SARS-CoV-2 infection during pregnancy and its impact on the newborn were, in the first months of the pandemic, unknown. Recent studies have provided information on the clinical involvement in the newborn and its evolution.
This work shows how passive immunity varies in the newborn in relation to the moment of maternal SARS-CoV-2 infection during pregnancy.
Population and methodObservational, prospective and longitudinal study in a third level hospital. Epidemiological and clinical data from mothers and their newborns were collected from May 2020 to June 2021.
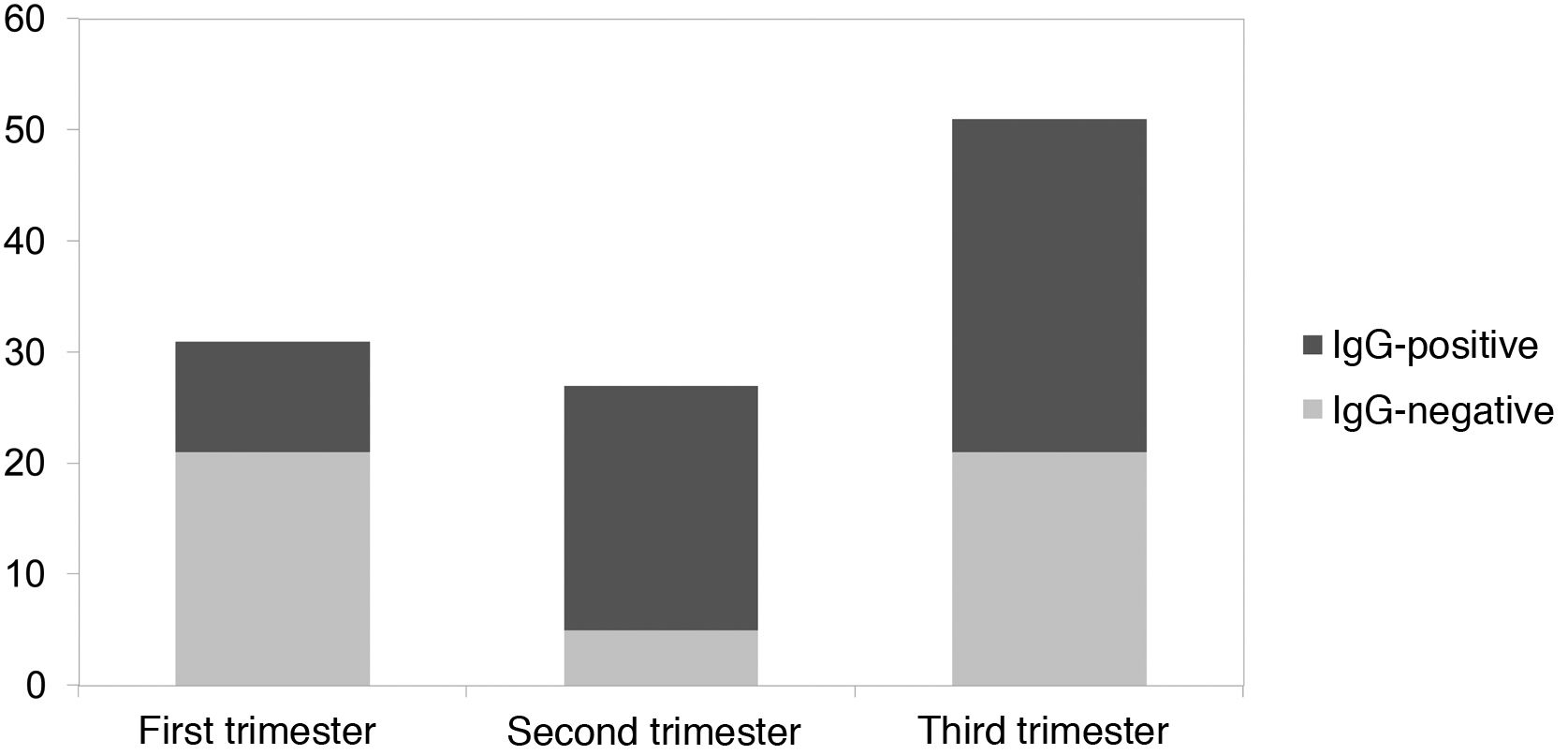
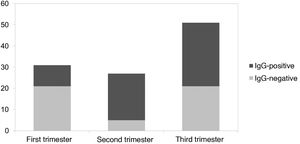
ResultsA total of 109 mothers and 109 neonates have been included. 28.4% of maternal infections were in the first trimester, 24.8% during the second and 58.8% in the third. 56% of maternal infections were symptomatic and only one pregnant woman with severe respiratory infection was admitted to intensive care. The mean gestational age of the newborns was 39 weeks, with a mean weight of 3232 g and a head circumference of 35 cm. Eight newborns born from mothers with SARS-CoV-2 required admission to the neonatal ICU: 2 due to jaundice, 2 due to respiratory distress, 1 due to moderate prematurity, and 3 due to other causes unrelated to infection attributable to SARS-CoV-2. IgG-type antibodies were positive in 56.9% of newborns. Of the mothers infected during the 1 st trimester, IgG were positive in 32.2% of the newborns, in the second trimester 81.5% were positive and in the third 58.8%. No neonate had positive IgM.
ConclusionsSARS-CoV-2 infection during pregnancy provides IgG antibodies to half of newborns. The presence of antibodies in the newborn is more likely when the infection has occurred in the second trimester of pregnancy.
