Although we lack enough evidence to justify supplementing with vitamin D in the prevention and treatment of COVID-19 infection, it is increasingly feasible that this hypothesis is valid. Two general underlying mechanisms should be considered. One would be the anti-infectious and immunomodulatory action that it exerts by improving intercellular barriers by stimulating innate immunity, as well as by modulating adaptive immunity. Also, vitamin D reduces the production of inflammatory cytokines, such as IL-2 and interferon-gamma (INFγ). More recently, multiple pleiotropic effects have been demonstrated on the actions of vitamin D at the anti-inflammatory and immunomodulatory level with positive results in studies with influenza, coronavirus, and other respiratory infections. An inverse relationship between serum vitamin D levels and the prevalence of the respiratory infectious disease has been described. Of interest, another mechanistic approach responds to considering the inhibition of the renin-angiotensin-aldosterone system (RAAS), which is exacerbated in COVID-19 infection because the virus binds to the enzyme ACE2, making more angiotensin II available to cause damage. Vitamin D inhibits mediators of RAAS – present in all cells of the body – and by inhibiting ACE activity and increasing ACE2, it lowers angiotensin II levels. We present studies with proposals for recommended doses of vitamin D, and although a single guideline is not specified, the possible benefits are promising. Finally, the purpose of this review is to share this idea with health professionals to ignite the debate and call for critical reflection, so that it can contribute to the undertaking of more and better clinical designs to validate the benefits of using high doses of vitamin D for the benefit of public health and especially in times of crisis for COVID-19.
Si bien carecemos de suficiente evidencia que justifique suplementar con vitamina D en la prevención y/o tratamiento de la infección por COVID-19, a la fecha resulta cada vez más factible que esta hipótesis sea válida. Dos mecanismos básicos generales deberían ser considerados. Uno sería la acción anti-infecciosa e inmuno-moduladora que ejerce mejorando las barreras intercelulares por estímulo de la inmunidad innata, así también por modulación de la inmunidad adaptativa. También, la vitamina D reduce la producción de citoquinas inflamatorias como IL-2 e interferón gamma (INFγ). Más recientemente se han demostrado múltiples efectos pleiotrópicos sobre las acciones de vitamina D a nivel anti-inflamatorio e inmuno-modulador. Esto explica resultados positivos en estudios con influenza, coronavirus y otras infecciones respiratorias. Se ha descripto relación inversa entre niveles séricos de vitamina D y prevalencia de patología infecciosa respiratoria. De interés, otro abordaje mecanístico responde a considerar la inhibición del sistema renina-angiotensina-aldosterona, que se exacerba en la infección por COVID-19 debido a que el virus se une a la enzima ECA2, quedando disponible más angiotensina II para causar daño. La vitamina D inhibe mediadores del SRAA -presente en todas las células del organismo-, y por inhibir la actividad ECA y aumentar la ECA2, disminuye los niveles de angiotensina II. Presentamos estudios con propuestas de dosis recomendadas de vitamina D y aunque no quede concretada una única guía, los posibles beneficios son promisorios. Finalmente, el propósito de la presente revisión es compartir esta idea con profesionales de la salud para encender el debate y llamar a la reflexión crítica, de modo tal que se pueda contribuir con el emprendimiento de diseños clínicos adecuados para validar los beneficios de utilizar altas dosis de vitamina D en beneficio de la salud pública y sobre todo en tiempos de esta emergencia por COVID-19.
In recent months, multiple therapeutic strategies have been implemented - some scientifically based and others even empirical- to address the COVID-19 pandemic, including antiretroviral drugs, steroids, immunomodulators, among others. Interestingly, the rational basis for empirical treatments is urgency, and the lack of clinical-pharmacological evidence for COVID-19. However, it is essential to remember that empiricism highlights the role of experience and evidence and, therefore, sooner or later constructs rigour of medical evidence or evidence-based medicine. We still lack sufficient evidence (some clinical studies) to support the benefits of deploying high doses of vitamin D in the population and/or in patients exposed to SARS-CoV-2. However, the hypothesis appears increasingly close to being validated.
