Infective endocarditis (IE) after transcatheter aortic valve implantation (TAVI) is an emerging complication. There are incomplete and disparate data on its incidence. We present the experience of a single-centre of incidence, mortality and associated factors of IE after TAVI.
MethodsA retrospective observational study of IE cases in people who received a TAVI, between 06/01/2009 and 11/01/2017, in a university hospital, during a median follow-up period of 15.3 months (interquartile range [IQR] 9.1–36.2). Incidence, clinical, microbiological and prognostic data, and factors associated with IE after TAVI were analysed.
ResultsEleven patients with IE of 200 TAVI were detected. Global incidence: 5.5% (2.77 cases per 100 patient-year). The median of days from TAVI to IE was 112 (IQR 36–578), the in-hospital mortality rate was 36.4%, and the one-year mortality rate was 54.5%. All the organisms identified were gram-positive (4 Enterococcus faecalis, 3 coagulase-negative Staphylococcus). The patients with IE after TAVI were significantly younger (median 78 years, IQR 73–80, versus 82 years, IQR 79–84, p=.002), they had a higher EuroSCORE (5.1±2.4 versus 3.2±1.2, p<.001), and they more frequently had a history of neoplasia (18.2% versus 4.2%, p<.03).
ConclusionsIn our area, IE after TAVI has an incidence greater than that described in multicentre series, this is in line with the trend published in the literature. It leads to high mortality and is associated with a worse baseline clinical situation.
La endocarditis infecciosa (EI) sobre transcatheter aortic valve implantation (TAVI) es una complicación emergente. Existen datos incompletos y dispares sobre su incidencia. Se aporta la experiencia en nuestro centro sobre incidencia, mortalidad y factores asociados de la EI post-TAVI y se compara con datos de la literatura.
MétodosEstudio retrospectivo observacional de los casos de EI diagnosticados en pacientes que habían recibido TAVI, entre el 1 de junio de 2009 y el 1 de noviembre de 2017, en un centro universitario tras una mediana de seguimiento de 15,3 meses (rango intercuartil [RIC] 9,1-36,2). Se analizaron la incidencia, los datos clínicos, microbiológicos y pronósticos, y los factores asociados a EI post-TAVI.
ResultadosSe detectaron 11 pacientes con EI de 200 TAVI. Incidencia global: 5,5% (2,77 casos por 100 años-paciente). La mediana de tiempo hasta la EI post-TAVI fue de 112 días (RIC 36-578), la tasa de mortalidad intrahospitalaria fue del 36,4% y la mortalidad al año, del 54,5%. Todos los microorganismos identificados fueron grampositivos (4 Enterococcus faecalis, 3 Staphylococcus coagulasa negativo). Los pacientes con EI post-TAVI eran significativamente más jóvenes (mediana 78, RIC 73-80, frente a 82, RIC 79-84, p=0,002), tenían un EuroSCORE mayor (5,1±2,4 frente a 3,2±1,2, p<0,001) y más frecuentemente antecedentes de neoplasia (18,2% frente al 4,2%, p<0,03).
ConclusionesEn nuestro medio, la incidencia de EI post-TAVI es mayor que la descrita en series multicéntricas, lo que concuerda con la tendencia publicada en la literatura. Conlleva una elevada mortalidad y se asocia con una peor situación clínica basal.
The treatment of severe aortic valve stenosis with transcatheter aortic valve implantation (TAVI) is an established treatment in patients with high surgical risk.1 The number of procedures carried out is increasing in Europe and now exceeds the number of surgical aortic valve replacement interventions.2 Infective endocarditis (IE) after TAVI, although uncommon, is not a rare complication, and it is also very serious.3 Developed countries are seeing a decrease in the incidence of IE following rheumatic heart disease but an increase in cases of IE associated with implantable cardiac devices and prosthetic valves.4
Up to November 2017, three multicentre series of post-TAVI IE (E-TAVI) had been published (PubMed, keywords: TAVI, endocarditis).5–7 The E-TAVI in these studies had an incidence of around 1.1 per 100 patient-years, affected people of around 80 years of age, was caused mainly by Enterococcus spp., Staphylococcus aureus and coagulase-negative Staphylococci and had a high mortality rate (20–47% of patients died during admission and 66% after 1–2 years of follow-up). Surgical treatment can be of benefit in 11–14% of cases.
In contrast to the above figures, the single-centre series8,9 report a higher incidence of E-TAVI (over 3% in the first year) but a lower mortality rate (20–22%). Puls et al.8 emphasise how difficult it is to diagnose E-TAVI, because of the relatively limited experience in this context, the age of the patients and the complexity of interpreting the echocardiography findings.
As TAVI is carried out with increasing frequency, E-TAVI can be expected to become a not uncommon clinical manifestation. There is continued debate about the actual incidence of E-TAVI, the difficulty of diagnosis and survival with or without surgical treatment. The aim of this study is to discuss the experience obtained at one university hospital in terms of E-TAVI incidence, bacteriology and prognosis and compare it to that of TAVI patients who did not develop endocarditis.