La infección por SARS-CoV-2 durante la gestación y su repercusión en el recién nacido eran, en los primeros meses de la pandemia, desconocidas. Recientes estudios han aportado información sobre la afectación clínica en el recién nacido y su evolución.
En este trabajo se muestra como varia la inmunidad pasiva en el recién nacido en relación al momento de infección SARS-CoV-2 materno.
Población y métodoEstudio observacional, prospectivo y longitudinal en hospital de tercer nivel. Se recogieron datos epidemiológicos y clínicos de las madres y sus recién nacidos desde mayo de 2020 hasta junio 2021.
ResultadosSe han incluido un total de 109 madres y 109 neonatos. El 28,4% de las infecciones maternas fueron en el primer trimestre, el 24,8% en el segundo y el 58,8% en el tercero. El 56% de las infecciones maternas fueron sintomáticas, solo una gestante con infección respiratoria grave ingresó en cuidados intensivos. La edad gestacional media de los recién nacidos fue de 39 semanas, con un peso medio de 3232 g y un perímetro craneal de 35 cm. Ocho recién nacidos hijos de madre con SARS-CoV-2 requirieron ingreso en UCI neonatal: 2 por ictericia, 2 por distrés respiratorio, 1 por prematuridad moderada y 3 por otras causas no relacionadas con infección atribuible a SARS-CoV-2. Los anticuerpos tipo IgG fueron positivas en el 56,9% de los recién nacidos. De las madres infectadas durante el 1er trimestre, las IgG fueron positivas en el 32,2% de los recién nacidos, en el segundo trimestre resultaron positivos el 81,5% y en el tercero el 58,8%. Ningún neonato presentó IgM positivas.
ConclusionesLa infección por SARS-CoV-2 durante la gestación proporciona anticuerpos IgG a la mitad de los recién nacidos. La presencia de anticuerpos en el recién nacido es más probable cuando la infección se ha producido en el segundo trimestre de gestación.
