Achromobacter xylosoxidans is a gramnegative bacillus resistant to multiple antibiotics, present both in the environment and in hospitals. This study describes an outbreak of colonizations and infections by A. xylosoxidans in the urodynamics unit of the Puigvert Foundation.
MethodsOn November 11, 2022, a patient developed a fever, and A. xylosoxidans was detected in their urine and blood. The case was linked to a recent urodynamic study. As a result, all A. xylosoxidans cases since 2018 were reviewed, and inspections were conducted in the unit, along with the collection of 24 environmental samples.
ResultsThe review identified 21 patients with A. xylosoxidans infections after urodynamic procedures since April 2022. Environmental microbiological controls revealed that pressure transducers were the likely source of infection. Corrective measures included the temporary closure of the unit, thorough cleaning with hypochlorite, use of single-use urinary catheters, daily replacement of equipment lines and pressure transducers, as well as other improvements in disinfection, handling, and workflows. A multidisciplinary team was formed to implement and supervise these actions.
ConclusionsThe measures resulted in the elimination of the outbreak and the safe resumption of activities in the unit. This incident highlights the importance of continuous surveillance and rapid response in clinical settings to prevent infections and improve patient safety.
Achromobacter xylosoxidans es un bacilo gramnegativo resistente a múltiples antibióticos, presente tanto en el medio ambiente como en hospitales. Este estudio describe un brote de colonizaciones e infecciones por A. xylosoxidans en la unidad de urodinamia de la Fundación Puigvert.
MétodosEl 11 de noviembre de 2022, un paciente presentó fiebre, y se detectó A. xylosoxidans en su orina y sangre. El caso se relacionó con un estudio urodinámico reciente. Debido a esto se revisaron todos los casos de A. xylosoxidans desde 2018 y se realizaron inspecciones en el servicio y se recogieron 24 muestras ambientales.
ResultadosEn la revisión de los casos se identificaron 21 pacientes con infecciones por A. xylosoxidans tras procedimientos urodinámicos desde abril de 2022. Los controles microbiológicos ambientales revelaron que los transductores de presión fueron las probables fuentes de infección. Las medidas correctivas incluyeron el cierre temporal del servicio, limpieza a fondo con hipoclorito, uso de sondas vesicales de un solo uso, cambio diario de líneas del equipo y transductores de presión, así como otras mejoras de desinfección, manipulación y circuitos. Se formó un equipo multidisciplinar para implementar y supervisar estas acciones.
ConclusionesLas medidas resultaron en la eliminación del brote y la reanudación segura de las actividades en la unidad. Este incidente subraya la importancia de la vigilancia continua y la respuesta rápida en entornos clínicos para prevenir infecciones y mejorar la seguridad del paciente.
Achromobacter xylosoxidans is a non-fermenting Gram-negative bacillus present both in the environment and in hospitals.1,2 Described in 1971 by Yabuuchi and Ohyama.3 It was initially classified within the genus Alcaligenes, but was later reassigned and became the first member of the genus we now know as Achromobacter, which includes 21 species.4
Achromobacter species are mainly found in aquatic environments and are particularly resistant to adverse conditions. They harbour intrinsic resistance mechanisms against beta-lactams, aminoglycosides, fluoroquinolones, aztreonam, tetracyclines and cephalosporins.5 In the hospital environment, they have been isolated in respiratory samples,6 pressure transducers,7 ultrasound gel and ultrasound transducers,8 and even chlorhexidine solutions.9
The majority of clinical isolates of this microorganism are asymptomatic colonisations.10 However, A. xylosoxidans-related infections have been described, including meningitis, both nosocomial and community-acquired pneumonia, peritonitis, endocarditis, gastrointestinal infections, pancreatitis, prosthesis infection, osteomyelitis, biliary tract sepsis, bacteraemia, ophthalmic disease and urinary tract infection (UTI).11
One possible way of acquiring a UTI is undergoing a urological procedure, such as a urodynamic study. A urodynamic examination may include several techniques and/or procedures, such as uroflowmetry, cystometry or measurement of bladder pressure during bladder filling and emptying, urethral pressure profile, pressure flow studies and video-urodynamics.12 All of these procedures are invasive and involve techniques that require urinary catheterisation, which can increase the risk of infection.13 The incidence of UTI after a urodynamic study ranges from 1.1% to 28.3%.14 The consequences of UTI related to urodynamic procedures can be severe and cause pyelonephritis or sepsis, requiring hospital admission. However, in most cases, they manifest as cystitis, which is quickly diagnosed and can be treated with oral antibiotics.14–16
Numerous outbreaks of A. xylosoxidans have been reported in different hospitals and departments. They tend to be associated with contaminated fluids, whether water, ultrasound gel or chlorhexidine-based disinfectants,1,6,8,9 probably due to their particular resistance to external agents.
