Since the advent of sulfonamides for the treatment of gonococcal infection in the 1930s, Neisseria gonorrhoeae has developed resistance to all the antibiotics used. The worldwide increase in gonorrhoea cases, as well as resistance to first-line drugs, make N. gonorrhoeae one of the top three "urgent" antibiotic-resistance threats for which optional treatments are needed.1 As alternatives to the current therapy, known antibiotics such as gentamicin and gemifloxacin are being tested,2–4 in addition to new drugs, all in different stages of development, such as solithromycin, zoliflodacin and gepotidacin.
With the aim of providing new information on the rate of resistance of N. gonorrhoeae to gentamicin and other antibiotics, we studied the antimicrobial susceptibility of all the strains isolated from the samples received at the Microbiology Department of Hospital Universitari i Politècnic La Fe, from March 2013 to March 2019.
The samples were seeded in chocolate agar (bioMérieux) and in Martin Lewis selective medium (Becton Dickinson). Isolated strains were identified by MALDI-TOF VITEK® MS (bioMérieux). In parallel, a real-time multiplex PCR (Anyplex™ II STI-7 Detection, Seegene) was performed from a direct sample, confirming the isolates. Susceptibility to benzylpenicillin, ceftriaxone, cefotaxime, cefixime, azithromycin and gentamicin was determined using the E-test method® (bioMérieux). With the exception of gentamicin, the minimum inhibitory concentrations (MIC) were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines in force in 2019.5,6 The MICs for gentamicin were classified as follows: susceptible: ≤4 mg/l; reduced susceptibility (intermediate): 8−16 mg/l, and resistant: ≥32 mg/l.4 A high level of resistance to azithromycin was established as when the MIC was ≥256 mg/l.7 The disc diffusion method was used to determine the susceptibility to ciprofloxacin and tetracycline, the result of which was interpreted according to the Clinical and Laboratory Standards Institute (CLSI).6 High level resistance to penicillin was tested by production of beta-lactamase with a chromogenic method (Cefinase®, Becton-Dickinson).
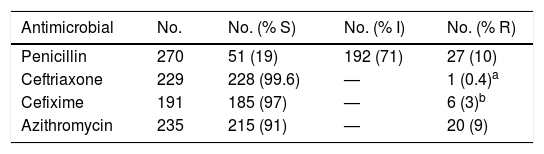
A total of 278 strains were isolated, corresponding to 218 urethral, 53 vaginal-cervical and four rectal exudates, two semen samples and an intrauterine device. Table 1 shows a summary of the sensitivity to the antibiotics tested, apart from gentamicin.Of the 65 strains in which gentamicin was tested, 59 (90%) were sensitive and 6 (10%) showed reduced or intermediate susceptibility. A total of 6.3% (15/238) of the strains produced beta-lactamase. Of the 20 (9%) strains resistant to azithromycin, one showed high-level resistance (MIC ≥256 mg/l).
Study of susceptibility of the N. gonorrhoeae strains isolated (EUCAST, 2019).
| Antimicrobial | No. | No. (% S) | No. (% I) | No. (% R) |
|---|---|---|---|---|
| Penicillin | 270 | 51 (19) | 192 (71) | 27 (10) |
| Ceftriaxone | 229 | 228 (99.6) | — | 1 (0.4)a |
| Cefixime | 191 | 185 (97) | — | 6 (3)b |
| Azithromycin | 235 | 215 (91) | — | 20 (9) |
I: reduced or intermediate susceptibility; R: resistant; S: susceptible; —: not defined.
The current recommendation for the treatment of gonococcal infection includes azithromycin combined with ceftriaxone in a single dose. Cefixime stopped being recommended as first choice after higher resistance rates were reported with this cephalosporin. Genetic alterations in the genome ofN. gonorrhoeaehave been described that could explain these findings.8 Cefixime no longer being recommended as first option considerably reduced its resistance rate, from 8.7% in 2010 to 2.1% in 2017,9 a figure very close to the 2% detected in our study. According to the latest 2017 European Centre for Disease Prevention and Control (ECDC) report, resistance rates for cefixime, ceftriaxone, and azithromycin have been stable for the past four years.9 In our study, we found one strain resistant to ceftriaxone and six to cefixime, corresponding to samples obtained in 2015 in the first case and 2016 and 2017 in the second. For azithromycin, the high percentage of resistance found is consistent with that reported by other authors in Spain.10 However, we believe that this may be the first time a strain with high-level resistance to azithromycin has been reported in Spain. We did not find any resistant strains for gentamicin.
In conclusion, although in our setting combined therapy with ceftriaxone and azithromycin appears to be effective in the treatment of gonococcal infection, it is important to continue monitoring the development of resistance to these antibiotics, especially strains with high-level resistance to azithromycin which could compromise the effectiveness of the treatment.
Please cite this article as: Castaño Aroca MJ, Acosta Boga B, Lozano Rodríguez N, Arcas RC. Estudio de sensibilidad antimicrobiana de Neisseria gonorrhoeae en el área de salud del Hospital Universitario y Politécnico La Fe de Valencia. Enferm Infecc Microbiol Clin. 2020;38:348–349.