To describe the experience using the combination abacavir, lamivudine plus non-boosted atazanavir (ABC/3TC+ATV) in a group of pretreated patients.
Patients and methodsWe performed a retrospective observational study to describe baseline characteristics and the evolution of patients who had received or were treating with ABC/3TC+ATV, from November 2004 and June 15th 2015, in the clinical setting.
ResultsOverall, 236 patients were included in the study. Median age (IQR) was 45 (42–50) years and 69% were male. The main reasons for using this combination were previous toxicity in 130 patients (56%), simplification in 60 (20%) and virologic failure in 29 (14%). Previous treatment was based in boosted protease inhibitor in 115 patients (48.7%), 3 analogs in 56 (28%) and non-analogous based in 19 (8.1%). Median treatment length was 2.2 years (IQR 0.8–5.3). A total of 66 (28%) patients continue receiving ABC/3TC+ATV (median time 5.7, IQR 2.2–8.3), treatment was changed in 170 patients (72%) (median time 1.6 years, IQR 0.7–3.6), and 22 (9.3%) patients were lost. Virological failure was assessed in 30 patients.
ConclusionIn selected patients, ABC/3TC+ATV is a durable and attractive therapeutic alternative.
Describir la eficacia en práctica clínica de abacavir, lamivudina y atazanavir sin potenciar (ABC/3TC+ATV) en pacientes pretratados.
Pacientes y métodosSe realizó un estudio observacional retrospectivo para describir las características clínicas y la evolución de los pacientes que, por prescripción facultativa, habían recibido tratamiento con ABC/3TC+ATV desde noviembre de 2004 hasta el 15 de junio de 2015.
ResultadosSe incluyeron 236 pacientes. La mediana de edad (IQR) fue de 45años (42-50) y el 69% eran varones. Los principales motivos para su indicación fueron toxicidad en 130 pacientes (56%), simplificación en 60 (20%) y fracaso virológico (FV) en 29 (14%). El tratamiento previo contenía un inhibidor de la proteasa (IP) en 115 pacientes (48,7%), 3 inhibidores de la transcriptasa inversa análogos de nucleósido (ITIAN) en 56 (28%) y 2ITIAN y un inhibidor de la transcriptasa inversa no análogo de nucleósido (ITINAN) en 19 (8,1%). Tras una mediana de 2,2años (IQR0,8-5,3), 66 (28%) pacientes continuaban con la misma pauta, se retiró en 170 (72%), en 30 de ellos por FV (12,7%) y en 22 (9,3%) por pérdidas de seguimiento.
ConclusiónEn pacientes seleccionados, ABC/3TC+ATV es una alternativa de simplificación eficaz y bien tolerada, usada principalmente para minimizar la toxicidad.
Despite the high efficacy and good tolerance of new antiretroviral therapy (ART) regimens, changes are still being made for a number of reasons: intolerance, toxicity, co-morbidities, drug interactions, simplification and treatment failure. The aim of the change is to achieve or maintain virological suppression and optimise ART according to resistance mutations of the virus and the patient's characteristics and preferences.1–3
Protease inhibitor (PI)-based regimens require, in most cases, co-administration of ritonavir (RTV) or cobicistat as a booster, increasing the risk of interactions and adverse effects.4–6 However, atazanavir (ATV) may be used unboosted with dosage adjustment provided that it is not accompanied by tenofovir difumarate (TDF). Although this option was not included in the summary of product characteristics in Spain until October 2015,7 unboosted ATV combined with abacavir (ABC) and lamivudine (3TC) has been used with good results in the ARIES,8,9 INDUMA10 and ASSURE11,12 studies, even though there are limited data from everyday clinical practice.13,14
In order to describe the experience of using ABC/3TC+ATV in the patient cohort resulting from the merger of Hospital Carlos III and Hospital Universitario La Paz, a retrospective study was conducted analysing the clinical characteristics and outcome in pre-treated patients who changed to an ABC/3TC+ATV regimen between 2004 and 2015.