MethodsStudy populationFrom 1 June 2009 to 1 November 2017 a total of 208 consecutive patients underwent TAVI in our centre's cardiology department. Eight patients who died peri-procedure within the first 48h were excluded (most of them in the first phase of the learning curve). All the included subjects had been diagnosed with severe symptomatic aortic stenosis and valve replacement surgery had been ruled out by a multidisciplinary team (Heart Team). Once the E-TAVI was diagnosed, the infectious diseases department was responsible for the antimicrobial treatment and the cardiovascular surgery department assessed the possibility of surgical treatment. The cases of endocarditis were identified retrospectively from computer records. Data related to the procedure, clinical and echocardiographic manifestations and outcome were retrieved from a prospectively updated database.
TAVI procedureA balloon-expandable Sapien prosthesis (Edwards Lifesciences) was implanted in 175 patients (87.5%) and 25 patients (12.5%) had self-expanding Portico (St. Jude Medical) and CoreValve (Medtronic) prostheses. The implant was transfemoral in all cases and antimicrobial prophylaxis was administered with cefazolin (1-g intravenous bolus 1h before the procedure). Mean follow-up after the procedure was 24.2 months (median: 15.3 months; P25–75: 9.1–36.2 months).
DefinitionsThe definitive diagnosis of E-TAVI was based on the modified Duke criteria.10 The deaths which occurred due to E-TAVI during hospitalisation were recorded as endocarditis-related deaths. Deaths occurring after hospital discharge were adjudicated individually after analysis of medical records. The cumulative incidence of E-TAVI related to time was calculated, as well as the overall rate per person-year.
Each patient's personal details and their previous general medical and cardiology history (transaortic gradient, aortic area, previous involvement of a valve other than the aortic valve and ejection fraction) were collected. The Charlson Comorbidity Index and the EuroSCORE (European System for Cardiac Operative Risk Evaluation) were calculated (calculated online at http://www.rccc.eu/ppc/indicadores/Charlson.html and in http://euroscore.org/calc.html, respectively).
The time between the procedure and the diagnosis of E-TAVI was quantified and the cases were classified as early (less than one year) or late. Last of all, the presenting symptoms, echocardiographic abnormalities, bacteria isolated in blood cultures, complications, antimicrobial treatment applied (empirical or adjusted to antibiogram) and outcome in terms of in-hospital death or death during follow-up were analysed.
Statistical analysisThe Kolmogorov–Smirnov test was used to evaluate the distribution normality of the quantitative variables. Continuous quantitative variables with a normal distribution are expressed in terms of mean and standard deviation. Continuous quantitative variables which did not have a normal distribution and discrete quantitative variables are expressed by median and interquartile range (25–75 percentile). Categorical variables are expressed in terms of absolute frequencies and percentages. According to whether distribution was normal or not, either Student's t-test or the non-parametric Mann–Whitney U test were used to compare the quantitative variables of the two study groups. The Chi-squared test and Fisher's exact test were used to compare qualitative variables. To assess the influence of different baseline variables on the E-TAVI rate and the rate of death during follow-up, a Cox regression analysis of survival free of these events with each of these variables (univariate analysis) was performed. The variables which in the univariate analysis were associated with a p<0.2 with a higher rate of events were included in a multivariate analysis (Cox regression, backward stepwise method based on the likelihood ratio).
p values <0.05 were considered significant. All statistical analyses were performed with the software programme SPSS v22 (SPSS 2016, Chicago, Illinois, United States).
Ethical considerationsThis was a retrospective observational study which had no influence on patient treatment. The data were treated completely anonymously following the instructions of the National Data Protection Authority Personal Data Protection Act 15/1999, of 13 December.
ResultsEleven cases of E-TAVI were diagnosed from 1 March 2013 to 31 January 2018, corresponding to an overall incidence of 5.5% (11/200). After a mean follow-up of 24.2 months, the cumulative incidence rate of E-TAVI was 2.77 per 100 patient-years (95% CI: 1.38–4.95). The cumulative incidence rates per 100 patient-years at 1, 2 and 3 years were, respectively, 4.30 (95% CI: 1.73–8.87), 4.32 (95% CI: 2.16–7.73) and 3.46 (95% CI: 1.73–6.20). The incidence was slightly higher in males (7.1%) than females (4.3%), but the difference was not significant.
