Certain polymorphisms in the non-muscle myosin IIA (MYH9) and apolipoprotein L1 (APOL1) genes have been associated to chronic kidney disease (CKD) in different populations. This study examined the association between the MHY9 rs2032487 and APOL1 rs73885319 polymorphisms and advanced CKD related to type 2 diabetes mellitus (T2DM) in a population of Gran Canaria (Canary Islands, Spain).
Patients and methodsPolymorphisms were genotyped in 152 patients with advanced CKD (estimated glomerular filtration rate [eGFR] <30ml/min/1.73m2) secondary to T2DM, 110 patients with T2DM onset ≥20 years before without advanced CKD (eGFR ≥45ml/min/1.73m2 and no proteinuria), and 292 healthy blood donors over 50 years of age without CKD or diabetes.
ResultsThe frequency of the risk allele for rs2032487 was 10.7% in patients with diabetes and advanced CKD, 7.1% in those with diabetes but without advanced CKD, and 6.1% in healthy subjects, with significant differences between the first and third groups (p=0.015). Among subjects with advanced CKD, 78.5% were homozygous for the protective allele, as compared to 87.9% in the other two groups (p=0.015 and p=0.016 respectively). The frequency of the risk allele for the rs73885319 polymorphism did not exceed 0.5% in any of the three groups.
ConclusionsThese data suggest that polymorphism rs2032487 is associated to advanced CKD related to T2DM in the population of Gran Canaria.
Ciertos polimorfismos de los genes de la miosina no muscular de tipo IIA (MYH9) y de la apolipoproteína L1 (APOL1) se han asociado con la enfermedad renal crónica (ERC) en distintas poblaciones. Este estudio evaluó la asociación entre los polimorfismos rs2032487 de MYH9 y rs73885319 de APOL1 con la ERC avanzada asociada a diabetes tipo 2 en una población de Gran Canaria.
Material y métodosLos polimorfismos se genotiparon en 152 pacientes con ERC avanzada (filtrado glomerular estimado [FGe]<30ml/min/1,73m2) secundaria a diabetes tipo 2, 110 pacientes con diabetes tipo 2 con evolución≥20 años sin ERC avanzada (FGe≥45ml/min/1,73m2 y ausencia de proteinuria) y 292 hemodonantes sanos de más de 50 años sin ERC ni diabetes.
ResultadosLa frecuencia del alelo de riesgo de rs2032487 fue de 10,7% entre pacientes con diabetes y ERC avanzada, 7,1% en aquellos con diabetes sin ERC avanzada y 6,1% en los sujetos sanos, alcanzándose diferencias significativas entre el primer y el tercer grupo (P=0,015). El 78,5% de los sujetos con ERC avanzada eran homocigotos para el alelo protector, frente al 87,9% en los ot dos grupos (P=0,015 y P=0,016, respectivamente). La frecuencia del alelo de riesgo del polimorfismo rs73885319 no superó el 0,5% en ninguno de los tres grupos.
ConclusionesEstos datos sugieren que el polimorfismo rs2032487 se asocia con la ERC avanzada asociada a diabetes tipo 2 en la población de Gran Canaria.
Type 2 diabetes mellitus (T2DM) is the most common cause of end-stage chronic kidney disease (CKD) in Western countries. In the Canary Islands (Spain), in particular, the incidence of end-stage CKD associated to diabetes is disproportionately high.1 According to calculations on the estimated population with diabetes in different Spanish Autonomous Communities, the risk of requiring renal replacement therapy (RRT) among people living in the Canary Islands is 3–5 times higher than among people living in other areas of Spain.2
The causes of this high incidence are not known, but it has been speculated that unidentified genetic factors might be implicated.3 In this regard, the analysis of uniparental genetic markers (mitochondrial DNA and chromosome Y),4,5 mobile autosomal DNA elements (Alu sequences)6 and human leukocyte antigen (HLA) haplotypes,5,7 suggests that the inhabitants of the Canary Islands come from the intermingling of aboriginals of the archipelago (which in turn represented a mixture of the populations from North Africa and Western Asia) with European colonizers mainly originating from the Iberian Peninsula – plus a small genetic contribution of sub-Saharan origin, probably from descendants of black individuals taken to the islands as slaves.
