The annual incidence of tuberculosis (TB) from Mycobacterium bovis in humans has considerably declined in industrialised countries since the early twentieth century. The objective of this study was to determine the epidemiological, clinical and microbiological characteristics of patients with this illness in Castile and León (CyL).
MethodsRetrospective study of all M. bovis TB cases in CyL over a 10-year period, comparing the risk factors, the epidemiology and the clinical course between pulmonary (PTB) and extrapulmonary TB (EPTB).
Results75 cases of TB were due to M. bovis: 45 PTB and 31 EPTB. The annual incidence of TB due to M. bovis was 0.3 cases per 100,000. It remained stable between the first and second five-year period (0.27 vs. 0.33, p=0.656). However, the overall incidence of TB fell in both five-year periods (13.58 vs. 10.71, p<0.0001). The mean age was 66.2+21.3 years, mainly men (63%) and Spanish patients (92%). PTB was significantly more frequent in men, aged over 66 years, with immunosuppressive conditions or who were smokers. Mortality was 9%, associated with higher age, immunosuppression or treatment different from that recommended by the WHO.
ConclusionsThe incidence of M. bovis TB in CyL was higher than that for Spain and for other European countries, and remained stable despite the decreased the TB due to MTC. It affected mostly Spanish-born patients who lived in rural areas and with a high mean age.
La incidencia anual de tuberculosis (TB) humana por Mycobacterium bovis ha disminuido considerablemente en los países industrializados desde inicios del siglo XX. El objetivo de este estudio fue conocer las características epidemiológicas, clínicas y microbiológicas de esta enfermedad en Castilla y León (CyL).
MétodosEstudio retrospectivo de los casos de TB por M. bovis de CyL en un periodo de 10 años, comparando la epidemiología, los factores de riesgo y la evolución entre las formas pulmonares (TBP) y extrapulmonares (TBEP).
ResultadosSe recopilaron 75 casos de TB por M. bovis: 45 TBP y 31 TBEP. La incidencia acumulada de TB por M. bovis fue de 0,3 casos por 100.000 habitantes. Se mantuvo estable entre el primer y el segundo quinquenio (0,27 vs. 0,33, p=0,656), a pesar del descenso de la incidencia global de la TB (13,58 vs. 10,71, p<0,0001). La edad media fue de 66,2+21,3 años, principalmente varones (63%) y nacidos en España (92%). TBP fue significativamente más frecuente en varones, mayores de 66 años, con inmunosupresión o fumadores. La mortalidad fue del 9%, asociada a la edad, a la inmunosupresión o a un tratamiento diferente al recomendado por la OMS.
ConclusionesLa incidencia de TB por M. bovis en CyL es superior a la de España y otros países europeos, y se mantuvo estable a pesar del descenso de la TB por MTC. Afectó mayoritariamente a población nacida en España que vivía en zonas rurales y con elevada media de edad.
Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex (MTC), is a zoonotic pathogen that primarily infects cattle. Humans are infected by inhaling aerosols contaminated with the mycobacterium, consuming unpasteurised dairy products or due to continuous and close contact with infected animals.1 Human-to-human transmission has occasionally been described both in immunocompetent and immunosuppressed patients.2,3 The MTC species are grouped into eight closely phylogenetically related lines. The clinical, radiological and pathological features of the main MTC species which cause tuberculosis (TB) in humans—M. tuberculosis, Mycobacterium africanum and M. bovis—are indistinguishable.1 Nevertheless, the differentiation thereof is of great epidemiological and therapeutic interest. Historically, taxonomic separation was based on phenotype characteristics, antibiotic resistance, geographical distribution and host affinity,1 but these have been replaced with faster, more accurate and more specific molecular methods.4,5
The annual incidence of M. bovis cases in humans has decreased considerably in industrialised countries since the beginning of the 20th century, as a result of eradication campaigns in animals. Currently, it represents less than 2% of all TB cases, although there may be areas and populations in which this prevalence is greater.6–8 Most of the cases diagnosed are attributed to the reactivation of a latent infection acquired either before the pasteurisation era or in non-industrialised countries.6–11
Bovine TB was once endemic in the autonomous community of Castile and León (CyL) but is now being eradicated thanks to the implementation of a nationwide bovine TB eradication programme since 1985.12 The prevalence of infected herds has been reduced from 5.11% in 2006 to 1.93% in 2015.13 However, there is limited information on the significance and evolution of human TB caused by M. bovis in this community. This study's endpoint was to determine the microbiological, clinical and epidemiological characteristics of M. bovis infection in CyL based on pulmonary and extrapulmonary locations, as well as to compare it with epidemiological reports of TB due to MTC in this autonomous community.