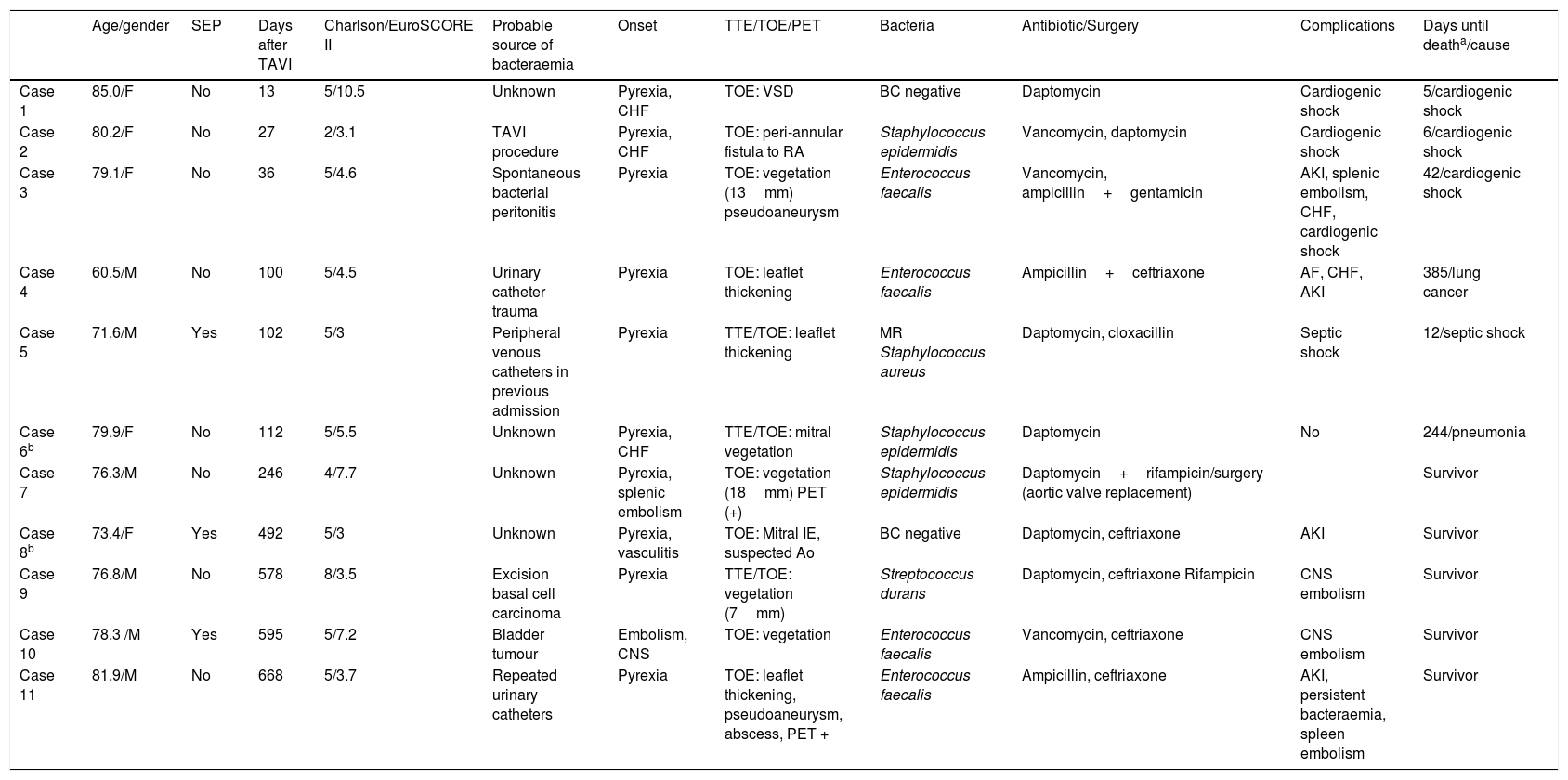
The characteristics of the cases of E-TAVI are shown in Table 1. All cases met confirmed E-TAVI criteria except two, which had probable E-TAVI. The patients were aged 60 to 85 years, with a median age of 78 (interquartile range [IQR] 73–80). Seven cases developed early endocarditis (less than one year after the procedure), with three of these cases being diagnosed during the same admission in which the TAVI was performed. The median time to E-TAVI was 112 days (P25–75: 36–578 days). The median Charlson Index was 5 and the mean EuroSCORE II was 5.1±2.4. Four patients (36.4%) died while in hospital for E-TAVI and associated with the procedure, but the one-year mortality rate climbed to 54.5% (two further patients died from causes unrelated to the E-TAVI). In all the cases in which the aetiological agent was detected, the bacteria isolated were Gram-positive cocci: four Enterococcus faecalis, three coagulase negative Staphylococci, one Staphylococcus aureus and one Streptococcus durans. An 18FDG-PET imaging test was performed in two cases, one of which was indicative of post-TAVI infection.