Outbreak investigations are an important component in the prevention and control of healthcare-associated infections. Using microbiology and epidemiology, the origin of existing outbreaks can be identified and new ones prevented. Even after the outbreak is over, the environmental and epidemiological research conducted can help increase knowledge about that particular microorganism and contribute to improving the tools to prevent new outbreaks, whether due to that microorganism or another.16
The aim of this work is to describe an outbreak of A. xylosoxidans in the urodynamics unit of Fundació Puigvert [Puigvert Foundation], and report the clinical and microbiological research and improvement actions that led to the complete resolution of the outbreak.
MethodsEpidemiological studyOn 11 November 2022, a patient developed a fever of above 38 °C at home and went to the Accident and Emergency department (A&E) of Fundació Puigvert, a specialist hospital dedicated to urology, nephrology, andrology and reproductive medicine in the city of Barcelona. Samples were taken for urine culture and two blood cultures. The next day, >100,000 cfu/mL of A. xylosoxidans were isolated in the urine culture, and days later, the two blood cultures were also positive for A. xylosoxidans, with the same antibiotype as the one isolated in urine.
Days later, on 14 November, the urodynamics department medical team contacted the infection control team to discuss the case, as they were relating it to two cases of patients who had attended A&E due to urinary tract infection with A. xylosoxidans isolated in urine culture a week earlier. All these patients had undergone a urodynamic study a few days before their visit to A&E.
Based on this information, it was decided to review all cases of A. xylosoxidans from recent years at Fundació Puigvert. For this purpose, we compiled the information accumulated in the VITEK® 2 library (bioMérieux), a system used in the microbiology laboratory for the detection and identification of microorganisms and for performing their antibiograms.
Microbiology studyOnce we had that information, we decided to visit the urodynamics department to carry out a visual inspection while they were performing a urodynamic procedure and identify possible points of environmental contamination.
In total, two samples of normal saline solution used to purge the system and which is not changed between patients, four samples of enzymatic disinfectant used to clean surgical material, a connector, two electrical cables connected to the catheter, two pressure valves and normal saline solution from the infusion pump, a sample from the bidet drain where the patient urinates during the swab collection procedure, eight samples from different surfaces in the unit (sink, floor, computer keyboard, work table and examination table) collected with Replicate Organism Detection and Counting plates with trypticase soy agar medium with lecithin and polysorbate 80 (Becton Dickinson), a recently unsealed catheter from the factory and another unsealed after the internal steam-based sterilisation process.
For the culture of catheters, connectors, switches and cables that might be contaminated by A. xylosoxidans, the Maki technique was adapted to these elements, as it was of interest to determine whether the surfaces of these items were colonised. In instruments in which the Cleri technique (catheters) could also be applied, the Cleri technique modified by Liñares et al. was carried out for catheters.17 The culture media used in all samples, except those from surfaces, were Columbia blood agar base (Becton Dickinson) and a selective chromogenic medium plate for the detection of extended-spectrum beta-lactamases (chromID® ESBL; bioMérieux), in which it was previously proven that the strain could grow due to its resistance to cephalosporins. All culture media used were incubated for 48 h at 35 °C in a CO2-enriched atmosphere (blood agar) or in air (ESBL plate). If there was no growth after that time, they were considered negative.
The VITEK® 2 system was used for the identification and study of sensitivity to antimicrobials. The criteria used for the interpretation of the antibiogram were those of the current European Committee on Antimicrobial Susceptibility Testing (www.eucast.org).
The work was approved by the Fundació Puigvert Ethics Committee on 22/09/2023. The confidentiality of the data was maintained in accordance with Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on data protection and Spanish Law 14/2007 on biomedical research, and the ethical principles of the Declaration of Helsinki, revised in October 2000, for medical research involving human subjects. We only used data from patients who gave consent.