In fact, it is known that vitamin D receptors (VDR) existed in very primitive organisms that lacked an appropriate dermal system to synthesize their specific ligand, vitamin D. Also, these organisms lacked devices such as the osteoarticular, cardiovascular systems, kidneys or even lungs.1 Therefore, the question arises as to their purpose, and a first approach to an answer would be that they would form part of a complex defence system. VDRs were originally described inside cells at the cytoplasmic level, but later and of special interest, they were also found in some fundamental organelles such as the mitochondria.2,3 This novel location reinforced the notion of non-genomic effects for vitamin D, especially if we consider that its most well-known actions (both genomic and non-genomic) are the result of hormone-receptor interaction at the cytoplasmic level. This binding, which undergoes regulation, forms a complex transcription factor which, translocated to the cell nucleus, produces the modulation of multiple genes mediating phosphocalcic metabolism.4,5
Of note, more than two decades ago, a diversity of actions was described for vitamin D that included both those related to phosphocalcic metabolism and other "pleiotropic" actions and that included inhibition of cancer cell proliferation, effects on hormone secretion and suppression of T-cell proliferation, as well as the modulation of certain cytokines.6 In this regard, more recently it has been shown that vitamin D and its metabolites are actively involved in the regulation of innate and adaptive immune responses, and therefore its deficiency is associated with a number of autoimmune and allergic infections.7 These findings justify the conjecture regarding evolutionary background and support the idea that its primary role is probably that of cellular and tissue defence through immune mechanisms and/or regulation of inflammatory processes. In addition, evolution also enabled it to interact with other systems that are fundamental to the maintenance of cellular homeostasis. Thus, vitamin D opposes and/or modulates the signalling pathways of another ancient system, the renin-angiotensin-aldosterone system (RAAS), which is characterised by regulating the internal environment and haemodynamics in superior organisms, but which, of central interest to this review, also functions as a complex pro-inflammatory system.1 Therefore, it is no coincidence that most mammalian cells express both VDRs and RAAS receptors and enzymes. Vitamin D, its metabolites and receptors on the one hand, and the RAAS on the other, would represent two sides of the same coin - the maintenance of the delicate balance of cell defence - as mediators of pro-inflammatory and anti-inflammatory processes.
In parallel to the existence of VDRs in almost all the cells of the organism, and not only at renal level, the presence of the 1-α-hydroxylase enzyme has also been demonstrated, suggesting a certain independence between circulating vitamin D levels with respect to intracellular levels.8 This concept complicates the understanding of the diagnostic/prognostic value of circulating vitamin D tests and its possible involvement in diseases not classically related to vitamin D such as cancer, multiple sclerosis and pre-eclampsia, among others.9
Actions of vitamin D linked to the axis of infection, inflammation, immune response and target organ injuryVitamin D as an anti-infective and immunomodulating hormoneAs previously mentioned, vitamin D can decrease the risk of infection by various mechanisms, which include participating in the integrity of a physical barrier and enhancing innate cellular and/or adaptive immunity.10
The barrier effect would be exerted through the stimulation of protein-coding genes related to cell integrity and junctions such as occludin (tight junctions), connexin 43 (gap junctions) and E-cadherin (adherens junctions).11 Here, it should be clarified that viruses generally alter the integrity of these barriers, which increases their degree of infectivity,12 and therefore the activity demonstrated for vitamin D of maintaining cell barrier integrity is promising.