Characteristics of the cases diagnosed with E-TAVI.
| Age/gender | SEP | Days after TAVI | Charlson/EuroSCORE II | Probable source of bacteraemia | Onset | TTE/TOE/PET | Bacteria | Antibiotic/Surgery | Complications | Days until deatha/cause | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 85.0/F | No | 13 | 5/10.5 | Unknown | Pyrexia, CHF | TOE: VSD | BC negative | Daptomycin | Cardiogenic shock | 5/cardiogenic shock |
| Case 2 | 80.2/F | No | 27 | 2/3.1 | TAVI procedure | Pyrexia, CHF | TOE: peri-annular fistula to RA | Staphylococcus epidermidis | Vancomycin, daptomycin | Cardiogenic shock | 6/cardiogenic shock |
| Case 3 | 79.1/F | No | 36 | 5/4.6 | Spontaneous bacterial peritonitis | Pyrexia | TOE: vegetation (13mm) pseudoaneurysm | Enterococcus faecalis | Vancomycin, ampicillin+gentamicin | AKI, splenic embolism, CHF, cardiogenic shock | 42/cardiogenic shock |
| Case 4 | 60.5/M | No | 100 | 5/4.5 | Urinary catheter trauma | Pyrexia | TOE: leaflet thickening | Enterococcus faecalis | Ampicillin+ceftriaxone | AF, CHF, AKI | 385/lung cancer |
| Case 5 | 71.6/M | Yes | 102 | 5/3 | Peripheral venous catheters in previous admission | Pyrexia | TTE/TOE: leaflet thickening | MR Staphylococcus aureus | Daptomycin, cloxacillin | Septic shock | 12/septic shock |
| Case 6b | 79.9/F | No | 112 | 5/5.5 | Unknown | Pyrexia, CHF | TTE/TOE: mitral vegetation | Staphylococcus epidermidis | Daptomycin | No | 244/pneumonia |
| Case 7 | 76.3/M | No | 246 | 4/7.7 | Unknown | Pyrexia, splenic embolism | TOE: vegetation (18mm) PET (+) | Staphylococcus epidermidis | Daptomycin+rifampicin/surgery (aortic valve replacement) | Survivor | |
| Case 8b | 73.4/F | Yes | 492 | 5/3 | Unknown | Pyrexia, vasculitis | TOE: Mitral IE, suspected Ao | BC negative | Daptomycin, ceftriaxone | AKI | Survivor |
| Case 9 | 76.8/M | No | 578 | 8/3.5 | Excision basal cell carcinoma | Pyrexia | TTE/TOE: vegetation (7mm) | Streptococcus durans | Daptomycin, ceftriaxone Rifampicin | CNS embolism | Survivor |
| Case 10 | 78.3 /M | Yes | 595 | 5/7.2 | Bladder tumour | Embolism, CNS | TOE: vegetation | Enterococcus faecalis | Vancomycin, ceftriaxone | CNS embolism | Survivor |
| Case 11 | 81.9/M | No | 668 | 5/3.7 | Repeated urinary catheters | Pyrexia | TOE: leaflet thickening, pseudoaneurysm, abscess, PET + | Enterococcus faecalis | Ampicillin, ceftriaxone | AKI, persistent bacteraemia, spleen embolism | Survivor |
AKI: acute kidney injury; BC: blood cultures; CHF: congestive heart failure; CNS: central nervous system; F: female; M: male; MR: methicillin resistant; RA: right atrium; SEP: self-expanding prosthesis; TOE: transoesophageal echocardiogram; TTE: transthoracic echocardiogram; VSD: ventricular septal defect.
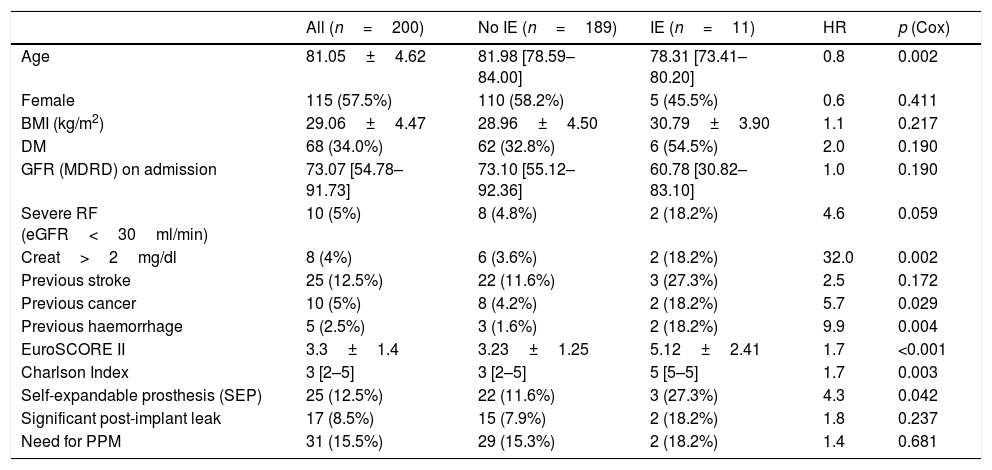
Table 2 shows the differences between patients with E-TAVI and those who, having had TAVI, did not develop IE during the follow-up period. Patients with IE received TAVI at a lower median age and were more often treated with a self-expanding prosthesis than those who were not diagnosed with IE. Patients with E-TAVI also had a worse baseline status, as reflected in a higher proportion of elevated plasma creatinine levels and previous cancer and haemorrhages, and by a significantly higher Charlson Index and EuroSCORE II.