Patients and methodsThis was a retrospective observational study. The inclusion criteria included patients from Hospital Carlos III or Hospital Universitario La Paz who had been prescribed ABC/3TC+ATV treatment between November 2004 and 15 June 2015. Patients had to be over 18 years of age and have received this regimen for a minimum of 3 months.
Retrospective data were collected from the medical records and outpatient database of the HIV Unit of the Internal Medicine Department of Hospital La Paz-Carlos III. The following data were collected: gender, age, race, country of origin, route of HIV transmission, chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection (when the PCR test was positive), history of ART, CD4+ lymphocyte nadir per cubic millimetre and CD4+ lymphocyte count at the time of the change and HIV viral load.
The patients’ outcome was evaluated until withdrawal of the combination (for any reason), loss to follow-up or the study end date. During follow-up we analysed whether the regimen had been discontinued and the reason for discontinuation, CD4+ count and viral load at the end of follow-up. The characteristics of patients who were still receiving ABC/3TC+ATV at the end of the study were analysed independently and compared to the characteristics of those patients who had discontinued the regimen due to virological failure (defined as viral load >50copies/ml at more than 2 consecutive follow-up visits).
The SPSS Statistics software version 17.0 was used for the statistical analysis. Qualitative variables were expressed as absolute numbers and percentages. Quantitative variables were expressed as median and interquartile range (IQR). Groups were compared using the Chi-square test, Student's t-test and non-parametric tests, as applicable.