Furthermore, and in relation to the immune system itself, it has been established that cells specialising in defence such as macrophages, monocytes, dendritic cells, T and B lymphocytes express VDRs and enzymes for the synthesis of vitamin D.13,14 The stimulus that vitamin D exerts on innate cell immunity takes place through the induction of antimicrobial peptides such as cathelicidin and β-defensin-2. Cathelicidin alters the membranes and acts on bacteria, viruses, fungi and even the Koch bacillus; in addition, it helps to reduce the so-called "cytokine storm" that occurs in severe viral infections such as those described for COVID-19,15 inhibiting the production of pro-inflammatory cytokines from Th1 cells such as tumour necrosis factor-alpha (TNF-α) and gamma interferon (INF-γ).16 The human β-defensin-2 peptide, in turn, is produced by epithelial cells and has powerful antimicrobial activity against gram-negative bacteria and Candida, which leads us to postulate that β-defensin-2 could contribute to reduce the frequency of skin infections and -of interest for this review- lung tissue infections.17
In relation to vitamin D and its action on adaptive immunity, this is exerted by suppressing the responses mediated by type 1 helper cells (Th1), reducing the production of pro-inflammatory cytokines such as interleukin-2 (IL-2) and INF-γ.18 It also promotes the production of anti-inflammatory cytokines by Th2 cells, collaborating with Th1 inhibition, and regulatory T cell induction.
Considering the above, it is worth mentioning that multiple studies have been able to demonstrate that people with chronic diseases have lower levels of vitamin D in relation to healthy subjects. It has even been discussed whether deficiency is a cause or a consequence of these conditions (vitamin D can be taken in its defensive role against injury). However, what is compelling in light of the evidence is that there is a strong association between deficiency and the presence of diseases non-classical for the spectrum of vitamin D. Studies of supplementation against placebo are scarce and most are conducted with low doses, over short periods of time, or in healthy subjects with normal vitamin D levels. These designs could barely establish benefits, while, as was to be expected, they demonstrated no difference and/or benefit when comparing individuals with similar conditions.19,20 However, an inverse relationship has been reported between vitamin D status and the onset of various viral diseases (dengue, hepatitis, herpes virus, influenza, respiratory syncytial virus, rotavirus, upper respiratory tract infections, enteric, urinary, pneumonia, otitis media, vaginitis, sepsis, hepatitis and HIV.21
Vitamin D and viral infectionsFlu (seasonal influenza)The influenza virus affects the respiratory tract by direct viral infection or by damage to immune system response. In addition, one-third of hospitalized patients with confirmed influenza have been reported to develop pneumonia, and this more prevalent in children and the elderly, or in chronic lung, heart, immunocompromised patients and smokers.22 It is known that survival of the virus is higher in seasons with low temperatures, but it has also been suggested that the increased incidence in winter may also be due to lower solar radiation and therefore to lower vitamin D levels.23 Then, and as a result of a poor level of vitamin D, fewer endogenous peptides with antibiotic properties (defensins and cathelicidins) would be synthesized such as those previously mentioned. In this regard, cathelicidin production is dose-dependent on the level of vitamin D. More specifically, 30 ng/mL of vitamin D was established as the minimum required for optimal cathelicidin induction.24 This same level of vitamin D was established as a cut-off value (Third National Health and Nutrition Examination Survey) for lower incidence of upper respiratory infections.25
Later, in 2018, the role of vitamin D in influenza was reviewed and as a result of analysis in studies of supplementation versus placebo, using different methodologies and dosages, positive results were established in most.26–28 In addition, the GrassrootsHealth study, based on questionnaires to 12,605 participants comparing the presence or absence of influenza syndrome in the last 6 months and vitamin D measurement, established that individuals with levels in the order of 60 ng/mL of vitamin D or higher had a 43% lower risk of diseases such as influenza compared to those with levels equal to or lower than 20 ng/mL (p < .0001).29 (https://www.grassrootshealth.net/project/our-scientists/).