Materials and methodsA retrospective study of all TB cases due to M. bovis collected in the laboratory information systems (LIS) of 14 public hospitals in CyL (autonomous community comprising 9 provinces and 14 Health Areas) between 2006 and 2015, both inclusive. One isolation was considered per patient. Patients with M. bovis BCG TB were excluded. Sample cultures were performed at each hospital. Phenotype and/or genotype identification and studies on antibiotic sensitivity to first-line anti-tuberculosis drugs were carried out in the reference laboratories of each Health Area. Fifteen strains (20%) were identified using GenoType MTBC hybridisation probes (HAIN Lifescience, Germany). Forty-four strains (59%) were identified at the Spanish National Mycobacteria Centre (Instituto de Salud Carlos III, Majadahonda, Madrid, Spain) using the genotype method described by Rodríguez et al.,7 based on the amplification of the gyrB gene and the detection of region of difference 1 (RD1), as well as the detection of the C169G mutation (H57D) in the pncA gene as an additional differentiating element for M. bovis and Mycobacterium caprae. In 16 strains (21%), mono-resistance to pyrazinamide (PZA) was considered the main identification criterion for M. bovis, alongside other morphological and biochemical characteristics unique to the species.1
The medical histories of all patients with TB due to M. bovis were reviewed. Demographic, bacteriological and clinical data were gathered, as well as data related to risk factors, treatment and outcome, in a database designed for this study. Moreover, the total number of confirmed TB cases was quantified by Health Area in order to estimate the proportion of TB due to M. bovis.
Patients were grouped into two categories according to disease location: pulmonary (PTB) and extrapulmonary (EPTB). The latter category included patients with or without concomitant pulmonary involvement and pleural TB.
The risk factors included in the study were: (a) rural life, patients living in populations of less than 2000 inhabitants or who worked in agriculture; (b) animal contact, people in direct contact with cattle; (c) travel abroad, patients who come from or who have travelled to a TB endemic country; (d) immunosuppressive conditions, patients with end-stage kidney disease or who have received organ transplants or immunosuppressant treatments or who have other immunosuppressant conditions indicated in their medical history, excluding patients co-infected with HIV or diabetes mellitus; (e) HIV status; (f) diabetes mellitus; (g) smoker; and (h) no risk factors, patients who had none of the aforementioned risk factors.
Treatments were divided into two categories: (a) the treatment recommended by the World Health Organisation (WHO) for PZA-resistant MTC,14 consisting of two months of isoniazid (INH), rifampin (RIF) and ethambutol (ETB), followed by seven months of INH and two months of RIF and (b) others, which include combinations of INH, RIF, ETB and PZA, other combinations containing PZA or treatments which lasted less than or more than nine months.
Patient outcomes were initially recorded as resolution, death, relapse or unknown. These were then reclassified as resolution and death in order to study the influence of various risk factors on outcome.
The results were analysed using the SPSS programme (Statistical Package for Social Sciences, version 20.0, SPSS Inc., Chicago, IL, USA). Qualitative variables were analysed using the χ2 test or Fisher's exact test, and quantitative variables using the Student's t-test. Differences were considered statistically significant if p was <0.05.
