target concentration? | >0.5mg/L>1–6mg/L>2mg/LA value > MIC (mg/L) of the isolate | 9.5%76%5%9.5% |
| 7 | Voriconazole TDM: Do you consider the information regarding the concentration of voriconazole N-oxide (main metabolite) useful? | No, is an inactive compoundNo, analysis not availableYes, is useful in estimating metabolic rate and predicting voriconazole exposureYes, the sum of both azoles is considered to assess the final exposureNo response | 14%43%24%5%14% |
| 8 | Voriconazole TDM: How often do you request CYP2C19 genotyping to assist monitoring? | Never, is uselessNever, is not availableSometimes, for special patients | 10%70%20% |
| 9 | Posaconazole TDM: What is the target concentration? | No TDM>0.35mg/L 48h after starting treatment (prophylaxis regimen)>0.5–0.7mg/L in steady state (after 7 days of treatment, prophylaxis regimen)>1–1.25mg/L in steady state>2mg/L (for gastro-resistant tablets) | 71%0%19%10%0% |
| 10 | Itraconazole TDM: What is the target concentration? | No TDM>0.5–1mg/L (by HPLC)>2mg/L (by HPLC)A value>MIC (mg/L) of the isolate | 95%5%0%0% |
| 11 | Itraconazole TDM: Do you consider the information regarding the concentration of hydroxyitraconazole (main metabolite) useful? | NoNo, analysis not availableYes, the sum of both azoles is considered to assess the final exposureNo response | 18%32%0%50% |
| 12 | Flucytosine TDM: What is the target concentration? | No TDM>30mg/L30–80mg/LA value>MIC (mg/L) of the isolate | 86%0%14%0% |
| 13 | If the trough concentration is below the target, do you… | Schedule a slight increase and re-monitorPlan to increase the dose by 50%Increase the frequency of administration | 53%31%16% |
| 14 | If the trough concentration is above the target, do you… | Reduce the dose by 50% if liver enzyme show any alterationSkip the next dose if liver enzyme show any alterationSkip next doseSchedule a new TDM | 74%10%10%6% |
| 15 | Which antifungal, from those listed below, do you want to monitor in your daily clinical practice? | Amphotericin BCaspofunginMicafunginAnidulafunginIsavuconazoleFluconazole | 16%11%0%0%68%5% |
TDM: therapeutic drug monitoring; LC: liquid chromatography; HPLC: high-pressure liquid chromatography.
Twenty-two people completed the questionnaire. Most responders were microbiologists (46%) or infectious diseases specialists (27%), and worked (64%) in large hospitals (>500 beds). In 45.5% of the cases, the TDM methodology was available onsite (hospital laboratory), which is convenient for measuring drug concentrations. In the remaining 45.5% of the cases, samples were sent to a reference laboratory; in 9.0% of the occasions, responders stated that the methodology was neither available onsite nor sent to a central laboratory.
Responders mainly used antifungal TDM to predict drug efficacy (76%), and sporadically for monitoring of toxicity (24%).
Liquid chromatography coupled with ultraviolet detection and tandem mass spectrometry was used in most of the cases (65%). Thirty five percent of responders recognized not knowing the method used by the laboratory. None of the responders reported on the use of a bioassay or the new enzyme immunoassay for voriconazole monitoring.9
Voriconazole resulted the most frequently monitored antifungal. A high percentage of responders (76%) used a voriconazole target (Cmin) in the range of 1–6mg/L in comparison to 24% who did not. Alternatively, the following targets were also reported: Cmin>0.5mg/L (9.5%), Cmin>2mg/L (5%), and Cmin>MIC for the identified isolate (9.5%).
Regarding voriconazole N-oxide, the major metabolite of voriconazole, most responders (57%) stated that they did not monitor its exposure, mainly because the TDM procedure was not available (43%) or because being an inactive metabolite, its contribution to voriconazole exposure was considered irrelevant for efficacy (14%). Surprisingly, 24% of responders considered voriconazole N-oxide concentration helpful to estimate voriconazole metabolic rate. It should be noted that around 5% of responders incorrectly considered this inactive metabolite as part of the pharmacodynamic target of voriconazole.