Coronavirus infectionsViruses such as influenza and coronavirus cause infections especially in winter, which can be particularly severe and even fatal due to pneumonia. Thus, during the current COVID-19 pandemic, a promising hypothesis has been postulated about increased caseloads and deaths in regions where average vitamin D levels are low.30 In line with this, during the Chinese winter fatal cases were higher at older ages (14.8% for individuals aged 80 or more), more in men than in women (2.8% vs. 1.7%) and more frequent with comorbidities (in older age, more chronic diseases).
But there are also other variables of interest for analysis, such as ethnicity. Currently the morbidity and mortality rates for COVID-19 in African-American or black individuals are the highest in many parts of the world. Specifically, a survey of the US community and Johns Hopkins University indicated that the infection rate is more than triple and the mortality rate is 6 times higher in predominantly black counties than in predominantly white counties.31 It should be noted that while this finding may be due to multiple confounding factors, such as the socioeconomic variable, the objective data that African Americans had low serum levels of vitamin D relative to the white population is undeniable.32 It is noteworthy that within the African-American ethnic group, more cases were established in states with less sun exposure and with colder weather.
The line of reasoning, therefore, leads us to conclude that in all cases with low or very low levels of vitamin D, the mechanisms will be favoured by which viruses such as influenza and coronavirus can more easily alter the lung epithelium through increased production of Th1 cytokines as part of the innate immune response to the viral infection itself.16 Similarly, low levels of vitamin D would favour the release of INF-γ,18 ultimately being responsible for damage in the late phase of SARS-CoV-2.33 Here the so-called "cytokine storm", favoured by vitamin D deficiency, will further complicate these viral infections, as reported for COVID-19, where Th2 cytokine bursts (IL-4 and IL-10) occur.15
Despite recent findings on possible antiviral effects of vitamin D, in-depth understanding of its complex mechanisms is still poor. This is due to the intricate relationship established between viral infections and vitamin D, which includes, among other factors, induction of antiviral status, immunoregulatory functional characteristics, interaction with cellular and viral factors, autophagy and apoptosis induction, genetic and epigenetic alterations. It seems that vitamin D interferes in a transitory way in the viral intracellular signalling pathways, causing an essential modulating effect in viral gene transcription.21
Anti-inflammatory and antioxidant actions of vitamin D at lung levelLung epithelial cells have high expression of the 1-α-hydroxylase enzyme, which allows local synthesis of 1,25-dihydroxyvitamin D, the most active form of vitamin D, also called calcitriol. Calcitriol inhibits the production and secretion of many cytokines from bronchial smooth muscle cells, such as platelet-derived growth factor, RANTES (regulated on activation, normal T cell expressed and secreted) and matrix metalloproteinases, leading to a reduction in proliferation and inflammation in lung smooth muscle cells. Vitamin D stimulates the synthesis of IL-10 by CD4+, CD25+, Foxp3+ and T-regulatory cells. At the same time, it inhibits the activation of dendritic cells by negatively regulating the expression of CD80/86 and CD40. Furthermore, and as previously mentioned, vitamin D stimulates the expression of cathelicidin and many other anti-infectious molecules.34,35
Supplementation with 1,25-dihydroxyvitamin D suppresses the recruitment of eosinophils and lymphocytes in the airways, decreases the production of IL-4 from the T cells and inhibits the migration of T cells by attenuating the inflammatory response.36 It also works as an adjuvant to other therapies, such as allergen immunotherapy.37 Simultaneous administration of vitamin D and dexamethasone in steroid-resistant asthma patients increased IL-10 synthesis to levels similar to those found in steroid-sensitive patients treated with dexamethasone alone.38