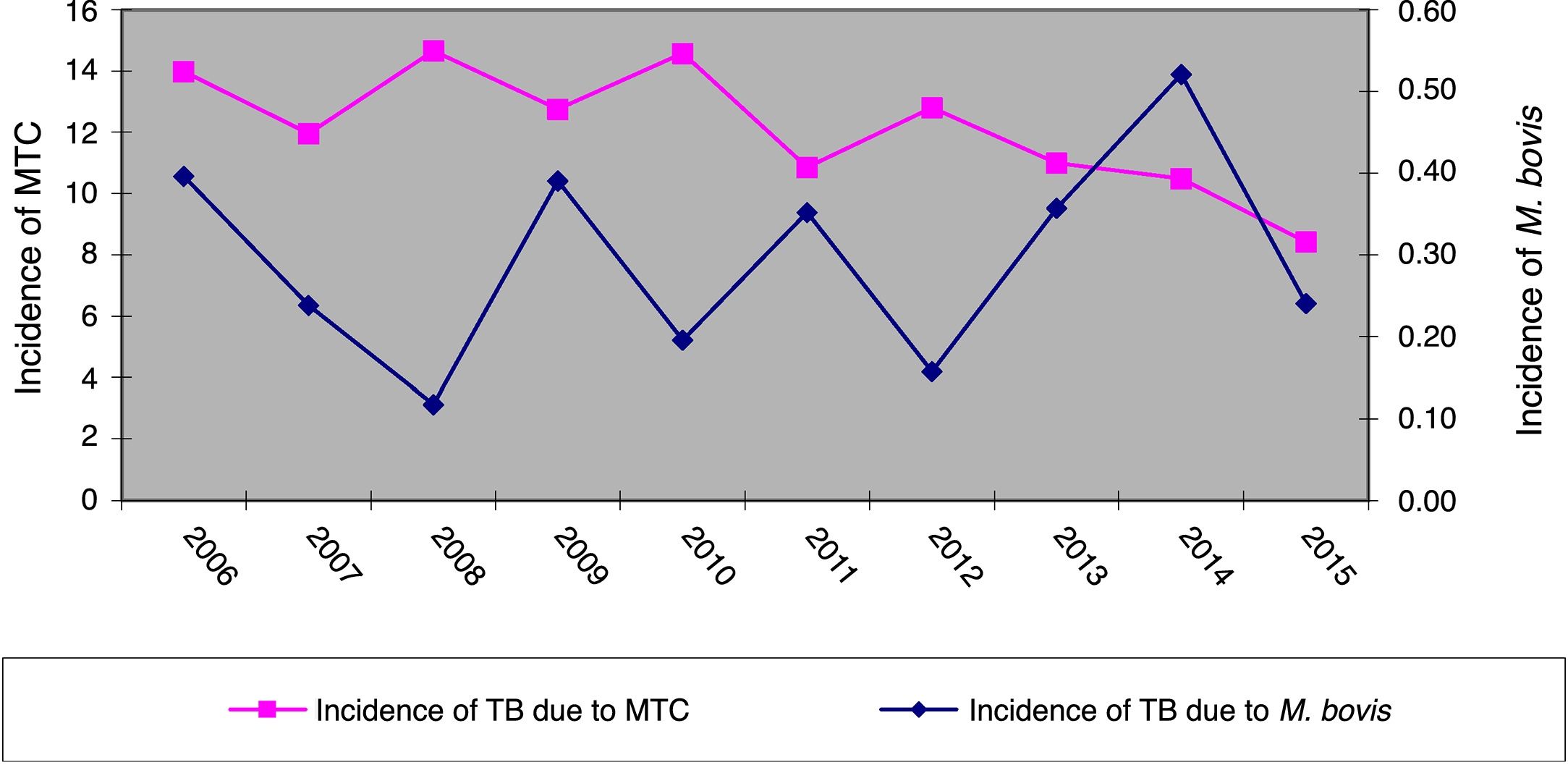
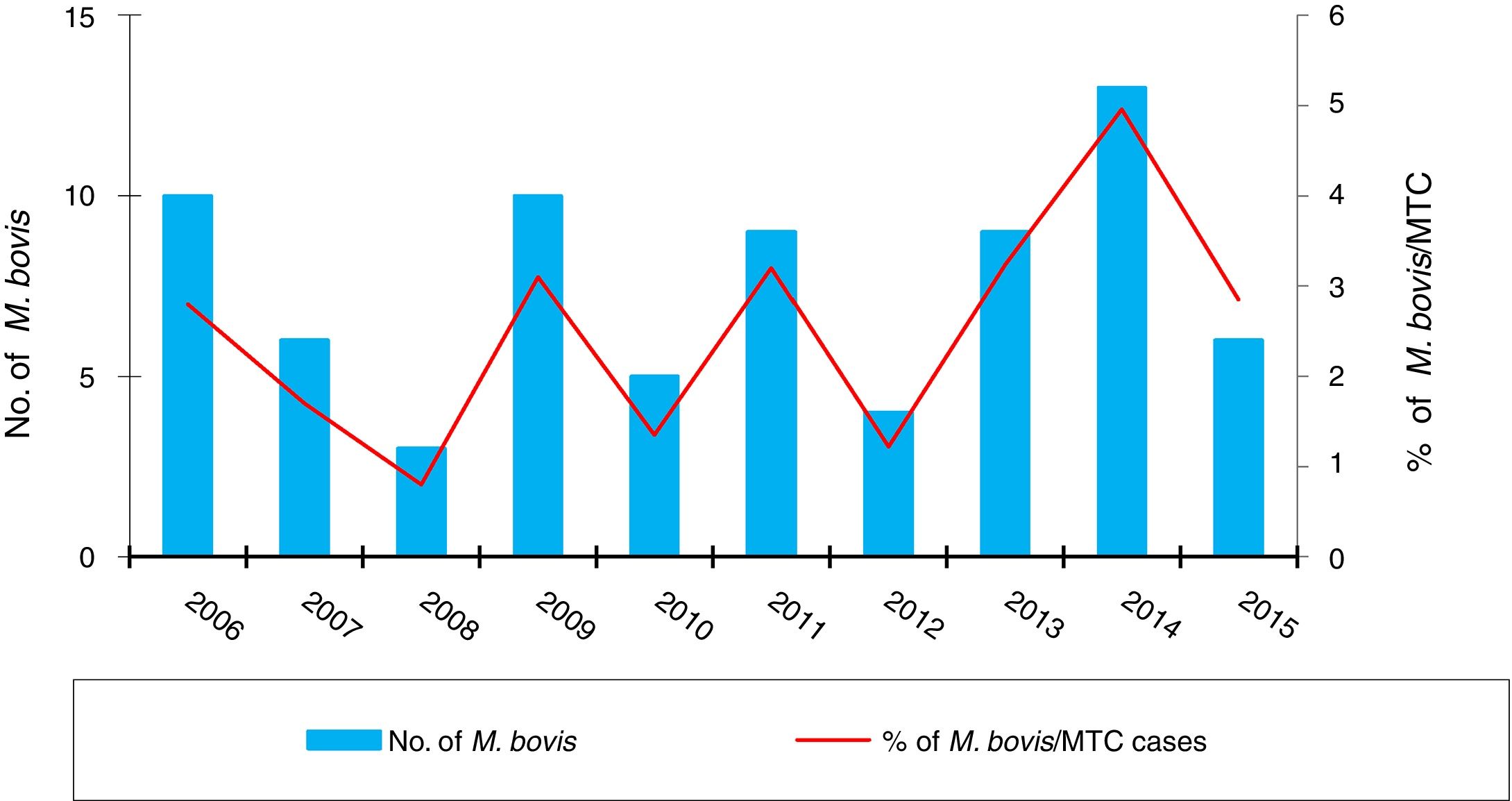
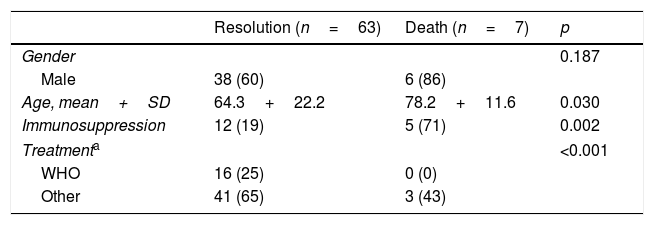
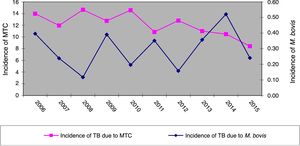
ResultsOver the 10 years included in the study, 3080 cases of TB were confirmed in CyL, of which 75 (2.43%) were due to M. bovis and 3 (0.10%) to M. caprae. The overall incidence of TB decreased significantly between the first and second five-year periods (13.58 vs. 10.71 cases per 100,000 inhabitants, p<0.0001). However, the incidence attributed to M. bovis remained stable (0.27 vs. 0.33 cases per 100,000 inhabitants, p=0.656) (Fig. 1). As a result, the proportion of TB due to M. bovis in relation to the total number of MTC cases showed a growing trend across both five-year periods (1.8 vs. 2.85%, p=0.001). This was more due to a decrease in MTC TB than to an increase in TB attributed to M. bovis (Fig. 2).