The SARS-CoV-2 pandemic reached Spain in March 2020. Lack of knowledge of the repercussions of the infection in the newborn (NB) was a matter of great concern. Whether or not this infection could be transmitted intrauterine and the severity of perinatal SARS-CoV-2 infection were unknown. Different studies have provided information on the vertical transmission of the infection and its severity and have shown that maternal-foetal transmission is very rare1–7 and that so too is horizontal transmission of the infection in the first weeks of life when the recommended hygiene measures are followed.8 Neonatal infection is usually asymptomatic9–11 and only isolated cases with more severe symptoms have been reported.12 With the information we have available, it is safe to maintain mother-child contact and encourage direct breastfeeding in women with SARS-CoV-2 infection.
Another aspect that is not fully understood is the acquisition of immunity in newborns of women with SARS-CoV-2 infection during pregnancy. The passage of immunoglobulins through the placenta begins at 12–14 weeks of gestation and gradually increases in the third trimester.13 Some studies provide information on the transfer of IgG to newborns of women infected with SARS-CoV-2 during pregnancy.14–17
The morbidity of newborns born to mothers with SARS-CoV-2 is apparently higher. Different studies have found an increase in prematurity in newborns born to mothers with SARS-CoV-2.18,19
Pregnant women with SARS-CoV-2 infection also present a more serious evolution of the infection, with an increase in admissions to Intensive Care. They also have a higher incidence of pre-eclampsia.10,20–22
The reason for this study is to provide epidemiological information on the characteristics of pregnant women with COVID infection and their newborns and to evaluate the acquisition of maternal antibodies in newborns.
Study method and populationStudy design and data collectionThis was an observational, prospective, single-centre, longitudinal study in a tertiary care hospital. The study was carried out in the period between May 2020 and June 2021. There are 1,800 births per year at our centre. All pregnant women with a positive polymerase chain reaction test for SARS-CoV-2 (RT-PCR) taken from a nasopharyngeal sample and the respective newborns at 24 h of life were included. The women were routinely screened for SARS-CoV-2 following admission to the obstetrics emergency department. Screening was also carried out on all pregnant women with exposure to SARS-CoV-2 or with symptoms consistent with infection during pregnancy.
During the data collection period, our centre continued to be a reference centre for all the pregnant women in our district, except for those with severe symptoms that required admission to an Intensive Care Unit. They were grouped at another hospital.
Patient pathwayPregnant women with positive screening for SARS-CoV-2 infection were isolated in a single room. A specific delivery room was set up and, in the case of caesarean section, a COVID operating theatre as well. In newborns of women positive for SARS-CoV-2, a PCR was performed on nasopharyngeal aspirate at 24 h of life and they were accommodated in a single room with their parents. Newborns who required admission to the Neonatal Unit were isolated in an individual box until SARS-CoV-2 infection was ruled out.
Pregnant women infected during pregnancy with negative PCR for SARS-CoV-2 at the time of delivery were treated as healthy pregnant women and followed the usual pathway.
No pregnant woman was vaccinated for SARS-CoV-2 during pregnancy.
The serologies of the newborns were performed coinciding with the extraction of blood for neonatal screening, at 48 h of life. Both IgG and IgM were tested. For the analysis of IgG, manual immunochromatography against the N protein was used during the first months and subsequently chemiluminescence against the S protein.
Data on epidemiological, clinical, and laboratory test variables (maternal symptoms, type of delivery, height, weight, head circumference, serology and microbiology of nasopharyngeal aspirate) were collected. Maternal symptoms were considered mild when they were limited to upper respiratory tract symptoms or fever, moderate in cases requiring respiratory support and severe if they required admission to the critical care unit.
Prematurity is defined as birth that occurs before the completion of the 37th week of gestation. It can be classified as extremely premature (24−27 + 6 W G), very premature (28–31 + 6 W G), moderately premature (32−33 + 6 W G) and late premature (34–36 + 6 W G). The term low weight is defined as newborns weighing less than 2,500 g or below the 10th percentile of the appropriate weight for their gestational age according to population studies.
The statistical analysis was performed with SPSS®, version 22. Descriptive statistics and contingency tables were used.
The study was approved by the centre's Research Ethics Committee. Informed consent was obtained from the pregnant women for their enrolment in the study. Throughout the research process, the principles enshrined in the Declaration of Helsinki were observed.