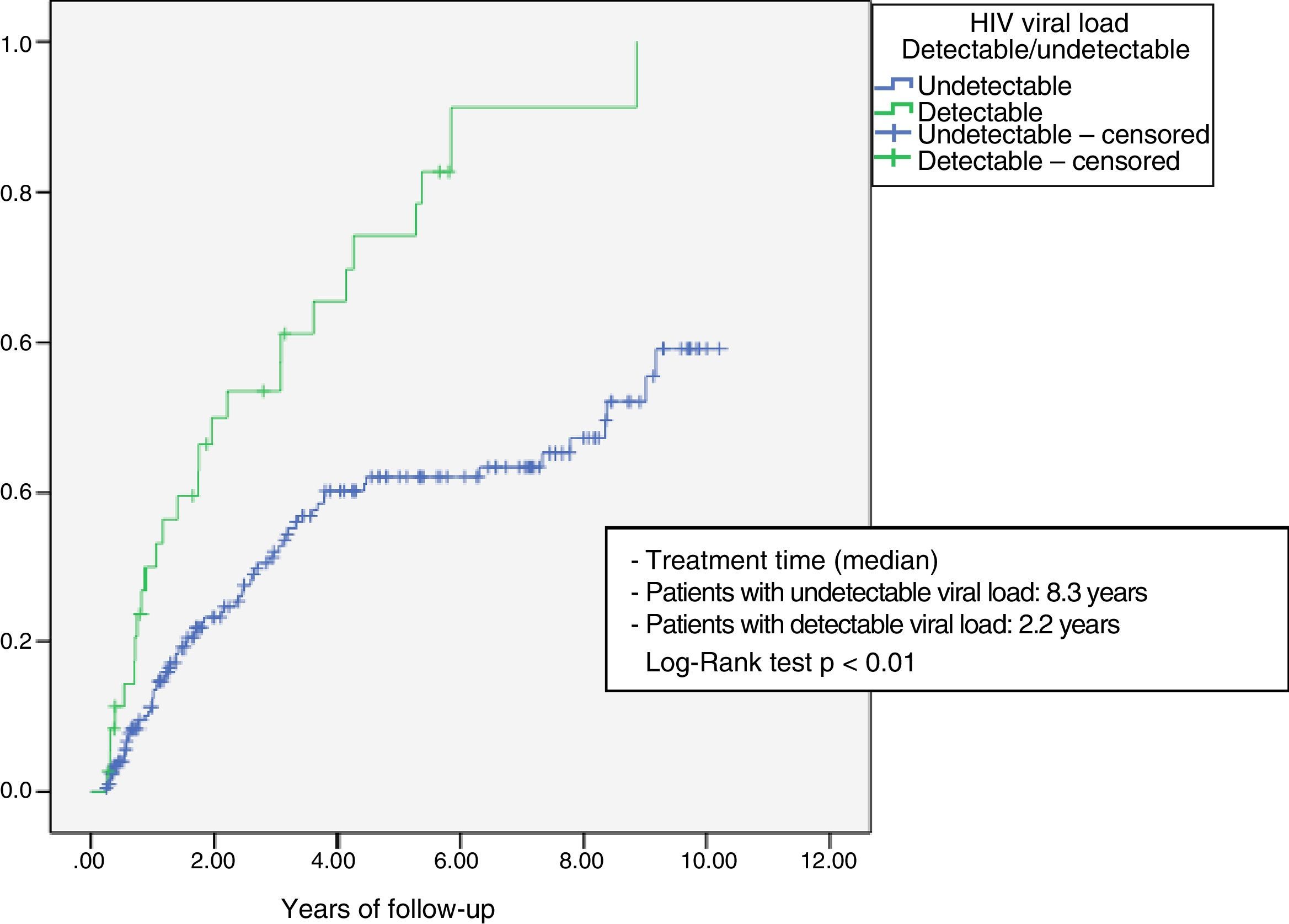
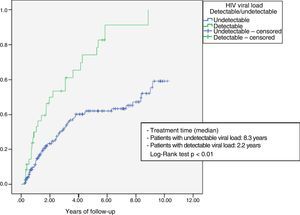
The probability of discontinuation of the combination due to poor outcome (virological failure or toxicity) was analysed using a Kaplan–Meier survival table, comparing patients who had started treatment with a detectable and undetectable HIV viral load using the log-rank test.
The protocol was approved by the Hospital's Ethics Committee and submitted to the Spanish Agency of Medicines and Medical Devices. It was classified as a “Post-Authorisation Safety Study – Any Design Other Than a Prospective Follow-Up Design” (abbreviated as EPA-OD in Spanish) and given code KVX+ATV-2015.
ResultsDuring the study period, 236 patients received ABC/3TC+ATV, 83.9% between 2005 and 2009 (198 patients), 10.1% between 2010 and 2013 (24 patients) and 5.93% after 2013 (14 patients). 69.1% were male, the median age (IQR) was 45 years (42–50) and 90.6% were white (Table 1). 50.8% contracted HIV through sexual intercourse and 42.4% through injection. Active chronic HCV infection was detected in 67 patients (28%) and 2 patients had HIV/HBV/HCV co-infection, with both receiving adefovir concomitantly with ART. The main reasons for starting the ABC/3TC+ATV regimen were toxicity from prior treatment in 130 patients (55.1%), simplification in 60 patients (25.4%) and virological failure in 29 patients (12.3%). Prior ART included 2 nucleoside reverse transcriptase inhibitors (NRTIs) and one PI in 115 patients (48.7%), 3 NRTIs (mainly zidovudine/3TC/ABC) in 56 patients (28%) and 2 NRTIs+non-nucleoside reverse transcriptase inhibitor (NNRTI) in 19 patients (8.1%).