There are few epidemiological data on CKD in Africa, particularly due to the scarcity of registries of patients on renal replacement therapy, and in most diagnosed cases the underlying etiology is not known.8,9 However, particularly from studies in Afro-American populations in the United States, it is known that black individuals have a greater risk of developing CKD, and that at least part of this risk could be conferred by variants of two adjacent genes located on chromosome 22q13: the gene encoding for the non-muscle myosin heavy chain IIA (MYH9) and the apolipoprotein L1 gene (APOL1). Identification of the MYH9 gene as a susceptibility locus for CKD was a finding of complete genome association studies in Afro-American individuals that related different polymorphisms of this gene, fundamentally the four intronic variants that conform the so-called E1 haplotype, to certain forms of CKD such as hypertensive nephroangiosclerosis, focal and segmental glomerulosclerosis, and nephropathy associated to HIV infection.10,11 Subsequently, however, other groups reported that CKD in these populations was more strongly linked to variants of the APOL112,13 gene, which are in linkage disequilibrium with those of MYH9, and with the E1 haplotype in particular – suggesting that the association between the MYH9 gene and CKD could in fact be explained by the variants in APOL1.
The present study constitutes exploratory research aimed at estimating the allelic frequencies of two single-base polymorphisms previously linked to CKD and corresponding to genes MYH9 and APOL1 (rs2032487 and rs73885319, respectively), in the southern area of the island of Gran Canaria – an area where the population results from the intermingling of European and African genetic traits, in order to also assess their potential relation to the presence of advanced CKD associated to type 2 diabetes.
Material and methodsStudy subjects. The study subjects were participants in the CERCA-Diabetes study (Characterization of Chronic Kidney Disease Associated to Diabetes), a project aimed at characterizing the population with CKD associated to type 2 diabetes in the southern area of the island of Gran Canaria. We included as cases with CKD secondary to T2DM the 152 patients first referred to the advanced CKD monographic clinic of Hospital Universitario Insular de Gran Canaria between February 2011 and February 2015, in whom the estimated glomerular filtration rate (eGFR) was less than 30ml/min/1.73m2 according to the Modification of Diet in Renal Disease (MDRD-4) formula, and in whom diabetes had been established as the main cause of CKD. The control group consisted of 110 patients with type 2 diabetes recruited in the Section of Endocrinology and Nutrition of the same center, and who met the following criteria: a more than 20 year old diagnosis of diabetes, eGFR ≥45ml/min/1.73m2, and urinary albumin excretion <300mg/g creatinine in first morning void urine, or <300mg in a 24-h urine sample.
In addition, the same polymorphisms were also analyzed in a collection of DNA samples from 292 healthy blood donors aged 50 years or older, without diabetes or CKD.
Study protocol. After a 9-h fast, the participants provided blood samples, first morning void urine, and 24-h urine samples. A data collection questionnaire including family and personal medical history, lifestyle and medical treatment was then completed. Lastly, the patients underwent a physical examination including anthropometric data and blood pressure measurements, taken twice in the sitting position over 10min. The average of the two readings was calculated for the statistical analyses.
A diagnosis of diabetic retinopathy and cardiovascular disease was accepted when either condition was documented in the medical records. Severe diabetic retinopathy was defined as a history of proliferative retinopathy subjected to panretinal photocoagulation or vitrectomy, or a history of clinically significant macular edema subjected to focal photocoagulation or intravitreal drug injection. Subjects were classified as smokers or non-smokers, with the inclusion of former smokers as non-smokers. Physical activity was measured by asking about exercise performed during work activity and leisure time using the questionnaire prepared for the 2006 Spanish National Health Survey.
The biochemical measurements were performed at the Department of Biochemistry of Hospital Universitario Insular. Methodological details of the laboratory studies have been published elsewhere.14
All subjects gave written informed consent prior to participation in the study. The project was approved by the Ethics Committee of Complejo Hospitalario Universitario Insular Materno-Infantil of Las Palmas de Gran Canaria (reference: CEIC-CHMI-491).