Concerning itraconazole monitoring, only 5% of the responders recognized to use this procedure. They adopted a Cmin>0.5–1mg/L as target for efficacy. Concentrations of the hydroxymetabolite (hydroxyitraconazole) were not evaluated by most responders mainly due to the lack of an available methodology in the local laboratory or because they considered that this compound does not significantly contribute to itraconazole activity. Furthermore, none of the responders used both values simultaneously as the effective target to clinical efficacy (0.5–1mg/L).
In this survey, most of responders did not routinely monitor posaconazole. Among those who used TDM (29%), none applied the early target (0.35mg/L) described as a fast and useful alternative (measured after 12–48h of treatment) instead of a trough>0.5mg/L proposed recently and measured after seven days post-initiation of therapy.1,10,11
Additionally, in this survey, 86% of the responders did not monitor the antifungal fluocytosine. Among the responders who used TDM, exposure between 30 and 80mg/L was chosen as the therapeutic range.
Clinicians were also asked about how they managed sub-therapeutic/supra-therapeutic concentrations of antifungals. According to the survey results, most responders (84%) decided to increase the dose causing the sub-optimal trough values, 53% of them scheduled a discrete dose increase and asked for a new sample to re-monitor exposure, meanwhile, the rest (31%) just increased the dose by 50%. It is worth mentioning that a small percentage of responders (16%) planned to increase the frequency of administration instead.
Concerning supra-therapeutic levels, for most responders (84%), checking the concentration of liver enzymes was mandatory for managing voriconazole dosage. Thus, a large percentage (74%) reduced the dosage by 50% whereas a smaller group (10%), skipped doses.
Most responders recognized that they did not use strategies based on CYP2C19 genotyping for voriconazole dosage adjustment, mainly because genotype characterization was not available at their hospital (70%) or because they considered it an useless strategy (10%).
Finally, responders were asked which systemic antifungals, among those not strictly recommended, would they consider more useful to monitor in daily clinical practice. For 68% of the responders exposure to isavuconazole should be measured, as well as (in order of priority) amphotericin B, caspofungin, and fluconazole.
DiscussionIn view of the results, this survey constitutes a proof of concept to estimate the implementation of antifungal TDM among Spanish professionals. It sets out a need of improvement to optimize its use with antifungal drugs and represents a starting point for future studies of these characteristics.
First of all, there are important limitations to point out. The data included in the study were obtained through a survey with defined questions, which may not exactly represent the clinician's reality. Second, the survey was sent by e-mail and its completion was voluntary, with an unrecorded response rate; this may have caused an unknown bias. We recorded personal practices and opinions, and these do not represent the general practice. It is important to highlight the high percentage of “no response” in some of the designed questions. In future designs for this type of surveys, we must give the responders the possibility to offer an alternative answer (out of the one initially established) to incorporate other possibilities and improve the quality of the results obtained.
However, our results allowed us to point out specific behaviour on antifungal TDM that deserve to be commented.
Following most recent expert recommendations, TDM should guide voriconazole dosage in critically ill adult patients, to achieve therapeutic exposure and to avoid toxicity. For other systemic antifungals with established therapeutic ranges, TDM may be useful, although in these cases, experts neither recommend nor discourage its use.1 Importantly, the analytical assay used for this procedure should be precise, accurate, robust and it is highly recommended to be available on site. In the past, TDM services were often centralized, which led to delays in reporting results. Currently, it is carried out at local laboratories for almost half, according to the responders of this survey, which allows having almost immediate results. However, a high proportion of antifungal TDM users do not have this method at hand, which prevents from making rapid changes to the antifungal regimen due to transportation issues and results reporting delays.12
Conventional methods for antifungal drug measurements include bioassay and liquid chromatography (LC-UV/PDA or LC–MS/MS). Performance, precision, and reproducibility of these techniques differ markedly.2,3 Personalized medicine is hastening the development of new diagnostic techniques to help control the therapeutic drug levels, as well as their toxic effects. Recently, a new enzyme immunoassay intended for the quantitative determination of voriconazole in human serum or plasma on automated clinical chemistry analysers was developed.9 This assay showed good correlation with the LC–MS/MS procedure, indicating it may be used as an alternative method for voriconazole quantitation in clinical laboratories lacking of sophisticated equipment. Recently, Falces-Romero et al. demonstrated the potential use of MALDI Biotyper system for voriconazole TDM.13 This highlights the importance of introducing new assays to avoid delays in results’ reporting and improve patient management.