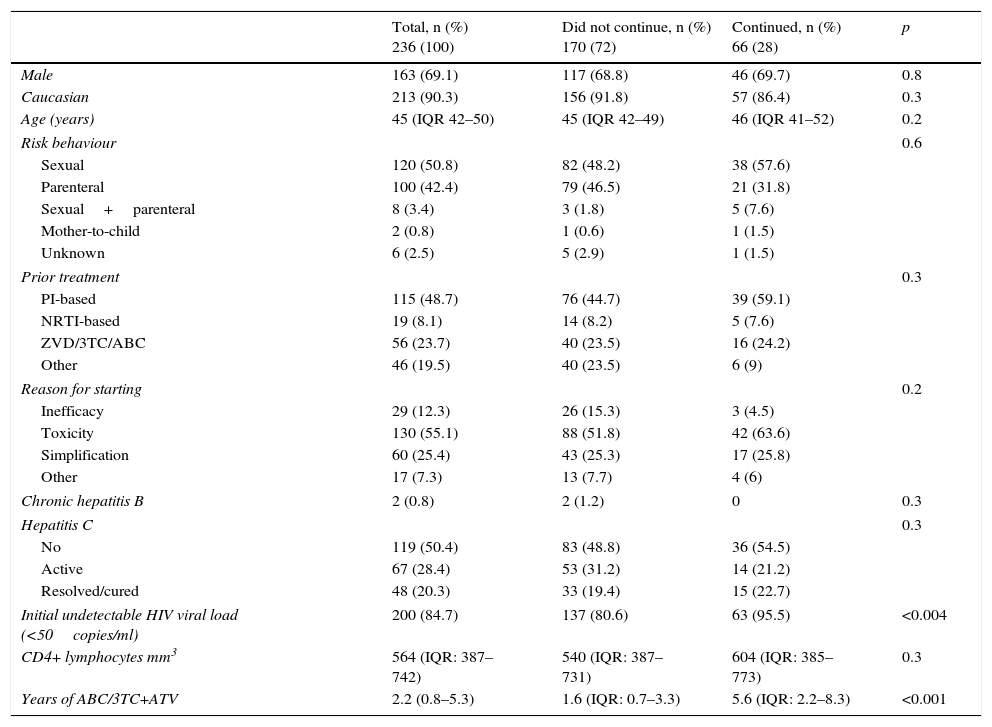
General characteristics of the overall series of 236 patients who received ABC/3TC+ATV and comparison of those patients who continued to receive ABC/3TC+ATV and those who were withdrawn from treatment for any reason.
| Total, n (%) 236 (100) | Did not continue, n (%) 170 (72) | Continued, n (%) 66 (28) | p | |
|---|---|---|---|---|
| Male | 163 (69.1) | 117 (68.8) | 46 (69.7) | 0.8 |
| Caucasian | 213 (90.3) | 156 (91.8) | 57 (86.4) | 0.3 |
| Age (years) | 45 (IQR 42–50) | 45 (IQR 42–49) | 46 (IQR 41–52) | 0.2 |
| Risk behaviour | 0.6 | |||
| Sexual | 120 (50.8) | 82 (48.2) | 38 (57.6) | |
| Parenteral | 100 (42.4) | 79 (46.5) | 21 (31.8) | |
| Sexual+parenteral | 8 (3.4) | 3 (1.8) | 5 (7.6) | |
| Mother-to-child | 2 (0.8) | 1 (0.6) | 1 (1.5) | |
| Unknown | 6 (2.5) | 5 (2.9) | 1 (1.5) | |
| Prior treatment | 0.3 | |||
| PI-based | 115 (48.7) | 76 (44.7) | 39 (59.1) | |
| NRTI-based | 19 (8.1) | 14 (8.2) | 5 (7.6) | |
| ZVD/3TC/ABC | 56 (23.7) | 40 (23.5) | 16 (24.2) | |
| Other | 46 (19.5) | 40 (23.5) | 6 (9) | |
| Reason for starting | 0.2 | |||
| Inefficacy | 29 (12.3) | 26 (15.3) | 3 (4.5) | |
| Toxicity | 130 (55.1) | 88 (51.8) | 42 (63.6) | |
| Simplification | 60 (25.4) | 43 (25.3) | 17 (25.8) | |
| Other | 17 (7.3) | 13 (7.7) | 4 (6) | |
| Chronic hepatitis B | 2 (0.8) | 2 (1.2) | 0 | 0.3 |
| Hepatitis C | 0.3 | |||
| No | 119 (50.4) | 83 (48.8) | 36 (54.5) | |
| Active | 67 (28.4) | 53 (31.2) | 14 (21.2) | |
| Resolved/cured | 48 (20.3) | 33 (19.4) | 15 (22.7) | |
| Initial undetectable HIV viral load (<50copies/ml) | 200 (84.7) | 137 (80.6) | 63 (95.5) | <0.004 |
| CD4+ lymphocytes mm3 | 564 (IQR: 387–742) | 540 (IQR: 387–731) | 604 (IQR: 385–773) | 0.3 |
| Years of ABC/3TC+ATV | 2.2 (0.8–5.3) | 1.6 (IQR: 0.7–3.3) | 5.6 (IQR: 2.2–8.3) | <0.001 |
Following a median follow-up of 2.2 years (IQR 0.8–5.3), 66 patients (27.9%) continued treatment with ABC/3TC+ATV and 30 patients (12.7%) experienced virological failure. Treatment was withdrawn in 118 patients due to reasons other than virological failure. It was withdrawn in 67 patients due to toxicity: nephrolithiasis (2), hyperlipidaemia (10), jaundice (9), digestive disorders (7), high risk of cardiovascular disease (11) and unspecified in all other patients. Low ATV levels were detected in 6 patients. Other causes for withdrawal included drug interactions in 30 patients (26 with undetectable viral load at the time of the change) and simplification in 21 patients (8.9%). 22 patients (9.3%) were lost to follow-up.