Genotyping. Genotyping was performed at the Human Genotyping Unit of the Spanish National Cancer Research Center using OpenArray technology (Thermo Fisher Scientific, MA, USA), following the instructions of the manufacturer. Briefly, DNA sample loading was performed on plates that included testing of the two polymorphisms considered (Taqman Assay®, Thermo Fisher Scientific), using the OpenArray® AccuFill™ System (Thermo Fisher Scientific). The QuantStudio™ 12K Flex System (Thermo Fisher Scientific) was used for sample amplification and fluorescein data reading. Hapmap samples of known genotype were used as internal process control. Genotypes were assigned using Taqman Genotyper software (Thermo Fisher Scientific).
Statistical analysis. Categorical variables were reported as frequencies and percentages, and continuous variables as means±standard deviation (SD) or as the median (interquartile range [IQR]) in the case of a non-normal data distribution. Percentages were compared using the chi-squared test or the Fisher exact test, means were contrasted with the Student t-test, and medians with the Wilcoxon test for independent groups. Hardy–Weinberg equilibrium testing was performed using the chi-squared test on the global genotyped individuals. The association between polymorphism rs2032487 and CKD was assessed in additive, dominant and recessive inheritance models, using as reference the control group of diabetics without advanced CKD. Taking T as the normal allele and C the risk allele, we defined three variables associated to them. Firstly, an ordinal variable with values 0 (TT), 1 (TC) and 2 (CC), and secondly a binary variable with values TT and TC+CC, assuming that C is a dominant allele. Finally, another binary variable was defined, with values TT+TC and CC, in this case assuming that C is recessive. Odds ratios (ORs) were obtained for each of these variables using logistic regression analysis. Lastly, in order to assess the possible independent effect of polymorphism rs2032487 upon the presence of advanced CKD, a multivariate logistic regression analysis was performed including other variables seen to be associated to the dependent variable in the univariate analyses. The selection of variables in the multivariate model was based on a full enumeration algorithm and on the Akaike information criterion. Results were presented with the OR, 95% confidence interval (CI) and p-values. Statistical significance was considered for p<0.05. The data were analyzed using the R version 3.3.1 statistical package (Vienna, Austria).15
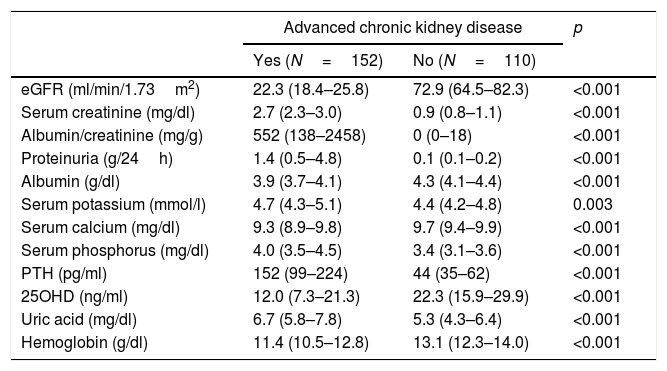
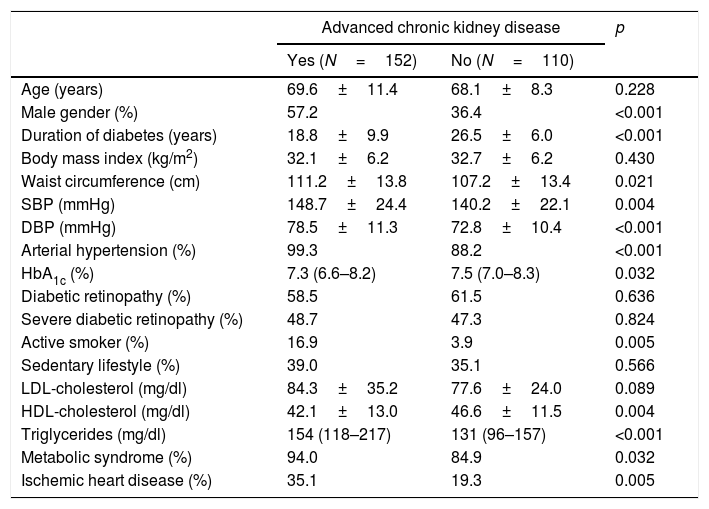
ResultsThe characteristics of the participants with type 2 diabetes, with or without advanced CKD, are summarized in Tables 1 and 2. Table 1 shows the results referred to different anomalous laboratory measurements in patients with advanced CKD, all of which proved significantly different between the two groups. Overall, it can be seen that although the inclusion of patients with mild forms of CKD in the control group was accepted, most of them had normal renal function. Table 2 shows other demographic, clinical and biochemical characteristics of both groups. Because of the requirement to include only individuals diagnosed with diabetes for at least 20 years in the control group, the duration of the disease was longer in this group than in the advanced CKD group. The proportion of men was significantly higher among the individuals with advanced CKD, which also exhibited lower glycosylated hemoglobin (HbA1c) levels, a greater cardiovascular risk (larger proportion of active smokers, higher blood pressure and waist circumference values, and poorer HDL-cholesterol and triglyceride levels), and a greater prevalence of established coronary disease.