ResultsThe data obtained showed a total of 34 patients with positive culture for A. xylosoxidans from November 2018, when the VITEK® 2 library began to be used, to 14 November 2022. The antimicrobial susceptibility of the A. xylosoxidans strain is shown in Table 1. The medical records of all patients who had given their consent were reviewed, including the Història clínica compartida a Catalunya [Catalonia Shared Medical Records], to look for a relationship between A. xylosoxidans and the urodynamics department. It was established that, since April 2022, the date of the first isolation of A. xylosoxidans, all patients with a positive culture for this microorganism had undergone a urodynamic study at our centre. We checked the time between the date of the urodynamic study and the positive culture for A. xylosoxidans, and the reason for performing the urine culture (Table 2).
Sensitivity profile of the Achromobacter xylosoxidans strain.
Patients with urine culture positive for Achromobacter xylosoxidans.
| Patient No. | Date of urodynamic study | Date urine culture positive for Achromobacter xylosoxidans | Days | Reason for urine culture |
|---|---|---|---|---|
| 1 | 18/3/2021 | 5/5/2022 | 46 | Routine |
| 2 | 8/4/2022 | 3/5/2022 | 25 | Routine |
| 3 | 21/4/2022 | 25/4/2022 | 4 | UTI |
| 4 | 10/5/2022 | 15/5/2022 | 5 | Acute prostatitis |
| 5 | 2/6/2022 | 7/6/2022 | 5 | UTI |
| 6 | 9/6/2022 | 16/6/2022 | 7 | UTI |
| 7 | 8/7/2022 | 17/7/2022 | 9 | Sepsis |
| 8 | 26/7/2022 | 1/8/2022 | 5 | UTI |
| 9 | 27/7/2022 | 30/1/2023 | 187 | Routine |
| 10 | 29/7/2022 | 8/8/2022 | 10 | AUR after urodynamics |
| 11 | 12/8/2022 | 14/12/2022 | 124 | Routine |
| 12 | 16/8/2022 | 19/8/2022 | 3 | UTI |
| 13 | 19/9/2022 | 22/9/2022 | 3 | UTI |
| 14 | 10/10/2022 | 14/10/2022 | 4 | UTI |
| 15 | 10/10/2022 | 14/10/2022 | 4 | Epididymitis |
| 16 | 17/10/2022 | 3/1/2023 | 76 | Routine |
| 17 | 27/10/2022 | 11/11/2022 | 15 | UTI |
| 18 | 4/11/2022 | 29/12/2022 | 25 | UTI |
| 19 | 9/11/2022 | 14/11/2022 | 5 | Routine |
| 20 | 14/11/2022 | 17/11/2022 | 3 | Routine |
| 21 | 17/10/2022 | 20/1/2023 | 95 | Routine |
UTI: urinary tract infection; AUR: acute urinary retention.
Routine: urine culture ordered at the request of the treating physician.
All the patients in whom A. xylosoxidans was isolated from 2022 onwards, a total of 21, were found to have undergone a urodynamic procedure at the Fundació Puigvert.
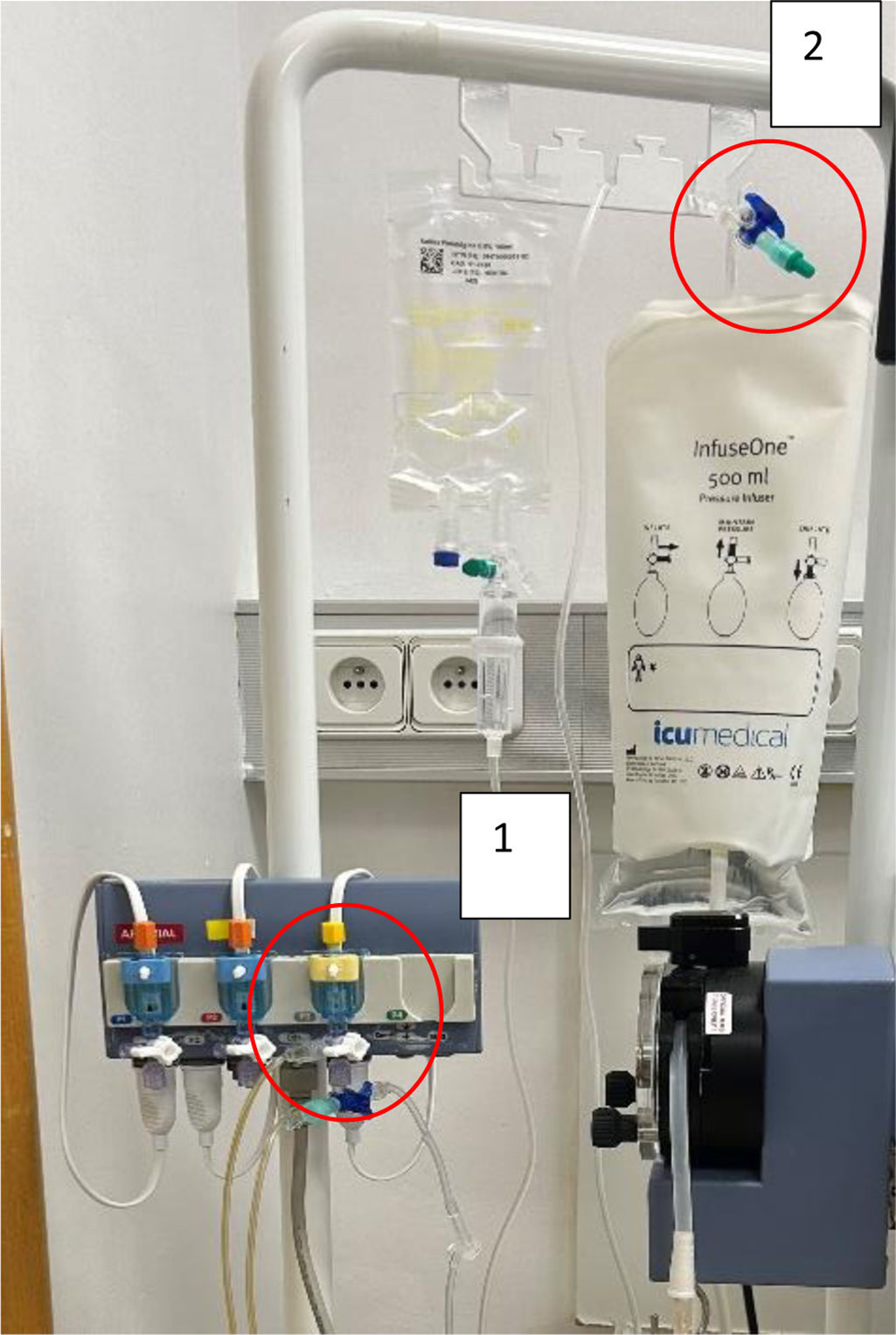
We collected a total of 24 environmental samples from different parts of the department used directly or indirectly in the urodynamic study, two of which tested positive for A. xylosoxidans. Fig. 1 shows the pattern of positive cases.