Fig. 1 shows the Kaplan–Meier survival analysis. The median time to treatment discontinuation due to toxicity or virological failure was lower in those patients who started treatment with detectable HIV viral load (2.2 years vs 8.3 years; Log-Rank test p<0.001).
The 66 patients who were still receiving ABC/3TC+ATV at the end of the study had received this regimen for a median of 5.7 years (IQR 2.2–8.3). Toxicity from prior ART was the reason for administering ABC/3TC+ATV to 42 of these 66 patients (63.6%), while simplification and other medical reasons were the reason for such administration in 17 patients (26%) and 4 patients (6%), respectively. A significant decrease in total cholesterol (191 vs 179mg/dl; p=0.013), LDL cholesterol (121 vs 106mg/dl; p=0.05), triglycerides (187 vs 137mg/dl; p=0.044) and creatinine (0.92 vs 0.82mg/dl; p=0.001) and an increase in CD4+ cells of 138cells/mm3 (604 vs 742; p=0.001) was observed from the start of treatment with this regimen in this group of patients who continued with the treatment.
Table 1 shows the general characteristics of the overall series and a comparison of the 66 patients who continued treatment with ABC/3TC+ATV at the end of the follow-up period and the 170 patients who changed regimen, regardless of the reason for such change.
We analysed the patients according to their initial viral load and found that 84.7% of patients had an undetectable viral load when starting the regimen. Of the 200 patients with virological suppression, 31.5% (66 patients) continued with the regimen versus 68.5% who required a new change of ART, mainly due to toxicity (31.5%) and drug interactions (13%). In those patients with a detectable viral load at the beginning of the new regimen (36 patients), treatment was changed again in 91.6% of them. The main reasons for such change in these patients were inefficacy (61.1%), toxicity (11.1%) and drug interactions (11.1%).
The 30 patients with virological failure failed after a median duration of treatment of 1.6 years (IQR 0.8–3.6). In this subgroup, the HIV viral load at the start was undetectable in only 8 patients (26.7%) and 30% of these patients were prescribed a rescue treatment. In 60% of the patients, HIV transmission was parenteral and no statistically significant differences (39.8% in the group without virological failure, p=0.384) were found.
DiscussionThis study shows that the ABC/3TC+ATV combination may be a useful regimen in certain circumstances. After a median follow-up of 2.2 years, 12.7% of withdrawals due to treatment failure and 11.8% due to toxicity were observed. Treatment was mostly discontinued for minor causes, possibly related to the availability of simpler therapeutic alternatives on the market.