Biochemical variables associated to CKD in the study population.
| Advanced chronic kidney disease | p | ||
|---|---|---|---|
| Yes (N=152) | No (N=110) | ||
| eGFR (ml/min/1.73m2) | 22.3 (18.4–25.8) | 72.9 (64.5–82.3) | <0.001 |
| Serum creatinine (mg/dl) | 2.7 (2.3–3.0) | 0.9 (0.8–1.1) | <0.001 |
| Albumin/creatinine (mg/g) | 552 (138–2458) | 0 (0–18) | <0.001 |
| Proteinuria (g/24h) | 1.4 (0.5–4.8) | 0.1 (0.1–0.2) | <0.001 |
| Albumin (g/dl) | 3.9 (3.7–4.1) | 4.3 (4.1–4.4) | <0.001 |
| Serum potassium (mmol/l) | 4.7 (4.3–5.1) | 4.4 (4.2–4.8) | 0.003 |
| Serum calcium (mg/dl) | 9.3 (8.9–9.8) | 9.7 (9.4–9.9) | <0.001 |
| Serum phosphorus (mg/dl) | 4.0 (3.5–4.5) | 3.4 (3.1–3.6) | <0.001 |
| PTH (pg/ml) | 152 (99–224) | 44 (35–62) | <0.001 |
| 25OHD (ng/ml) | 12.0 (7.3–21.3) | 22.3 (15.9–29.9) | <0.001 |
| Uric acid (mg/dl) | 6.7 (5.8–7.8) | 5.3 (4.3–6.4) | <0.001 |
| Hemoglobin (g/dl) | 11.4 (10.5–12.8) | 13.1 (12.3–14.0) | <0.001 |
eGFR: estimated glomerular filtration rate.
Demographic and clinical characteristics of the patients.
| Advanced chronic kidney disease | p | ||
|---|---|---|---|
| Yes (N=152) | No (N=110) | ||
| Age (years) | 69.6±11.4 | 68.1±8.3 | 0.228 |
| Male gender (%) | 57.2 | 36.4 | <0.001 |
| Duration of diabetes (years) | 18.8±9.9 | 26.5±6.0 | <0.001 |
| Body mass index (kg/m2) | 32.1±6.2 | 32.7±6.2 | 0.430 |
| Waist circumference (cm) | 111.2±13.8 | 107.2±13.4 | 0.021 |
| SBP (mmHg) | 148.7±24.4 | 140.2±22.1 | 0.004 |
| DBP (mmHg) | 78.5±11.3 | 72.8±10.4 | <0.001 |
| Arterial hypertension (%) | 99.3 | 88.2 | <0.001 |
| HbA1c (%) | 7.3 (6.6–8.2) | 7.5 (7.0–8.3) | 0.032 |
| Diabetic retinopathy (%) | 58.5 | 61.5 | 0.636 |
| Severe diabetic retinopathy (%) | 48.7 | 47.3 | 0.824 |
| Active smoker (%) | 16.9 | 3.9 | 0.005 |
| Sedentary lifestyle (%) | 39.0 | 35.1 | 0.566 |
| LDL-cholesterol (mg/dl) | 84.3±35.2 | 77.6±24.0 | 0.089 |
| HDL-cholesterol (mg/dl) | 42.1±13.0 | 46.6±11.5 | 0.004 |
| Triglycerides (mg/dl) | 154 (118–217) | 131 (96–157) | <0.001 |
| Metabolic syndrome (%) | 94.0 | 84.9 | 0.032 |
| Ischemic heart disease (%) | 35.1 | 19.3 | 0.005 |
DBP: diastolic blood pressure; SBP: systolic blood pressure.