It is common and practice to evaluate and compare a single concentration against a stablished target for antifungal dosing adjustment. Sampling time is traditionally performed at the steady state, at the end of a dosing interval (e.g. Cmin or trough sample) and blood samples are considered good surrogates for target concentrations at the site of infection. Optimal targets for clinical efficacy or toxicity have been reported for most antifungals. Concerning voriconazole, two recent meta-analyses suggest a Cmin between 1 and 6mg/L as the best index associated with clinical efficacy.14,15 However, this was not considered by one quarter of the responders (24%). The reasons for not following this target are not clear, but since the survey was not designed to approach this particular question, we hypothesize that responders may not be familiar with the most up-to-date recommendation and were using targets previously adopted. A Cmin target of 2–6mg/L is recommended to guide voriconazole dosing in the recent position paper related to antimicrobial TDM.1 The recommended range of trough concentrations should be included in the laboratory report, therefore, adding this information as a footnote may help clinicians better interpret the results.
In a routine TDM, measuring drug metabolites in the same run may help to identify low metabolite concentrations as indicators of non-adherence, poor metabolism, or metabolic interference by other compounds.16 On the other hand, high metabolite concentrations may suggest previous exposure or rapid metabolism.17 These different possibilities should be kept in mind when performing TDM. In this survey, only a quarter of responders considered voriconazole N-oxide concentration helpful to estimate its metabolic rate. Around 5% of the responders incorrectly considered this inactive metabolite as part of the voriconazole target. This strategy should be reviewed, as it misrepresents the actual voriconazole exposure in the patient.
Contrarily to voriconazole, metabolism of itraconazole by CYP3A4 generates a major active metabolite, hydroxy-itraconazole, which is present in the systemic circulation in equal or higher concentrations than itraconazole and, interestingly, with similar antifungal activity.18,19 Several studies have shown that this metabolite contributes to CYP3A4 inhibition and needs to be considered in the quantitative rationalization of the treatment, although there is no common criteria.5 Thus, the concentration of the metabolite should be measured as itraconazole TDM. The measurement of this metabolite, alone or in association to itraconazole, is rarely performed by responders, which may underestimate this azole exposure in the patient.
TDM-guided dosing to prevent flucytosine toxicity is highly recommended. This is a synthetic antifungal used in combined therapy for the treatment of cryptococcal meningitis. It is not easily available in Spain and probably one of the causes of why only 14% of responders reported flucytosine TDM. A Cmax>80–100mg/L has been associated with toxicity and concentration<25mg/L may induce resistance.1,5
There is no consensus on the pertinence of performing TDM for posaconazole. The introduction of new formulations (delayed release oral tablets or intravenous formulations) with improved bioavailability makes it necessary to reassess the role of TDM, although most experts recommend it to promote effective posaconazole exposure.11 There is still limited results from prospective cohort studies prescribing new posaconazole formulations to support TDM. However, posaconazole oral suspension is still widely used and available worldwide. Following most recent expert's recommendation, when this formulation is used, TDM is mandatory if there are concerns regarding gastrointestinal absorption, uncertainty about compliance, or suspicious of breakthrough infection. However, although a low number of responders monitored posaconazole exposure (29%) when prescribing this drug, we encourage clinicians to make use of this methodology, surely available in analytical laboratories, such as it is for voriconazole. Although none of the questions specifically asked for the reason to choose among a list of not strictly recommended TDM, responders selected isavuconazole, probably because it is a relatively new antifungal and clinical evidence is still lacking in selected patient populations. Subjects with critical illness, sepsis, low or high body weight, polypharmacy, hepatic impairment, renal replacement therapy or other extracorporeal devices, long-term administration (which is usually required in proven invasive fungal disease, chronic pulmonary aspergillosis), and on oral treatment may benefit from exposure monitoring. Several pharmacokinetic studies concluded there is little need for routine TDM,20 although recently reported real-world concentrations differed from those reported in clinical trials, particularly in cases of prolonged therapy.21 Interestingly, these reports describe cut-off values for isavuconazole predicting adverse effects leading to discontinuation. The significance of these observations remains unclear, but may indicate the need of TDM.