The efficacy and toxicity of the many different antiretroviral therapies has improved between the initial years and today as persistently undetectable viral loads can now be achieved, sometimes with just one tablet.15 To reach this situation, modern regimens, some of which are very complex, have had to be designed to achieve good results since undetectable viral loads were hard to achieve, either due to inefficacy or toxicity. In the case of PIs, one of the main problems was complications due to the compulsory use of RTV or cobicistat as a booster. As ATV is the only PI that can be administered unboosted, it has sometimes been a useful solution for patients who do not require TDF. Since this indication was not approved in the summary of product characteristics in Spain until October 2015,7 it has only been used in specific cases, with close patient supervision, and in situations where it was difficult to create a regimen within current clinical guidelines.1–3 There are other simplification studies, such as INDUMA,10 ARIES8,9 or ASSURE,11,12 that demonstrate the efficacy of the ABC/3TC+ATV regimen versus regimens with 2 NRTI+ATV/r. However, there are limited data on the use of this combination in everyday clinical practice.13,14 Data from our study could provide a scenario in which it could be used, taking into account the fact that there are now more therapeutic alternatives than were available when data were first collected from the patients described herein. Furthermore, the regimen was also used in other scenarios, such as prior treatment failure, for which even less data are available. We must also highlight that response in this group of patients was acceptable, with virological failure in 31% of the patients (data not shown).
It is important to point out that, even at the end of the long follow-up period, only 28% of patients continued to receive the treatment. Durability of the regimen, even in those patients who were withdrawn from treatment, was good and the median duration of use in this group was 1.6 years and almost 6 years in those patients who continued to receive the regimen, with gradual withdrawal observed on the survival curve.
Furthermore, after more than 2 years of follow-up, only 12.7% of patients showed virological failure. It is therefore a fairly safe regimen since toxicity was the reason for withdrawal in only 11.8% of cases. The main reasons for discontinuation of treatment were: drug interactions, simplification or at the physician's request for unspecified reasons. This, together with the fact that only 10% of patients began the regimen after 2013, may reflect the development of more convenient treatments (particularly involving a single tablet) with less drug interactions and not the fact that this combination is in an ineffective or poorly tolerated regimen. Treatment was discontinued in 6 patients due to low ATV levels with undetectable viral load. It must be clarified that monitoring of pharmacological levels is not standard clinical practice. However, several efficacy and toxicity studies relating to ATV levels have been conducted at Hospital Carlos III and pharmacological monitoring was performed in these patients.16
Our study has significant limitations, mainly because it is retrospective, which entails biases such as screening or loss of data. Even so, this combination should be considered in certain circumstances, such as situations where access to new drugs is restricted or there are problems using boosters.
In summary, in certain patients infected with HIV, changing to the ABC/3TC+ATV regimen for simplification and/or toxicity reasons has proven to be a long-term, effective therapeutic alternative. The development of new drugs, especially those formulated as a single tablet, could explain reduced use of this combination over recent years. However, more studies are required to evaluate the efficacy of this regimen, particularly in patients with virological failure in whom response was lower.
FundingNo funding was received to complete this article.
Conflicts of interestMa Eulalia Valencia has provided consultancy services and received payment for lectures from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dome and ViiV Healthcare.
Luz Martín Carbonero has provided consultancy services and received payment for lectures from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dome and ViiV Healthcare.
Victoria Moreno has provided consultancy services and received payment for lectures from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dome and ViiV Healthcare.
José Ignacio Bernardino has provided consultancy services and received payment for lectures from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dome and ViiV Healthcare.
Ma Luisa Montes has provided consultancy services and received payment for lectures from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dome and ViiV Healthcare.
Rocío Montejano is in receipt of a Rio Hortega grant from the Fondo de Investigaciones Sanitarias [Medical Research Fund].
Please cite this article as: Valencia ME, Martín-Carbonero L, Moreno V, Bernardino JI, Montes ML, Montejano R. Abacavir/lamivudina+atazanavir no potenciado en la práctica clínica diaria: doce años de experiencia. Enferm Infecc Microbiol Clin. 2018;36:29–33.