Significant reductions in serum IgE and eotaxin levels have also been described in an asthma model by treatment with vitamin D.39 In addition, airway infiltration of inflammatory cells, serum levels of IL-6, TNF-α and IL-1β, as well as expression of apoptotic protein associated with Bcl2, caspase-3, TLR4, NF-κB and phosphorylated NF-κB p65 were reduced. Thus, vitamin D raised serum levels of IL-10, reducing the inflammatory and apoptotic response in that asthmatic mouse model.40 Of interest, vitamin D suppressed the synthesis of 8-isoprostane (8-iso), IL-6 and granulocyte colony-stimulating factor and macrophages in human bronchial epithelial cells exposed to contaminating particles. In addition, it increased expression of the antioxidant pathway gene G6PD and levels of oxidised glutathione, and therefore it is inferred that vitamin D appears to protect the lungs and airways in asthmatic pathology through its anti-inflammatory and antioxidant effects.41
In a murine model of bleomycin-induced lung inflammation, calcitriol reduced early lung inflammation by attenuating immune cell infiltration, suppressing inflammatory cytokine secretion, blocking nuclear translocation of nuclear factor kappa B (NF-κB) p65, inhibiting phosphorylation of pulmonary p38 MAPK and protein kinase B (Akt), attenuating alpha-smooth muscle actin (a marker for epithelial-mesenchymal transition in the lungs, which promotes fibrosis), and decreased phosphorylation of transforming growth factor beta 1 (TGF-β1) regulated by augmentation and Smad.42 Calcitriol also caused a 40% reduction in neutrophil recruitment to the lungs in an acute lung injury animal model. This anti-inflammatory effect of vitamin D could be mediated by inhibition of IL-8 secretion at the pulmonary level.43
The administration of vitamin D to neonatal rats with hyperoxia-induced lung injury (as a model of bronchopulmonary dysplasia) caused attenuation of this injury through several protective actions, such as preserving the integrity of the lung structure, decreasing inflammation by negatively regulating TLR4 activation, reducing extracellular matrix deposition and inhibiting lung cell apoptosis.44 Vitamin D has also been shown to have immunomodulatory and anti-inflammatory effects in the treatment of cystic fibrosis of the respiratory tract by reducing the expression of CD279 (PD-1) on CD4+ and CD8 + T cells. In addition, vitamin D decreased the frequency of CD8+ and mucosa-associated invariant T cells that co-express CD38 and human leucocyte antigen D activation markers. Therefore, treatment with vitamin D would prevent the progression of lung damage associated with cystic fibrosis of the airways.45
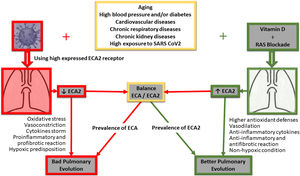
Oxidative stress caused by tobacco smoke worsens the progression of chronic obstructive pulmonary disease (COPD). In this regard, vitamin D has also been proposed as a natural anti-inflammatory and antioxidant capable of improving the prognosis of this lung disease in patients who smoke.46 In fact, it has been observed that COPD patients have lower plasma levels of vitamin D than healthy patients, suggesting a possible correlation between poor antioxidant defence and the development of this lung disease.47 Of central interest to this review paper, some years ago our group raised for discussion a global pandemic of vitamin D deficiency as a possible explanation for the high cellular inflammatory activity induced by the RAAS.1 The original discussion involved a significant number of diseases, mainly cardiovascular, but all with a similar inflammatory basis. Now, with the focus on acute lung inflammation caused by COVID-19, the Irish Longitudinal Study on Ageing (TILDA 2020) reinforces the idea that appropriate supplementation of vitamin D, especially in older people, may be beneficial to the population of vulnerable groups during this COVID-19 pandemic.48 In this sense, vitamin D inhibits Skp2 protein, which plays a central role in the viral replication mechanism of COVID1949 and uses autophagy blockage for its accelerated replication and infectivity. The virus induces the abovementioned Skp2, which in turn inactivates Beclin-1, an essential component of the autophagy process (Fig. 1).
The figure gives a simple summary of the main pathways involved in the clinical progression at lung level of positive COVID-19 patients, its correlation with ACE/ACE2 receptors, and how their decoupling would determine a poor outcome at lung level. Furthermore, both RAAS blockage and vitamin D implementation are outlined, highlighting the potential impact of vitamin D on the restoration of signalling pathways and possible improved pulmonary clinical outcomes for COVID-19 positive patients.