Prevalence of different clinical variables pre- and post-procedure in all patients, patients without IE and patients with IE. Influence of these variables on E-TAVI-free survival.
| All (n=200) | No IE (n=189) | IE (n=11) | HR | p (Cox) | |
|---|---|---|---|---|---|
| Age | 81.05±4.62 | 81.98 [78.59–84.00] | 78.31 [73.41–80.20] | 0.8 | 0.002 |
| Female | 115 (57.5%) | 110 (58.2%) | 5 (45.5%) | 0.6 | 0.411 |
| BMI (kg/m2) | 29.06±4.47 | 28.96±4.50 | 30.79±3.90 | 1.1 | 0.217 |
| DM | 68 (34.0%) | 62 (32.8%) | 6 (54.5%) | 2.0 | 0.190 |
| GFR (MDRD) on admission | 73.07 [54.78–91.73] | 73.10 [55.12–92.36] | 60.78 [30.82–83.10] | 1.0 | 0.190 |
| Severe RF (eGFR<30ml/min) | 10 (5%) | 8 (4.8%) | 2 (18.2%) | 4.6 | 0.059 |
| Creat>2mg/dl | 8 (4%) | 6 (3.6%) | 2 (18.2%) | 32.0 | 0.002 |
| Previous stroke | 25 (12.5%) | 22 (11.6%) | 3 (27.3%) | 2.5 | 0.172 |
| Previous cancer | 10 (5%) | 8 (4.2%) | 2 (18.2%) | 5.7 | 0.029 |
| Previous haemorrhage | 5 (2.5%) | 3 (1.6%) | 2 (18.2%) | 9.9 | 0.004 |
| EuroSCORE II | 3.3±1.4 | 3.23±1.25 | 5.12±2.41 | 1.7 | <0.001 |
| Charlson Index | 3 [2–5] | 3 [2–5] | 5 [5–5] | 1.7 | 0.003 |
| Self-expandable prosthesis (SEP) | 25 (12.5%) | 22 (11.6%) | 3 (27.3%) | 4.3 | 0.042 |
| Significant post-implant leak | 17 (8.5%) | 15 (7.9%) | 2 (18.2%) | 1.8 | 0.237 |
| Need for PPM | 31 (15.5%) | 29 (15.3%) | 2 (18.2%) | 1.4 | 0.681 |
BMI: body mass index; Creat: creatinine; DM: diabetes mellitus; GFR: glomerular filtration rate; HR: hazard ratio; IE: infective endocarditis; PPM: permanent pacemaker; RF: renal failure.
The quantitative variables that follow a normal distribution are shown as mean±SD. The quantitative variables that do not follow a normal distribution are shown as median [25th percentile−75th percentile]. The categorical variables are presented as observed frequencies (%).
In the multivariate analysis, a history of cancer [HR: 6.5; 95% CI: 1.3–32.7; p=0.022) and the EuroSCORE II (HR: 1.7; 95% CI: 1.3–2.3; p<0.001) were independently associated with a higher rate of E-TAVI.
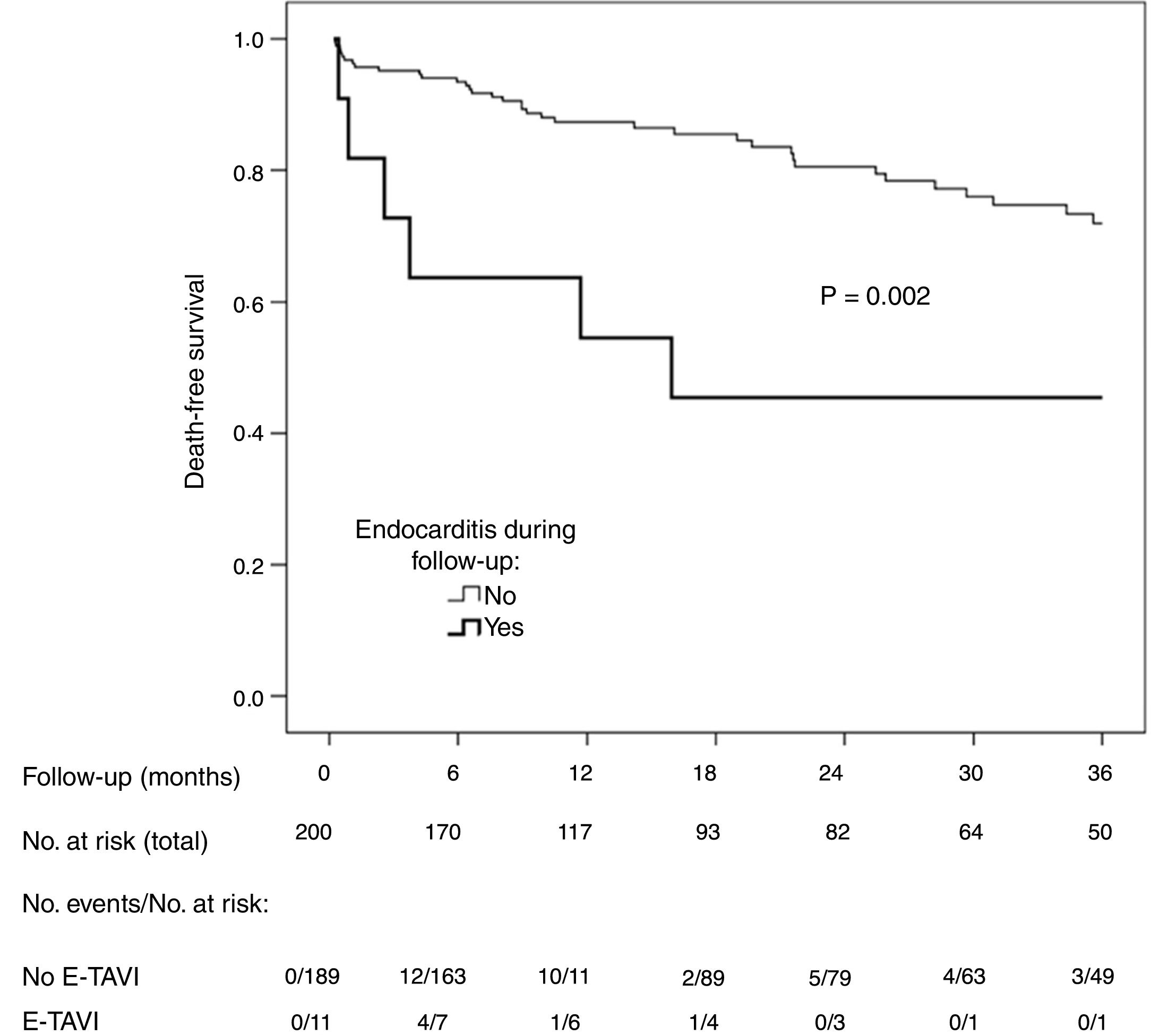
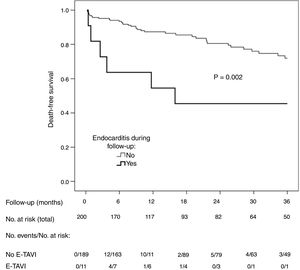
A total of 51 patients (25.5%) died during follow-up (12.6 deaths per 100 patient-years). The incidence of death was higher among the patients with E-TAVI (54.5% vs 23.8%; p=0.033) (Fig. 1). E-TAVI (HR: 3.2; 95% CI: 1.3–7.7; p=0.011), being female (HR: 2.0; 95% CI: 1.1–3.9; p=0.036), diabetes mellitus (HR: 1.8; 95% CI: 1.0–3.1; p=0.049) and a history of COPD (HR: 3.8; 95% CI: 1.9–7.6; p<0.001) were independently associated with a higher mortality rate after TAVI.