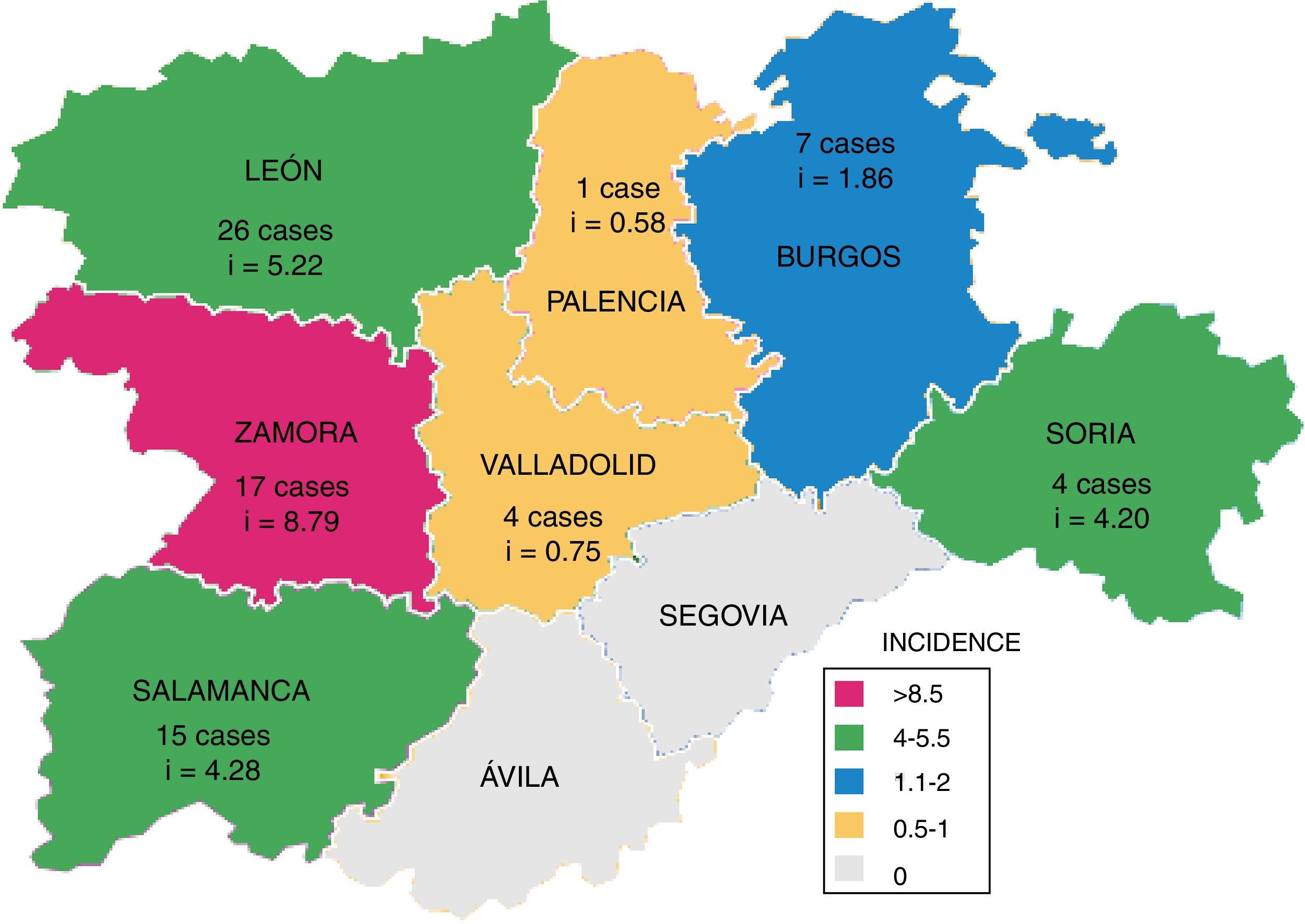
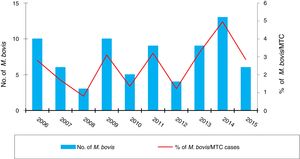
Fig. 3 shows the number of cases of TB due to M. bovis and the cumulative incidence per province, which was significantly higher in westerly provinces (León, Zamora and Salamanca; p=0.031).
PTB was predominant in this population, accounting for 44 cases (59%). Of the 31 patients who presented with EPTB, 5 also had PTB. Disseminated (26%) and lymphatic TB (26%) were the most common forms of EPTB. Others included bone and/or joint TB (5 cases), cutaneous TB (4 cases), genitourinary TB (2 cases), pleural TB (one case) and digestive TB (one case).