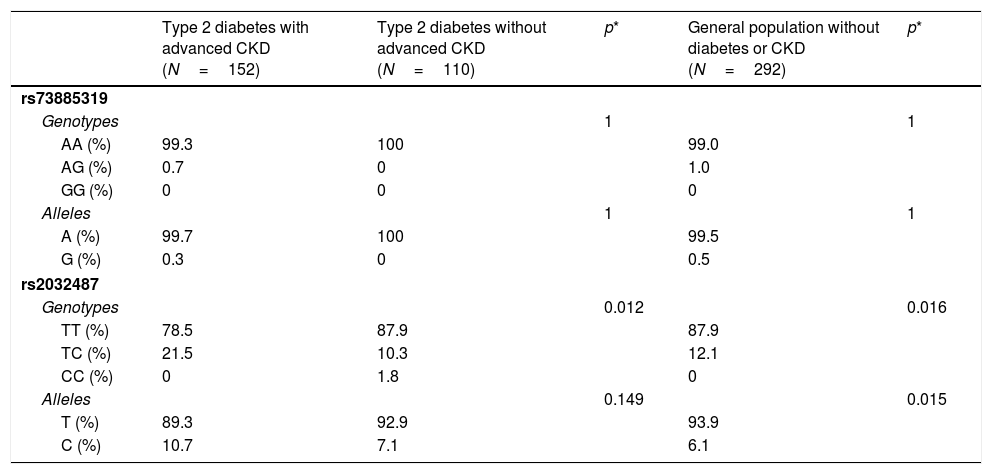
Virtually all the genotyped individuals, including the healthy blood donors, were homozygous for the A allele of polymorphism rs73885319 (Table 3). Only one individual in the group with diabetes and advanced CKD, and three healthy blood donors, were heterozygous. These data confirmed that the risk allele of this variant (the G allele) is virtually absent in Gran Canaria, as occurs in other Caucasian populations; it therefore was not subjected to further analysis.
Table 3 also shows the genotypic and allelic frequencies of polymorphism rs2032487 in the different groups analyzed. The distribution did not deviate from Hardy–Weinberg equilibrium (p=0.717 for all genotyped individuals). The comparison between groups of diabetic patients with and without advanced CKD showed statistically significant differences, at the expense of a lower frequency of the TT genotype among the subjects with CKD (78.5% versus 87.9%; p=0.012). The genotypic frequencies in the control group of healthy subjects were virtually identical to those seen in the diabetic group without CKD, and therefore also significantly different from those of the diabetic group with advanced CKD (p=0.016) (Table 3). The differences in allelic frequencies between the two groups with diabetes were not significant, probably because of the limited number of subjects studied, but statistical significance was reached when the patients with diabetes and advanced CKD were compared against the healthy blood donors (p=0.015) (Table 3).
Genotypic and allelic frequencies of polymorphisms rs73885319 and rs2032487 in the different populations analyzed.
| Type 2 diabetes with advanced CKD (N=152) | Type 2 diabetes without advanced CKD (N=110) | p* | General population without diabetes or CKD (N=292) | p* | |
|---|---|---|---|---|---|
| rs73885319 | |||||
| Genotypes | 1 | 1 | |||
| AA (%) | 99.3 | 100 | 99.0 | ||
| AG (%) | 0.7 | 0 | 1.0 | ||
| GG (%) | 0 | 0 | 0 | ||
| Alleles | 1 | 1 | |||
| A (%) | 99.7 | 100 | 99.5 | ||
| G (%) | 0.3 | 0 | 0.5 | ||
| rs2032487 | |||||
| Genotypes | 0.012 | 0.016 | |||
| TT (%) | 78.5 | 87.9 | 87.9 | ||
| TC (%) | 21.5 | 10.3 | 12.1 | ||
| CC (%) | 0 | 1.8 | 0 | ||
| Alleles | 0.149 | 0.015 | |||
| T (%) | 89.3 | 92.9 | 93.9 | ||
| C (%) | 10.7 | 7.1 | 6.1 | ||
CKD: chronic kidney disease.