Coronavirus infection produces a high risk of complications and mortality in the elderly, hypertensive, diabetic or patients suffering from previous heart or lung diseases. Initial reports of patient progress in China, where the epidemic first broke out, showed that patients with these clinical conditions had three to four times more respiratory complications, hospitalisations, and mortality than those without such preliminary conditions.50,51
One of the most frequent complications is high blood pressure, and as a relevant risk factor it led to various speculations. In this regard, and with results from the basic sciences, it was hypothesized that drugs that block the RAAS (angiotensin converting enzyme inhibitors [ACEi] and angiotensin II receptor blockers [ARB]) could increase the risk for patients with COVID-19. This would be based on their mechanism of action, which would increase the production of angiotensin converting enzyme 2 (ACE2).52 Experimental work showed that the use of these drugs increases levels of ACE2.53 ACE2 is the receptor to which the coronaviruses, both SARS-CoV and SARS-CoV-2 (COVID-19), bind to enter the cell.54,55 The hypothesis raised by the authors and restated in a more recent editorial letter is that increased ACE2 could increase viral load, which in turn would explain the increase in morbidity and mortality.
This hypothesis did not prosper, among other reasons, because the first reports from China did not state whether infected patients receiving ACEi or ARBs had poorer clinical outcomes. In fact, to date there is a lack of data showing a causal relationship between increased ACE2 and increased mortality from COVID-19. Available studies do not describe whether patients were receiving ACE inhibitors or ARBs, and only indirect information suggests that approximately 33% of them were on such treatments. Nor has it been shown that hypertension, and even diabetes, were independent predictors of risk.56
The international scientific societies very judiciously agreed that there is no evidence to suggest that RAAS blockage treatments should be modified, and therefore it is accepted that discontinuing treatment carries an extremely high risk. In fact, there is evidence to the contrary, and it validates the decision of these societies. In fact, hypertensive patients hospitalized due to COVID-19 were studied, and the use of ACE inhibitors/ARBs was associated with a lower risk of all-cause mortality compared to patients not treated with ACE inhibitors/ARBs. The authors acknowledge that interpretation of the study should take into account possible confounding factors; however, they conclude that the use of ACE inhibitors/ARBs is unlikely to be associated with a higher risk of mortality in COVID-19 positive patients.57
In contrast to the hypothesis of not using ACEi/ARBs in hypertensives, other authors have proposed a different approach. Here the hypothesis would be a possible protective action of RAAS blockers in COVID-19 infection.58,59 Along these lines, two clinical trials, not yet initiated, have been registered to evaluate the action of losartan in the course of viral infection (# NCT04312009 and # NCT04311177 Clinicaltrials.gov).
The hypothesis on the usefulness of RAAS blockers also stems from the mechanics used by the virus to enter the cell via ACE2 receptors and the concomitant reduction in intracellular levels of ACE2. ACE2, unlike the classical angiotensin converting enzyme (ACE), degrades angiotensin 2 so that the virus-induced reduction exacerbates the pathogenic action of the higher concentration of angiotensin 2 in the lung.60 ACE2 levels exert a protective action on the lung parenchyma. In this respect, there is evidence that high levels of ACE2 at the level of the lung tissue are relevant in the defence process against respiratory viral infections. The mechanism identified would be that ACE2 decreases the massive release of cytokines and the consequent inflammatory infiltration that leads to the known serious respiratory complications.61 Therefore, the initial suggestion to discontinue RAAS blockers may be a counterproductive strategy for patient outcome, not only by destabilizing their blood pressure levels at a complex clinical time but also through the possible risk of eliminating the protective factor of increased ACE2 in COVID-19-induced lung pathogenesis.