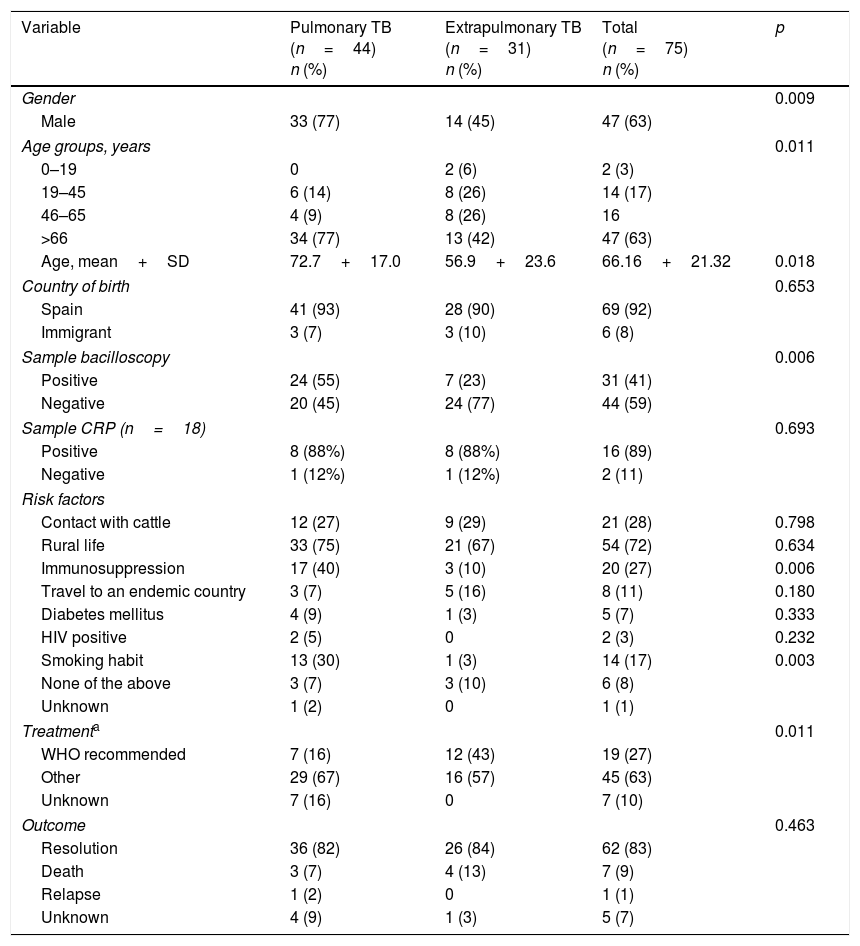
The microbiological, clinical and epidemiological characteristics of TB due to M. bovis are shown in Table 1, based on the location of the disease. PTB was significantly more common in men and in patients with a higher mean age. There were only two cases of childhood TB, where both children had been born in Spain and travelled to their parents’ country of origin (Morocco). Most patients had been born in Spain (92%) and the rest came from Morocco (3 cases), Romania (one case), Thailand (one case) and “unspecified” (one case). No association was found between disease location and the patient's country of origin (p=0.687). Patients with more bacilliferous forms presented with PTB (p=0.006).
Characteristics of pulmonary and extrapulmonary Mycobacterium bovis infections in Castile and León, 2006–2015.
| Variable | Pulmonary TB (n=44) n (%) | Extrapulmonary TB (n=31) n (%) | Total (n=75) n (%) | p |
|---|---|---|---|---|
| Gender | 0.009 | |||
| Male | 33 (77) | 14 (45) | 47 (63) | |
| Age groups, years | 0.011 | |||
| 0–19 | 0 | 2 (6) | 2 (3) | |
| 19–45 | 6 (14) | 8 (26) | 14 (17) | |
| 46–65 | 4 (9) | 8 (26) | 16 | |
| >66 | 34 (77) | 13 (42) | 47 (63) | |
| Age, mean+SD | 72.7+17.0 | 56.9+23.6 | 66.16+21.32 | 0.018 |
| Country of birth | 0.653 | |||
| Spain | 41 (93) | 28 (90) | 69 (92) | |
| Immigrant | 3 (7) | 3 (10) | 6 (8) | |
| Sample bacilloscopy | 0.006 | |||
| Positive | 24 (55) | 7 (23) | 31 (41) | |
| Negative | 20 (45) | 24 (77) | 44 (59) | |
| Sample CRP (n=18) | 0.693 | |||
| Positive | 8 (88%) | 8 (88%) | 16 (89) | |
| Negative | 1 (12%) | 1 (12%) | 2 (11) | |
| Risk factors | ||||
| Contact with cattle | 12 (27) | 9 (29) | 21 (28) | 0.798 |
| Rural life | 33 (75) | 21 (67) | 54 (72) | 0.634 |
| Immunosuppression | 17 (40) | 3 (10) | 20 (27) | 0.006 |
| Travel to an endemic country | 3 (7) | 5 (16) | 8 (11) | 0.180 |
| Diabetes mellitus | 4 (9) | 1 (3) | 5 (7) | 0.333 |
| HIV positive | 2 (5) | 0 | 2 (3) | 0.232 |
| Smoking habit | 13 (30) | 1 (3) | 14 (17) | 0.003 |
| None of the above | 3 (7) | 3 (10) | 6 (8) | |
| Unknown | 1 (2) | 0 | 1 (1) | |
| Treatmenta | 0.011 | |||
| WHO recommended | 7 (16) | 12 (43) | 19 (27) | |
| Other | 29 (67) | 16 (57) | 45 (63) | |
| Unknown | 7 (16) | 0 | 7 (10) | |
| Outcome | 0.463 | |||
| Resolution | 36 (82) | 26 (84) | 62 (83) | |
| Death | 3 (7) | 4 (13) | 7 (9) | |
| Relapse | 1 (2) | 0 | 1 (1) | |
| Unknown | 4 (9) | 1 (3) | 5 (7) | |
The most common risk factors in these patients were living in a rural environment (72%) and having direct contact with cattle (28%). The predisposition to PTB was greater in patients who smoked and in those who had immunosuppressive conditions.