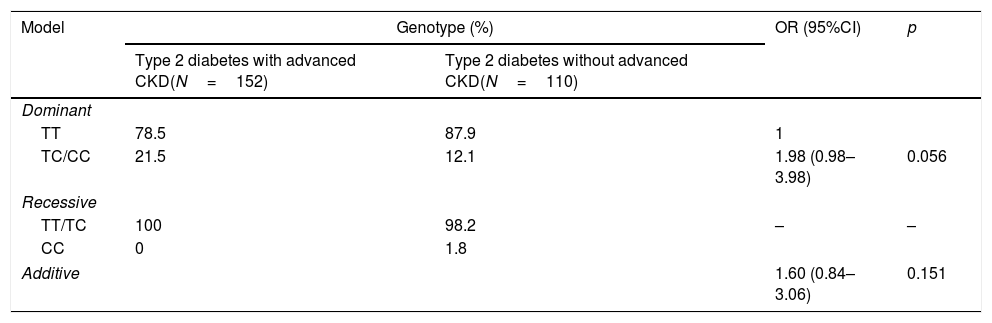
On applying different genetic models to verify the association between polymorphism rs2032487 and advanced CKD, it was not possible to evaluate the recessive model, since only two subjects were homozygous for risk allele C. Neither of the other two models was significantly associated to the presence of advanced CKD (Table 4).
Analysis of the relationship between polymorphism rs2032487 and advanced chronic kidney disease associated to type 2 diabetes according to the hereditary model.
| Model | Genotype (%) | OR (95%CI) | p | |
|---|---|---|---|---|
| Type 2 diabetes with advanced CKD(N=152) | Type 2 diabetes without advanced CKD(N=110) | |||
| Dominant | ||||
| TT | 78.5 | 87.9 | 1 | |
| TC/CC | 21.5 | 12.1 | 1.98 (0.98–3.98) | 0.056 |
| Recessive | ||||
| TT/TC | 100 | 98.2 | – | – |
| CC | 0 | 1.8 | ||
| Additive | 1.60 (0.84–3.06) | 0.151 | ||
CKD: chronic kidney disease.
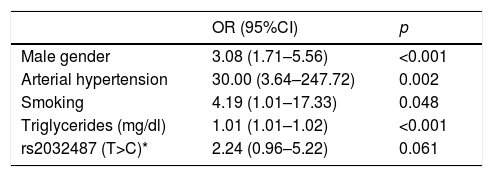
In a multivariate model including polymorphism rs2032487 together with all the variables associated to CKD among subjects with diabetes, the effect of the polymorphism was no longer significant, and only the male gender, high blood pressure, smoking and triglyceride levels remained associated to CKD (Table 5). Polymorphism rs2032487 was excluded from the model when smoking was included in the latter.
Variables associated to advanced chronic kidney disease in patients with type 2 diabetes in the multivariate analysis.
| OR (95%CI) | p | |
|---|---|---|
| Male gender | 3.08 (1.71–5.56) | <0.001 |
| Arterial hypertension | 30.00 (3.64–247.72) | 0.002 |
| Smoking | 4.19 (1.01–17.33) | 0.048 |
| Triglycerides (mg/dl) | 1.01 (1.01–1.02) | <0.001 |
| rs2032487 (T>C)* | 2.24 (0.96–5.22) | 0.061 |
Variations in genes MYH9 and APOL1 represent a significant part of the increased risk of non-diabetic CKD in black individuals. In these populations, the risk haplotypes of both genes are strongly co-segregated, and the participation of each of them in the risk of kidney disease is not precisely known. Nevertheless, both the initial studies in Afro-Americans in the United States12,13 and other later studies in West African populations16 suggest a predominant role of polymorphic variants of the APOL1 gene. However, some polymorphisms of MYH9 also showed an association to non-diabetic CKD in a predominantly Caucasian population in the United States, in which the frequency of risk variants of APOL1 was extremely low17 – thus evidencing an independent role of MYH9 as a factor responsible for the increased risk of CKD.