Our survey reveals some interesting data on the use of TDM for dose adjustment in cases of under- or over-exposure. Theoretically, TDM should guide dosing adjustment; thus, when the concentration does not reach the established target the dose ought to be increased. Similarly, a decrease of the dosage is necessary when drug concentration is higher than that of the target. However, the magnitude of the increase or decrease has not been well defined yet. Park WB et al. established a strategy on a voriconazole TDM group of patients based on a 100% increase of the dose if the level was below 1mg/L.22 A similar strategy (increase the dose, slightly or by 50%) was followed by around 84% of the responders, while 16% increased the frequency of administration, which has shown to provide positive results with posaconazole dose adjustment. In the same line, Park WB et al. established a 50% dose reduction if drug levels were above 5.5mg/L. The authors also suggested skipping a dose if drug levels were>10mg/L or in case that any adverse effect appeared, followed by a 50% dose reduction until therapeutic levels were reached again.22 Whereas most of the responders seemed to follow one of the two above-mentioned strategies for managing voriconazole over-exposure, it remains unclear how under-exposure is handled in most cases, which offers an opportunity for learning and research.
Personalized medicine has also influenced antifungal TDM via genotyping of CYP2C19 variants. A meta-analysis published by Li X et al. found no significant association between these variants and either voriconazole daily maintenance dose or adverse outcomes.23 However, more recently, other authors reported that CYP2C19 genotype-guided dosing with TDM is a potential solution to optimize voriconazole efficacy while avoiding treatment failures and common toxicities.24,25 However, the introduction of CYP2C19 genotyping methods into medical practice may be most helpful for problem solving, improvement of the outcomes and reduce unnecessary medical costs.26–28 In this survey, we appreciated that this methodology remains out of the reach of most of the responders. This highlights the importance of introducing new technologies to optimize healthcare resources and promote more accurate and safer patient care.
Recent guidelines held that TDM-guided dosing may be an important tool for antifungal stewardship programmes, mainly in the context of haematological disorders and intensive care units.1 These strategies aim to dosage control to ensure efficacy and avoid toxicity, and also contribute to cost reduction and, probably, reduce the risk of the emergence of resistant isolates.29 Data on the effect of antifungal stewardship programs is still limited, but point towards the relevance of TDM, also for antifungals for which there is no strict recommendation. According to the answers given by responders in this survey, monitoring the exposure of isavuconazole, amphotericin B, caspofungin and fluconazole would be interesting in daily clinical practice (despite the fact that a specific question about antifungal stewardship programme was not included).
In conclusion, systemic antifungal TDM is commonly used among this representation of Spanish clinicians interviewed, mainly for voriconazole. Monitoring is more likely used to determine dose efficacy and less to minimize drug toxicity. Surprisingly, TDM of other azoles and major metabolites is not demanded or is infrequently done. Most responders rarely used flucytosine and then, they rarely demanded monitoring. Despite the need for further comprehensive studies reflecting more accurately the reality of antifungal monitoring in Spain, appropriate use of TDM may be an objective of antifungal stewardship programmes.
Ethical approvalNone required.
Transparency declarationsNone to declare.
FundingThis study received support from the Health Research Fund (FIS, reference number AESi PI16CIII/00014, MPY 1347/16; Government of Spain).
Conflicts of interestAuthors declare no conflicts.
The authors would like to thank GEMICOMED for their assistance in the distribution of this survey and the respondents for their time in completing it; their contributions and opinions were cornerstones for the completion of this work.
We also thank D.R. Jaloveckas for English-language editorial work.