DiscussionThe data provided by the experience in one university hospital after 200 TAVIs and a median follow-up of over one year reveal an incidence of E-TAVI greater than that described in multicentre series. Our data also show E-TAVI to be associated with a poorer baseline status compared to patients who did not develop IE. E-TAVI tends to affect people in their late seventies and has a high mortality rate of over 50% after one year.
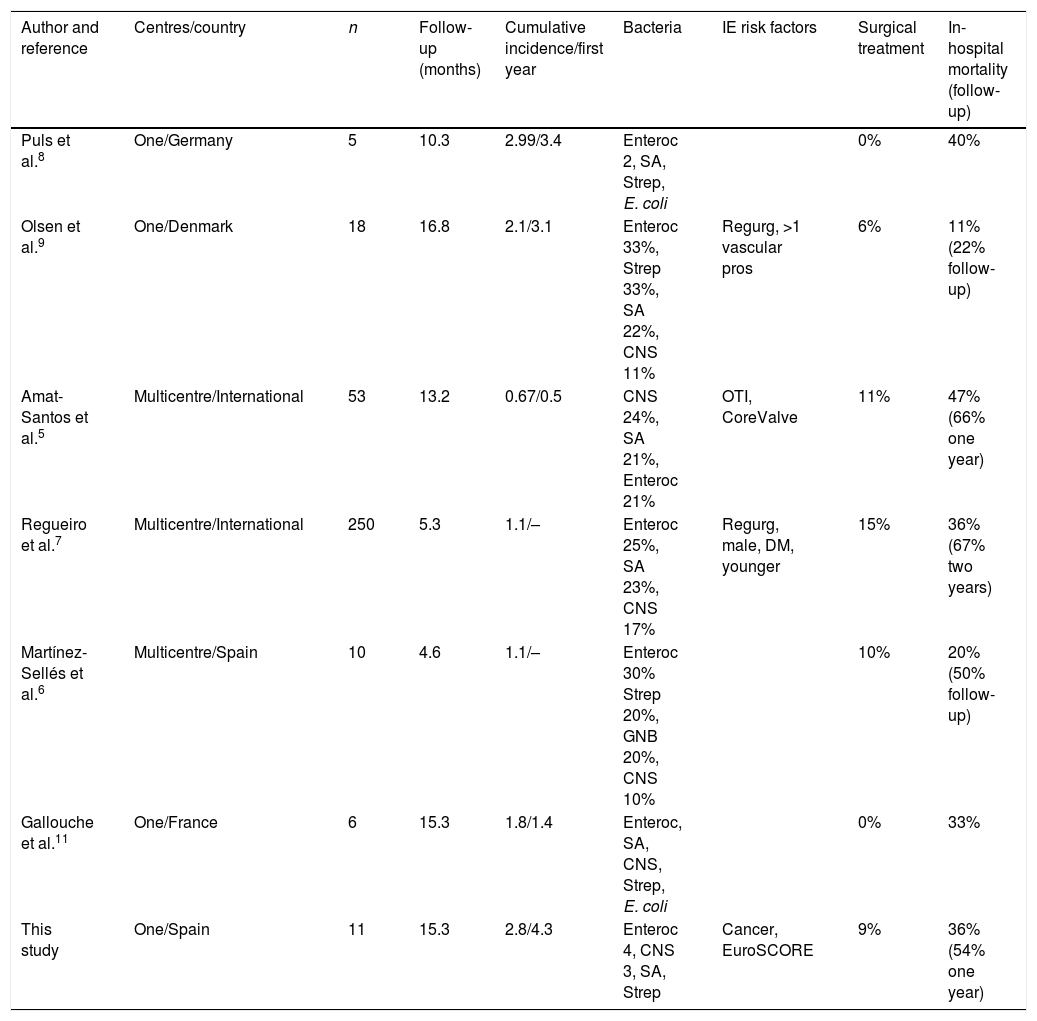
The incidence of E-TAVI in the literature varies greatly from one series to another. Eight years after the technique was introduced in our hospital, IE has been diagnosed in more than 5% of patients. The rate per 100 patient-years (2.77) is greater than that described in multicentre series (0.6–1.1%) and more in line with the rates reported by single hospitals (1.8–2.1%).6,7,9,11 In a previous single-centre study, the cumulative incidence of E-TAVI was 3.4%.8 The possibility should be considered that multicentre series underestimate the incidence of E-TAVI, given that the symptoms of IE in these populations can be extremely atypical and some cases might not be recorded as such unless there is very strict follow-up and a systematic search for symptoms. In contrast, in the single-centre series, systematic reviews of these patients looking specifically for the signs and symptoms and causes of death may be more thorough, especially taking into account that many of these patients do not come from the referral centre area, necessitating very specific access and follow-up of the series. Furthermore, the inclusion of patients right from the introduction of the technique, with the learning curve included, may have led to a somewhat higher incidence. Moreover, the change in working culture in a haemodynamics laboratory by moving towards what is essentially a more surgical environment involves a learning process. Also having an effect is the move towards simplifying the procedure, by avoiding intubation for example, which has proven effective in reducing hospital stay, infections and in-hospital mortality rates.12 The single-centre series, as in our case, may reflect the search specifically dedicated to the complication of IE with coordinated collaboration between the infectious diseases and cardiology departments, and not simply searching the database of complications for a single unit or department. This collaboration has been found to be effective in reducing hospital stays, mortality rates and defining of infectious processes.13