Seventy-four of the M. bovis strains were mono-resistant to PZA and one strain presented INH and PZA resistance. Only 19 patients (27%) received the treatment recommended by the WHO, which was more common among patients with EPTB (p=0.011) and in those who recovered (p<0.001). Forty-one patients (63%) presented with satisfactory outcomes with a treatment that either included PZA in the first two months and/or did not have a duration of nine months. We were unable to determine the outcome of seven patients (10%) due to either a change in Health Area or failure to attend the review.
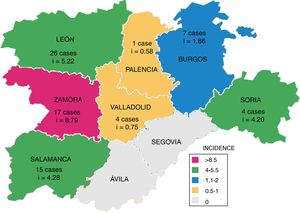
The overall mortality of these patients was 9% (Table 2): four patients died before starting the treatment and three during treatment. Mortality was higher in males, although no significant differences were found. The deceased patients had a mean age that was higher than those who recovered and immunosuppressive conditions were also significantly more prevalent. The underlying diseases of these five deceased patients were: diabetes (one case), end-stage kidney disease (one case), rectal cancer (one case), haematological malignancy (one case) and severe COPD treated with corticosteroids (one case).
Outcome of patients with tuberculosis due to Mycobacterium bovis.
| Resolution (n=63) | Death (n=7) | p | |
|---|---|---|---|
| Gender | 0.187 | ||
| Male | 38 (60) | 6 (86) | |
| Age, mean+SD | 64.3+22.2 | 78.2+11.6 | 0.030 |
| Immunosuppression | 12 (19) | 5 (71) | 0.002 |
| Treatmenta | <0.001 | ||
| WHO | 16 (25) | 0 (0) | |
| Other | 41 (65) | 3 (43) | |
In the 10 years between 2006 and 2015, the mean annual incidence of confirmed M. bovis TB cases in CyL was higher than that of Spain and other European countries.15 Likewise, the proportion of M. bovis infections in relation to the total number of TB cases (2.43%) was also higher than in Asturias (1.4% in 2006–2014),16 Spain overall (1.9% in 2004–2007),7 the Netherlands (1.4% in 1993–2007)6 and the United Kingdom (1.5% in 2003).10 The risk of zoonotic TB in CyL may be greater than in other regions, since it is an important livestock-producing region and 26.4% of the population live in rural areas.
In our population, as in other regions with low TB rates, the efforts aimed at eradicating TB have led to a fall in M. tuberculosis TB. However, they have not managed to reduce M. bovis infections, indicating that these two species behave in different ways.8,17 The fact that other domestic and wild animals may be a source of infection for humans could also have an impact in this regard.1,10,18
The greatest incidence of human M. bovis infections were found in the most westerly provinces of the community, while the greatest prevalence of herds infected with bovine TB were located in the south of CyL.12,13 This disparity may be due to differences in how the disease was detected in each Health Area.