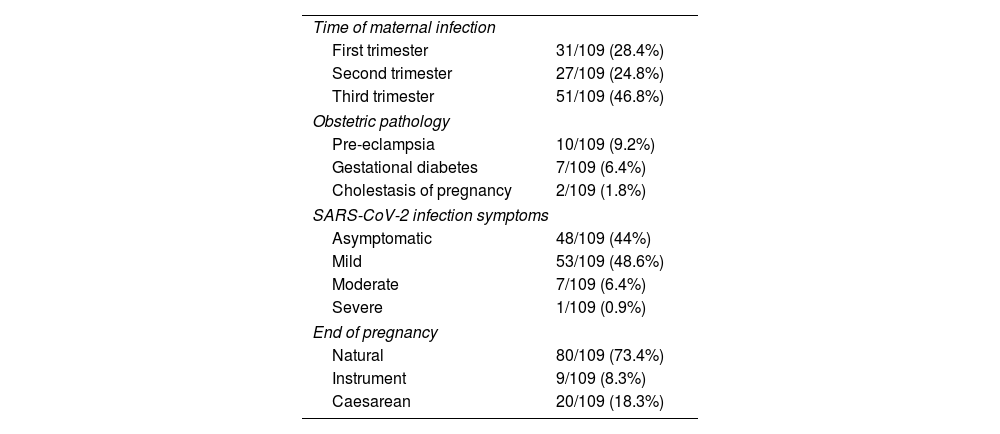
ResultsA total of 109 mothers and 109 newborns were included between May 2020 and June 2021. 28.4% (31/109) of maternal infections were diagnosed in the first trimester; 24.8% (27/109) in the second and 46.8% (51/109) in the third. Of the latter two, 15 pregnant women (29.4%) were diagnosed at the time of admission for delivery. 56% of the maternal infections were symptomatic; 7.3% of them required admission with respiratory support. Only one pregnant woman required admission to the Intensive Care Unit. Regarding obstetric disease, 9.2% had pre-eclampsia, 6.4% gestational diabetes and 1.8% cholestasis of pregnancy. The epidemiological and clinical data are summarised in Table 1.
Maternal characteristics.
| Time of maternal infection | |
| First trimester | 31/109 (28.4%) |
| Second trimester | 27/109 (24.8%) |
| Third trimester | 51/109 (46.8%) |
| Obstetric pathology | |
| Pre-eclampsia | 10/109 (9.2%) |
| Gestational diabetes | 7/109 (6.4%) |
| Cholestasis of pregnancy | 2/109 (1.8%) |
| SARS-CoV-2 infection symptoms | |
| Asymptomatic | 48/109 (44%) |
| Mild | 53/109 (48.6%) |
| Moderate | 7/109 (6.4%) |
| Severe | 1/109 (0.9%) |
| End of pregnancy | |
| Natural | 80/109 (73.4%) |
| Instrument | 9/109 (8.3%) |
| Caesarean | 20/109 (18.3%) |
With regard to the newborns, the mean gestational age was 39 weeks ± 1.3 weeks (range 30 + 5–41 + 2), with a mean birth weight of 3,232 ± 512 g (range 1,115−4,295 g) and a head circumference of 35 cm. 6% of the neonates were premature, six late premature and one very premature. 7.6% had low birth weight and 3.4% had a head circumference < p3. Delivery was by caesarean section in 18.3% of cases. The mean Apgar score at five minutes was 10 (range 6–10). Two patients (1.8%) required advanced neonatal resuscitation.