The two positive samples were obtained from the transducer key that measures bladder pressure during the study (Fig. 2, point 1) and from the saline solution key that purges the entire system (Fig. 2, point 2).
After the microorganism was isolated, a special meeting of the hospital hygiene and infections committee was held, where they investigated the cause of the pressure transducers being contaminated by A. xylosoxidans. They concluded that it had occurred because of the infrequency (monthly) with which the transducers were changed. As they were kept for so long and subjected to so much manipulation in each test, it was deemed that as an aqueous medium, they were at high risk of becoming contaminated. It was also found that, although the sterilisation process was correctly followed, the catheters used for the test were reused more often than the manufacturer recommended. Taking these points into account, the following improvement actions were decided on immediately, listed in chronological order:
- a
The urodynamics department will be closed down until the indicated cleaning and disinfection are completed and the above improvements have been implemented.
- b
Carry out a deep clean and subsequent disinfection with sodium hypochlorite 4% in the urodynamics facilities, to eliminate other possible sources of contamination not detected with the already performed environmental microbiological controls.
- c
Use single-use urinary catheters.
- d
In the future, the microbiology department will notify the infection control nurse of all new isolates of A. xylosoxidans in the hospital for investigation, indefinitely.
- e
Create a multidisciplinary working team to evaluate all the steps of the urodynamic study and generate improvement actions.
It took three days to carry out actions a, b and c so the urodynamics department could be reopened and resume operating.
A multidisciplinary working team was created, made up of the nurses from the urodynamics unit, the supervisor of the service, the quality and patient safety managers at the centre, as well as the infection control team, made up of the head and deputy head of the microbiology department and the infection control nurse.
The final improvement actions implemented over one month were as follows:
- a
Use of single-use urinary catheters.
- b
Change pressure transducers and lines daily.
- c
Protect the connections between the transducer lines with swabs and antiseptic solution.
- d
Disassemble the monitor once a week for cleaning.
- e
Purge the transducer lines with sterile normal saline solution before placing them in the disinfectant solution.
- f
To purge the system, use one syringe for each patient.
- g
Use single-dose povidone-iodine solution.