The predominance of PTB is a finding that can be compared to other populations in our setting,7,10,11 with the exception of the Netherlands, where EPTB accounted for 59% of the cases.6 Historically, M. bovis infections were primarily located in the digestive and lymphatic systems. The reduced incidence of EPTB could be due to the fact that air is the main mode of transmission now that milk pasteurisation has become widespread,10,17 as well as greater difficulties in diagnosis, which ultimately reduces clinical suspicion until the advanced stages of the disease.19,20
EPTB was more common in women, as in other studies on TB caused by M. bovis7,16 or M. tuberculosis.20 The reasons for this are not well known, although some authors have linked it to socioeconomic factors, different infection pathways in each gender, hormonal factors and cellular immunity.6,21
The advanced age of the patients and the presence of immunosuppressive conditions suggest the reactivation of an old infection which occurred prior to the pasteurisation era. Thus, in regions where bovine TB has been virtually eradicated, the residual cases appearing in older patients are associated with the reactivation of a dormant infection, while TB occurring in younger patients is considered a primary infection.9,21 However, in the study by Palacios et al.16 47% of the genotypes in human strains were shared by cattle, suggesting a recent transmission irrespective of age. The most common spoligotypes in the bovine TB strains of CyL are SB0121, SB0134 and SB0339.22 The first two have been identified in both humans and cattle in Asturias16 and England.11 Unfortunately, the lack of spoligotyping among our strains did not allow us to draw conclusions in this regard.
Diabetes mellitus has been strongly correlated with PTB,20 and HIV with EPTB.23 However, this association was not evident in our study, probably due to the limited number of patients with these risk factors.
Although 63% of our patients were not treated as per the WHO recommendation, they had satisfactory outcomes. The low resistance of MTC to first-line anti-tuberculosis drugs (less than 6%24,25) could explain the patients’ improvement, despite delayed microbiological identification. Quickly and reliably identifying the mycobacterium species and the immediate notification of the clinician could improve these results.
The mortality of the disease was lower than in similar studies.6,16 This was probably related to multiple factors,6,10,16 such as advanced age and/or immunosuppression.
In comparison to TB due to M. bovis, the patients with MTC TB in CyL in 201525 had a lower mean age (52.7+24.5) and a higher percentage of immigrants (17.5%), childhood TB (6.7%) and pulmonary forms (73%). The main risk factor was contact with a TB-positive patient (13.41%). The provinces with the highest incidences were Palencia, León and Zamora.
One of the limitations of this study was the lack of knowledge on patient chest X-ray patterns, as well as the disease resolution time.
In conclusion, the incidence of M. bovis TB in CyL remained stable despite the reduction in MTC TB and the fact that bovine TB is undergoing eradication. The disease mostly affected the population that had been born in Spain who had a higher mean age. The pulmonary form was the most prevalent. Detecting M. bovis genotypes in the population would help to detect sources of infection and prevent the transmission thereof. In our opinion, close collaboration between human and animal health is necessary in order to control and eradicate this disease.
AuthorshipT. Nebreda Mayoral wrote the manuscript, which was reviewed by the co-authors. T. Nebreda Mayoral, M.F. Brezmes Valdivieso, N. Gutiérrez Zufiaurre, S. García de Cruz, C. Labayru Echeverría, R. López Medrano, L. López-Urrutia Lorente, A. Tinajas Puertas and O. Rivero Lezcano took part in designing the study and analysing the data.
Conflicts of interestWe declare that we have no conflicts of interest.
We would like to thank Dr M.S. Jiménez (Spanish National Mycobacteria Centre, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain) for her help in identifying Mycobacterium bovis. And thank you to all other members of the GRUMICALE (Grupo de Micobacterias de Castilla y León [Castile and León Mycobacteria Group]): B. Nogueira González, Hospital Clínico Universitario de Valladolid, Valladolid; R. Sánchez Arroyo, Hospital de Ávila, Ávila; S. Hernando-Real, Hospital de Segovia, Segovia; O. Rivero-Lezcano, Complejo Asistencial Universitario de León, León; B. Ullivarri Francia, Hospital Santiago Apóstol, Miranda de Ebro-Burgos; R. Rodríguez-Tarazona, Hospital Santos Reyes, Aranda de Duero-Burgos; I. Antolín Ayala, Hospital de Medina del Campo, Medina del Campo-Valladolid, for their interest in searching for Mycobacterium bovis cases and providing details on the TB cases from their Health Areas.
Please cite this article as: Nebreda-Mayoral T, Brezmes-Valdivieso MF, Gutiérrez-Zufiaurre N, García-de Cruz S, Labayru-Echeverría C, López-Medrano R, et al. Tuberculosis por Mycobacterium bovis en la población de Castilla y León (España), 2006–2015. Enferm Infecc Microbiol Clin. 2019;37:19–24.