However, in a recent randomised multicentre study14 with 145 patients who had TAVI (Core Valve) and 135 cases who had surgical implantation, even in lower-risk populations, with a five-year follow-up, the incidence of IE was 11.3% for TAVI cases and 5.8% for surgical cases. These are higher incidences than those reported in intermediate-risk populations, such as PARTNER 2,1 and in other multicentre studies.5–7 In the surgical series, the Swedish registry data base SWEDEHEART, with more than 26,580 patients,15 showed that the incidence of IE was higher in the first year (0.99%). The probable higher incidence of IE after TAVI than after valve surgery could be explained by the fact that, after TAVI, the old valve is left as residual tissue, with nooks and crannies which are more susceptible to contamination by circulating microorganisms.
In two patients (cases 6 and 8), the main echocardiographic criterion for IE was the involvement of the native mitral valve, without data on the aortic prosthesis. Both cases have been included as E-TAVI because they met diagnostic criteria for definitive IE and for the consequent infection of the aortic prosthesis if there is vegetation in the mitral valve. This situation has been described before by Martínez-Sellés et al.,6 who reported four IE exclusively on the mitral valve out of a total of ten cases post-TAVI. Another limitation of the study derives from the difficulty in defining the cause of death. In a series of autopsies in deceased patients after TAVI in whom cardiogenic shock predominated initially as cause and then septic shock after 30 days, unexpected findings were revealed in more than half of the cases, with some of them being attributable to undiagnosed IE.16
The fact that the follow-up was longer after TAVI, with a median of over one year, similar to that published in other single-centre studies and higher than that reported in some multicentre studies (around six months),5–9 may have contributed to a higher cumulative incidence. Our data relating to age, the predominance of Gram-positive microorganisms as causative agent and the high mortality rate are completely consistent with the published data (Table 3). The relative importance of Enterococcus spp., more common as an aetiological agent in E-TAVI than in IE over native valve or conventional prosthetic valve, may be a consequence of advanced age (around 80 years) and the associated prevalence, in this population, of urinary (prostatic, bladder) and colon problems.3 The inguinal approach for TAVI is also a factor to be taken into account, as most cases of IE occur early. A possible correlation with the procedure cannot therefore be ruled out.