In terms of diabetes-related CKD, the seminal studies that discovered the MYH9 gene, using genetic mapping by mixed linkage disequilibrium, found no association between this gene and the end-stage CKD attributed to diabetes in the Afro-American population.10,11 However, the authors of one of these studies conducted additional research, specifically analyzing a larger population with a clinical diagnosis of end-stage CKD secondary to type 2 diabetes, and were able to demonstrate an association between CKD and two MYH918 haplotypes. New studies associating complete genome and candidate gene analysis19,20 have corroborated the relationship between variants of MYH9 and end-stage diabetic nephropathy in Afro-Americans, but also in this case, as in non-diabetic CKD, the relationship was significantly attenuated on adjusting for the effect of APOL1.21 Information on the role of MYH9 in CKD secondary to diabetes in Caucasian populations is scarce. A study conducted in Americans of European descent found an association between terminal CKD and three E1 haplotype polymorphisms (rs4821480, rs2032487 and rs4281481).22 These results were not confirmed in a smaller population in the United Kingdom,23 though in this case the study sample mainly consisted of patients with non-advanced CKD.
The data recorded in the present study suggest that this variant of the MYH9 gene could also be related to an increased risk of advanced CKD in patients with type 2 diabetes in the population of Gran Canaria.
These results should be regarded as preliminary, given the considerable limitations of the study. Firstly, only a single E1 haplotype polymorphism was investigated, and future studies including other variants of the gene would be advisable. Specifically, and as noted by other authors,24 the E1 haplotype could be inferred more reliably simply by additional analysis of polymorphism rs3752462. On the other hand, it is also possible that in our population the risk polymorphisms do not exhibit the same degree of linkage disequilibrium seen in other populations. In this regard, a study conducted in Spain on the general population in the region of Asturias,25 eGFR <60ml/min/1.73m2 was found to be associated to polymorphism rs3752462, but not to rs4821480, which is in linkage disequilibrium with rs2032487. The second major limitation of this study is the small sample size involved, particularly considering the low frequency of the rs2032487 risk allele. It should be noted that, for the frequencies seen, the sample size required to achieve a statistical power of 80%, with a significance value of 0.05, would have been 978 subjects per group – a number far above the sample size of our study. In fact, it was not possible to analyze the possible association of polymorphism with a recessive genetic model, given the rarity of the CC homozygous genotype. This aspect may be relevant, since most published studies agree that the effect of intronic variants of MYH9 upon the susceptibility to CKD is based on a recessive hereditary pattern.10,11,18,22,24
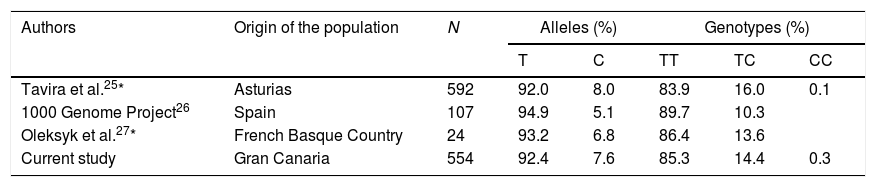
Although a small proportion of people in the Canary Islands are known to retain genetic traits of sub-Saharan ancestors, the frequency of the APOL1 risk genotype was minimal, as in Caucasian populations,17,22 and we observed no co-segregation between MYH9 polymorphism and that of APOL1. As mentioned above, the frequency of the African ancestral allele (C) of rs2032487 was also low, compared to populations in black Africa, and globally similar to that recorded in the Iberian population of the 1000 Genome Project26 and in other European populations.25,27Table 5 summarizes the genotypic and allelic frequencies of polymorphism rs2032487 in different populations close to that evaluated in our study. It thus seems highly unlikely that variations in MHY9 may have an impact upon the increased risk of CKD associated to type 2 diabetes seen in Gran Canaria. New steps should be taken to search for other possible genes that increase susceptibility to CKD in the population of the Canary Islands with type 2 diabetes. Considering that the sample universe is limited and may even be genetically heterogeneous among different islands, it could be useful to design studies allowing the use of massive genotyping strategies without requiring the large sample sizes usually needed in procedures of this kind, for example by using broad genomic association studies applied to large pedigree studies with rigorous phenotyping28 (Table 6).