- h
Follow the recommendations in the summary of product characteristics or technical data sheets of the products and materials used during the procedure.
This paper describes an outbreak of urinary tract infections and colonisations caused by A. xylosoxidans present in a urodynamic examination apparatus, and the measures that allowed it to be under control. The results of the epidemiological study revealed that the patients who developed A. xylosoxidans colonisation or infection after a urodynamic study represented all cases since April 2022 at Fundació Puigvert. This led to a thorough review of the studies and the identification of a temporal association between urodynamic studies and infections, thereby confirming the origin of the outbreak.
As shown in Table 2, most of the urine cultures were collected from patients showing symptoms of infection (UTI, epididymitis, sepsis and acute prostatitis), a total of 13 (59%, 95% CI: 47.6–70.4 %). Nine patients had urine cultures taken and did not have symptoms (41%, 95% CI: 29.6–52.4 %). In all urodynamic procedures performed at our centre, prior antibiotic prophylaxis is given with 3 g of fosfomycin. If the patient is allergic to fosfomycin, the physician uses another antibiotic from among those indicated in the centre's prophylaxis protocol as an alternative.
Although A. xylosoxidans grew in some samples more than 30 days after the urodynamic study was performed, and this might suggest they were not related, these cases were included in the epidemiological study of the outbreak because the sensitivity profile of the microorganism was the same, and there were no positive cultures for A. xylosoxidans in any of the centre's other departments. These patients were considered to have become colonised and were not detected until they showed symptoms of infection.
Once it was known that the origin could be a urodynamic study, the microbiological analysis was carried out, following the same lines as other studies on outbreaks of A. xylosoxidans,1,2,7–9,18–21 which report that the microorganism is concentrated mainly in media with the presence of water. The environmental samples collected in the urodynamics unit focused on sites with these characteristics, as well as the points mentioned above. Two points with positive culture for A. xylosoxidans were identified: the transducer tap that measures the bladder pressure, and the tap for the normal saline solution used in the process. In the study by Tian et al.,22 where they report an outbreak of Achromobacter spp. in a urology unit, they found the microorganism in a very similar place to the one we report here: the connections of the medical pressuriser used in operating theatres. Our findings show that the equipment used during urodynamic studies that connected the distal end of the catheter to the computer responsible for converting pressure into measurable parameters was the source of the microorganism, probably because this equipment was changed monthly.
Once the outbreak was addressed and the two positive cultures pointing to the possible source of contamination were obtained, a multidisciplinary team was created with all the healthcare professionals who form part of the urodynamic process to evaluate and improve the procedures. Several improvement actions were implemented, including thorough cleaning and disinfection of the urodynamics unit to try to eliminate other possible sources of contamination not found in the microbiological analysis. Also implemented were the use of single-use urinary catheters, daily change of lines and pressure transducers, and additional measures to prevent contamination during the procedure.
These measures were implemented within five days and resulted in the complete resolution of the outbreak and the safe resumption of activity in the urodynamics unit. Furthermore, the improvement actions implemented not only addressed the immediate problem, but also updated existing protocols to prevent future outbreaks and improve patient safety in the unit.
In subsequent follow-up, it was found that new cases of A. xylosoxidans were detected in patients who had undergone a urodynamic examination prior to the date of detection of the outbreak (two in total).
This work highlights the importance of continuous surveillance and rapid response to such events in specialised clinical settings, such as urodynamics units, where invasive techniques are performed. The temporal association between the cases of A. xylosoxidans infection and the urodynamic studies, through review of medical records, underscores the importance of continuous monitoring of invasive clinical procedures with a high risk of infection and early detection of possible outbreaks. Epidemiological and microbiological analysis based on existing literature made it possible to identify the source of the outbreak. This understanding was crucial to implementing effective improvement actions for infection control and prevention. In addition, the creation of a multidisciplinary team to generate long-term prevention measures prevented future outbreaks and helped ensure patient safety and quality of care in the urodynamics unit.
The successful management of this outbreak not only led to the elimination of A. xylosoxidans colonisation in the urodynamics unit to date, but also laid the groundwork for safer clinical practices and the prevention of similar future events. The isolation of A. xylosoxidans in patient samples should alert to the existence of a possible hospital source of the microorganism which can be controlled.
FundingThis study has received no specific funding from public, private or non-profit organisations.
None.
We would like to thank all the healthcare professionals involved in the multidisciplinary team for their participation in analysing and controlling the outbreak and for helping rapidly create and implement the improvement actions described here.