Published series of post-TAVI IE.
| Author and reference | Centres/country | n | Follow-up (months) | Cumulative incidence/first year | Bacteria | IE risk factors | Surgical treatment | In-hospital mortality (follow-up) |
|---|---|---|---|---|---|---|---|---|
| Puls et al.8 | One/Germany | 5 | 10.3 | 2.99/3.4 | Enteroc 2, SA, Strep, E. coli | 0% | 40% | |
| Olsen et al.9 | One/Denmark | 18 | 16.8 | 2.1/3.1 | Enteroc 33%, Strep 33%, SA 22%, CNS 11% | Regurg, >1 vascular pros | 6% | 11% (22% follow-up) |
| Amat-Santos et al.5 | Multicentre/International | 53 | 13.2 | 0.67/0.5 | CNS 24%, SA 21%, Enteroc 21% | OTI, CoreValve | 11% | 47% (66% one year) |
| Regueiro et al.7 | Multicentre/International | 250 | 5.3 | 1.1/– | Enteroc 25%, SA 23%, CNS 17% | Regurg, male, DM, younger | 15% | 36% (67% two years) |
| Martínez-Sellés et al.6 | Multicentre/Spain | 10 | 4.6 | 1.1/– | Enteroc 30% Strep 20%, GNB 20%, CNS 10% | 10% | 20% (50% follow-up) | |
| Gallouche et al.11 | One/France | 6 | 15.3 | 1.8/1.4 | Enteroc, SA, CNS, Strep, E. coli | 0% | 33% | |
| This study | One/Spain | 11 | 15.3 | 2.8/4.3 | Enteroc 4, CNS 3, SA, Strep | Cancer, EuroSCORE | 9% | 36% (54% one year) |
CNS: coagulase-negative Staphylococci; DM: diabetes mellitus; Enteroc: Enterococcus spp.; GNB: Gram-negative bacilli; OTI: orotracheal intubation; Pros: prosthesis; Regurg: valvular regurgitation; SA: Staphylococcus aureus; Strep: Streptococcus spp.
The echocardiographic diagnosis of E-TAVI is more difficult because of the lack of data compared to other types of IE. Diagnostic sensitivity may improve with three-dimensional echocardiography.17 Nuclear medicine techniques, such as 18F-FDG-PET/CT, with a sensitivity of 75%, may facilitate diagnosis.18 Only one patient (9.1%) had surgical treatment, consistent with previous reports which range from 6% in Olsen et al.9 to 15% in Regueiro et al.7 The need for surgical replacement of the aortic valve after TAVI will become increasingly important and may create problems with the surgical technique, as it can differ from the conventional procedure. Under optimal conditions, Guenther et al.19 obtained acceptable survival with multidisciplinary support.
The association of E-TAVI with a younger age at the time of the implant has already been described by Regueiro et al.7 and contrasts with the other factors independently related to E-TAVI, which indicate a worse clinical status (higher proportion of cancer and higher EuroSCORE II). In the above series, the EuroSCORE was associated with a higher mortality rate.7 The presence of cancer has been associated with a higher mortality rate in patients with IE.20 It has also been suggested that IE could be a warning sign, particularly in cancers of gastrointestinal origin.21 Despite the limitations imposed by the retrospective nature of our study in a single centre and the limited number of IE, the data provided show that E-TAVI is becoming increasingly more common and is not only challenging to diagnose and treat, but requires a high index of suspicion and planned and coordinated collaboration between cardiology and infectious diseases. Collating information on the re-admission of these patients in the centres of origin can also help prevent underestimation of the real incidence of E-TAVI. It would be helpful to have cumulative incidence studies with longer follow-up periods to determine the magnitude of the incidence calculated in this study. One basic factor if the incidence of E-TAVI is to be reduced is to reinforce bacteraemia prevention measures in patients undergoing a TAVI, particularly peri-procedure, but also with insertion of urinary catheters or intravenous cannulation, and in cases possibly related to gastrointestinal disease.
It can be concluded that, in our setting, the incidence of E-TAVI is greater than described in multicentre series, and this is in line with the trend reported in the literature. E-TAVI has a high mortality rate and is associated with a poor baseline status (a high EuroSCORE and the presence of cancer).
Conflicts of interestThe authors declare that they have no conflict of interests.
Please cite this article as: Rodríguez-Vidigal FF, Nogales-Asensio JM, Calvo-Cano A, González-Fernández R, Martínez-Carapeto A, Gómez-Sanchez I, et al. Endocarditis infecciosa después de TAVI: aportaciones de la experiencia en un único centro sobre la incidencia y los factores asociados. Enferm Infecc Microbiol Clin. 2019;37:428–434.