Comparison of allelic and genotypic frequencies of polymorphism rs2032487 between the study population and other populations of the Iberian Peninsula.
| Authors | Origin of the population | N | Alleles (%) | Genotypes (%) | |||
|---|---|---|---|---|---|---|---|
| T | C | TT | TC | CC | |||
| Tavira et al.25* | Asturias | 592 | 92.0 | 8.0 | 83.9 | 16.0 | 0.1 |
| 1000 Genome Project26 | Spain | 107 | 94.9 | 5.1 | 89.7 | 10.3 | |
| Oleksyk et al.27* | French Basque Country | 24 | 93.2 | 6.8 | 86.4 | 13.6 | |
| Current study | Gran Canaria | 554 | 92.4 | 7.6 | 85.3 | 14.4 | 0.3 |
Imputed data of the polymorphism rs4821480 genotype, which is found in absolute linkage disequilibrium with rs2032487.
The MYH9 gene encodes for myosin 9, one of the subunits that conform non-muscle myosin type IIA. Myosin type IIA forms part of the actin–myosin complex, a key element in cell motility and cytoskeletal integrity. In the kidney, the MYH9 gene is mainly expressed by podocytes, as well as by mesangial cells and peritubular capillaries and arterioles, and it has been postulated for some time that the abnormal expression of MYH9 could induce podocyte fragility, thereby promoting proteinuria and renal failure.24 Several studies have confirmed this hypothesis in murine models of animals carrying MHY9 inactivating mutations, and which experience podocyte dysfunction, glomerulosclerosis, and accelerated development of CKD, mainly when exposed to different agents that cause kidney injury, such as angiotensin II, reduced renal mass, or certain nephrotoxic drugs.29–31 The extent to which these findings can be used to explain the association between different intronic mutations in MHY9 and the risk of diabetic and non-diabetic CKD is not yet clear. In any case, the existing evidence suggests that the development of albuminuria or glomerular damage in the context of alterations in MHY9 expression might require second stimuli, such as high blood pressure or a loss of functioning nephrons; it is thus possible that pathogenicity only results in the presence of environmental factors. In our multivariate analysis, the association between polymorphism rs2032487 and advanced CKD was no longer significant upon adjusting for other factors, mainly smoking – thus effectively suggesting an interaction between this MHY9 variant and certain environmental agents. The results of the multivariate analysis must be considered with caution, since they are based on point values of different variables whose effect upon CKD risk is a result of many years of exposure. In any case, many existing data have related smoking to the progression of diabetic nephropathy. Glomerular hypertrophy, glomerulosclerosis, mesangial expansion, glomerular filtration barrier alterations, elevated albuminuria and impaired glomerular filtration have been observed among smokers with diabetes.32 In fact, there is some evidence that smoking induces podocyte damage in patients with diabetes and microalbuminuria.33 Future studies will be needed to investigate whether the intronic variants of MHY9 promote renal damage, facilitating the harmful effect of other environmental factors upon the podocytes and the glomerular filtration barrier.
In conclusion, although its allelic frequency was not different from that seen in other European populations, polymorphism rs2032487 of the MHY9 gene was associated to advanced CKD in patients with type 2 diabetes mellitus in the southern area of Gran Canaria. The risk haplotypes of this gene should be studied in larger populations in order to assess their role as markers of kidney damage risk in populations with diabetes in our setting.
Financial supportThis work was funded by the Spanish Ministry of Science and Innovation (IP 11 1880). The Human Genotyping Laboratory, member of the CeGen, PRB2-ISCIII, was supported by grant PT13/0001/0005 of EP I+D+i 2013–2016, funded by ISCIII and ERDF (European Regional Development Fund).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Boronat M, Tugores A, Saavedra P, Garay P, Bosch E, Lorenzo D, et al. Asociación entre el polimorfismo rs2032487 del gen de la cadena pesada de la miosina no muscular tipo IIA (MHY9) y la enfermedad renal crónica secundaria a diabetes tipo 2 en población de las Islas Canarias. Endocrinol Diabetes Nutr. 2019;66:639–646.