There is another way of counteracting the consequent elevation of the RAAS, especially by inducing ACE2. This can be achieved by administering appropriate doses of vitamin D. Higher levels of either element are inversely associated with lower levels of the other. There is ample evidence that administration of vitamin D attenuates RAAS activity at the circulating level, but significantly more so at the tissue and intracellular level.1 Thus, vitamin D would block the inflammatory cascade by reducing activity of the RAAS.62
Vitamin D reduces the activity of ACE and increases the activity of ACE2, which has a protective effect at the lung level, restoring the balance of ACE2. This restoration of balance mediated by vitamin D has been key in reducing respiratory events in experimental models.63 Low levels of vitamin D are associated with an increase in respiratory infections. In controlled clinical trials the administration of vitamin D has also demonstrated a protective effect on infections in healthy subjects and patients with chronic obstructive pulmonary disease (COPD).26,64–67
Of particular interest, different trials on the subject have been published, and systematic reviews through meta-analyses. Most of them report benefits in reducing respiratory conditions, and several used vitamin D supplements.68–77 Potential benefits have also recently been reported in dengue virus infections78,79 (Fig. 1).
Suggested behaviours according to vitamin D levelsIn this respect, it has been shown that the degree of protection against infection increases as vitamin D levels increase, but this relationship has not yet allowed an adequate cut-off level to be set. However, an observational study reported that values of 38 ng/mL are appropriate to decrease the risk of acute viral respiratory infections.80 On the other hand, some authors suggest maintaining a vitamin D level of at least 30 ng/mL or even keeping it in a range between 40 and 60 ng/mL to reduce infectious processes. Thus, it was possible to establish that post-surgical in-hospital infections were three times higher with vitamin D levels lower than 30 ng/mL,81 and that these infections decreased by 33% for each 10 ng/mL increase in vitamin D levels.82
According to the medical evidence, routine clinical behaviour suggests that in the case of a severe vitamin D deficiency there should be a two-stage therapeutic approach: first using a high loading dose and then a lower maintenance dose.83 In this regard, a so-called "loading dose" of vitamin D has been reported in order to reach a target level where a plasma concentration of vitamin D of 30 ng/mL can be achieved using different dosage regimens (daily, weekly, fortnightly, monthly). Of interest, in patients with elevated inflammatory markers, such as the obese, it has been established that the necessary intake should be 2 to 3 times higher than that established for the general population, and at least 1.5 times higher for the overweight than the general population.84
The current emerging circumstances of the COVID-19 pandemic require scientifically/rationally based and even, as previously mentioned, empirical therapeutic behaviours. As for what we know about vitamin D, its protagonism remains modest, but we are gradually gaining more data to support it as an adjuvant strategy in an attempt to achieve rapid and effective protection against the risk of infection by SARS-CoV-2. In this sense, there are different positions, such as daily doses over a short time, or the use of an initial loading dose followed by high doses of vitamin D for a short period of time, allowing in each case, and in times of pandemic, the achievement of concentrations within appropriate ranges of between 30 and 50 ng/mL or higher. More specifically, strategies have been proposed, such as that suggested by Grant et al.,30 with a dose of 10.000 IU/day for one month in order to reach the target of levels between 40 and 60 ng/mL rapidly, and then continue with 5.000 IU/day for a few more weeks.