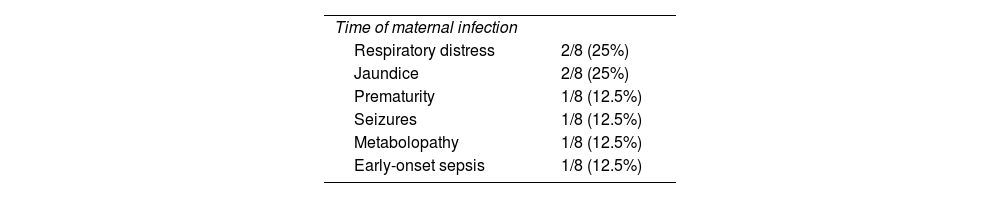
The causes of admissions to Neonatology are detailed in Table 2. Eight newborns (7.3%) required admission and respiratory distress was the most frequent cause, followed by pathological jaundice. The mean hospital stay was 11 ± 9 days (range 2–26 days). None of the patients died.
The PCR for SARS-CoV-2 in nasopharyngeal aspirate performed at 24 h of life in the newborns of women with positive screening at the time of delivery was negative in all cases. At 15 days of life, a nasopharyngeal aspirate was repeated in 18/30 (60%) of these newborns with a mother positive at the time of delivery, with only one positive case found.
No newborns presented positive IgM-type antibodies. IgG were positive in 56.9% of the newborns. Regarding the time of SARS-CoV-2 infection during the pregnancy of the mothers who contracted the infection in the first trimester, 32.2% of the newborns presented positive IgG; in the second trimester, 81.5% of newborns were positive and in the third trimester, 58.8% of newborns. This is summarised in Fig. 1.
DiscussionThe data obtained in the literature and in our study on vertical infection by SARS-CoV-2 does not seem to have serious consequences for the newborn, and nor does acquisition through community transmission.1–3 In our study, no case of vertical infection with virus detection in neonatal samples has been confirmed and nor have type M immunoglobulins been found in newborns that would indicate perinatal neonatal infection. Only one asymptomatic case of horizontal infection was detected in the first 15 days of life.
The epidemiological data found in our series on prematurity and low birth weight do not differ from the general population data.23 This is supported by various articles18,19,24 that show that there is no increased risk of premature delivery in asymptomatic pregnant women or in those with mild symptoms.
Only 60% of children born to mothers infected with SARS-CoV-2 during pregnancy had specific IgG against the virus. The transplacental passage of immunoglobulins is known to increase with advancing gestation13 and we would therefore expect to find a higher percentage of newborns with seroconversion during the third trimester. However, in our study, the majority of newborns acquired antibodies if the mother had presented the infection during the second trimester compared to the first and third trimesters. This is probably due to the fact that pregnant women with SARS-CoV-2 infection at the time of delivery are also included as part of the third trimester group. If we analyse newborns with infected mothers at the time of delivery, 67% of the cases have negative IgG (10/15). From these data, it can be deduced that the time between maternal infection and obtaining antibodies is a determining factor for the transfer of antibodies to the newborn. In this sense, we found a higher proportion of asymptomatic pregnant women (51%) in the third trimester compared to the second trimester (18.5%). This is due to the targeted screening of symptomatic patients or those with close contacts during the second trimester, as opposed to the general screening performed in the third. For these reasons, another determining variable in the transplacental passage of antibodies would be the viral load of the pregnant woman.
Vaccination during pregnancy is capable of generating immunity in newborns25,26 and ideally it should be done before the third trimester of pregnancy in order to protect the newborn sufficiently, as our study indicates. More studies on the acquisition of immunoglobulins with vaccination in both the mother and the newborn are necessary and the main factors associated with their seroconversion, such as the time elapsed from infection to delivery and viral load, must be determined, as suggested by this work.
The limitations of the study include the fact that it is a single-centre, retrospective study. It should also be noted that at that time in our centre, the value of the cycle threshold (Ct), a variable that could be correlated with the viral load of the pregnant woman and its consequent greater or lesser transplacental passage of antibodies, was not specified.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Presented at the XXVIII Congreso de Neonatología y Medicina Perinatal [28th Congress of Neonatology and Perinatal Medicine], held online on 25–29 October 2021.