The proposed level of high doses is striking, as it dismisses possible toxic effects; however, studies show that a dose of 10,000 IU/day for 4-6 weeks is free of adverse effects. In detail, Amir et al.85 found this in Canadian women with breast cancer and bone metastases. Similarly, the research team led by Dr Holick86 - one of the leading groups in studies with vitamin D - supplemented cancer patients with high doses of vitamin D and did not establish toxicity; instead, it improved the intestinal microbiota of treated patients. The same group worked with 10,000 IU/daily for 6 months - without causing hypercalcaemia - and achieved vitamin D levels in the order of 78.6 ± 13 ng/mL.87
Psychiatric patients were also treated at a hospital in Cincinnati, Ohio, with doses of 5,000 or 50,000 IU/day for 16 months, and as a result they found no adverse effects; it was only recommended that if the patient was also receiving calcium supplementation, the dose of calcium should not be high so as to minimize the risk of hypercalcaemia.88
The stakes are higher in other papers with initial dose proposals of 100,000 IU if serum concentrations of 20 ng/mL are desired, initial dose of 300,000 IU if concentrations of 30 ng/mL are desired, and even initial doses of 500,000 IU have been suggested in healthy adults.89,90
As a corollary, Dr Alipio91 has just published results that provide substantial information for physicians and health policy makers. To be specific, he concludes that vitamin D supplementation improves the clinical outcomes of patients infected with COVID-19 based on the increased likelihood of a mild outcome when the serum level of vitamin D is increased, while a decrease in serum levels of vitamin D is associated with a poorer clinical outcome.
Conclusions and perspectivesAs a result, and in the face of this devastating epidemic for which effective treatments are still lacking, this review compiles and proposes examining the potentially protective effect of high daily doses of vitamin D to rapidly increase blood and tissue levels, with the aim of countering overloading of the RAAS and thus improve the course of COVID-19 infection, its respiratory complications (Fig. 1) and even complications involving other organs. The purpose is to open and create an appropriate debate on the behaviour of indicating vitamin D for the general population, particularly to those most exposed, and as mentioned, to achieve increased serum and tissue levels to counteract the imbalance of some of the components of the RAAS and also manifest its own anti-inflammatory effects (Fig. 1).
We believe that this strategy at a population level could provide another weapon in the armoury against the virus and with no adverse effects, as demonstrated in the review of more than 76,000 patients included in controlled trials with vitamin D intake. Therefore, a dose to obtain rapid increases in plasma levels of vitamin D could be between 5,000 and/or 10,000 IU daily, or from 50,000 to 100,000 IU per week.92 Given the tentative nature of the proposed dose, the use of lower doses in children or young adults at low risk of exposure to the virus could be considered. In this regard, our working group is making progress in the development of controlled protocols with different populations of people at risk or already infected, evaluating physiological parameters and clinical events. We accept that this intervention does not claim to eliminate the virus; however, its potential to make viral entry more difficult and, if it has taken place, to improve patient outcomes is promising. In other words, vitamin D intake could improve the conditions of patients to place them in a better position to cope with and increase their chances against COVID-19 and even other equivalent infectious conditions.
As described above, we believe that the recommendation is supported by multiple reports. On the same lines, Grant el al.93 very recently proposed raising serum concentrations through vitamin D supplementation and claim that this could reduce the incidence, severity and risk of death from influenza, pneumonia and the current COVID-19 epidemic. Moreover, Rhodes et al.94 proposed supplementation with vitamin D at least for those in the northern hemisphere who are most at risk of severe illness and death. The UK Association of Dieticians95 and editorials in international scientific journals make the same recommendation.
Finally, the purpose of sharing this idea with health professionals is to quickly ignite debate and call for critical reflection so that we can contribute towards conducting more and better clinical designs without delay to validate this hypothesis for the benefit of public health and especially during this COVID-19 emergency.
FundingThis work was funded by grants from the Council of Research and Technology of the University of Cuyo (SECyT), Mendoza, Argentina, and from ANPCyT FONCyT, both awarded to Walter Manucha. Scholarship No. PICT 2016-4541.
AuthorshipAll authors contributed equally to the conception and design of the review, with substantial input on data, analysis and interpretation of content, the drafting and critical review of the article for intellectual content.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Mansur JL, Tajer C, Mariani J, Inserra F, Ferder L, Manucha W. El suplemento con altas dosis de vitamina D podría representar una alternativa promisoria para prevenir o tratar la infección por COVID-19. Clin Investig Arterioscler. 2020. https://doi.org/10.1016/j.arteri.2020.05.003