Due to the currently growing rate of obesity, it is important to maintain good control of food intake. The main purpose of the present study is to determine the influence of physical exercise on appetite, changes in hormone concentrations, and changes in certain neuronal regions. To achieve this, a literature search was conducted using different data bases. The results show how exercise produces changes in the appetite perception, in the amount of energy intake, and in different weight-control related hormones, as well as in specific neuronal responses. In conclusion, it can be shown that exercise leads to changes in appetite, hunger, and energy intake. In addition, exercise decreases the ghrelin levels and increases concentrations of leptin. Likewise, it is shown how physical exercise alters the responses of certain neuronal regions after visualising specific food elements decreasing so the appetite or the intake of them.
Debido a los problemas de obesidad que hay en la actualidad, es importante llevar un buen control de la ingesta alimentaria. El propósito del presente estudio es conocer la influencia que tiene el ejercicio físico sobre el apetito, los cambios generados en las concentraciones de diferentes hormonas y la alteración de determinadas regiones cerebrales. Para ello se ha realizado una revisión bibliográfica a través de diferentes bases de datos. En cuanto a los resultados, se aprecia que el ejercicio produce cambios en el apetito, en la cantidad de ingesta de energía, en diferentes hormonas relacionadas con el control del peso así como en determinadas respuestas neuronales. Como conclusión, se puede afirmar que el ejercicio disminuye el apetito, el hambre y la ingesta de energía. Además, el ejercicio disminuye los niveles de grelina y aumenta las concentraciones de leptina. Asimismo, se muestra como el ejercicio físico altera la actividad de ciertas regiones del cerebro tras la visualización de determinados alimentos, con lo que disminuyen el apetito o la ingesta.
Obesity is a disease which has taken the form of an epidemic and affects all populations. It is linked to the obesogenic lifestyle we are surrounded by, in which the combination of overeating and lack of physical activity results in an excessive accumulation of fat and increases morbidity and mortality rates among affected people.1 As a consequence, a large number of research projects have tried to evaluate the physiological mechanisms involved in the control of energy expenditure and food intake, in an attempt to reduce the effect of obesity and maintain normal serum and histological levels of fat.2,3
The last 10 years have seen great advances in terms of understanding the neurohormonal and biochemical mechanisms that regulate appetite. The most important finding is the multifactorial nature of these mechanisms, whereby afferent brain stimuli, intracerebral integration of peripheral signals and efferent orders all work to establish a balance between appetite and satiety.4
One of the substances produced by adipose tissue is leptin, a hormone composed of 167 peptide amino acids. It is found mainly in white adipose tissue, but is also present in other tissues such as the stomach, placenta and mammary gland.5 Leptin is considered to be a controller of body weight because it transmits information to the hypothalamus about the amount of energy stored in the adipose tissue and suppresses the appetite, which then affects energy expenditure.6
In addition to leptin, adipocytes also release a peptide known as ghrelin. Exogenous administration of ghrelin induces the release of growth hormone, stimulates food intake and increases body weight.7 In this context, when fasting, leptin concentrations decrease, which stimulates the appetite and modulates the size of the intake and the perception of taste. By contrast, the amount of ghrelin increases before meals, when fasting or suffering from cachexia. Ghrelin exercises its role in the regulation of dietary intake through different mechanisms. These include the fact that it competes with leptin and also its interaction with the vagus nerve,20 from where it can generate neuronal activation in the nucleus of the solitary tract and the dorsal motor nucleus which causes gastric motility and secretion and, in short, induces appetite and the consumption of food.21 The hormone leptin is also involved in appetite regulation. Leptin causes activation of the catabolic effector systems. These catabolic systems reduce adiposity through inhibition of appetite. This then stimulates energy expenditure and disables anabolic effector systems, whose aim is to increase body adiposity (increasing appetite), and consequently favours the lipolysis process in adipose tissue.22 Therefore, both ghrelin and leptin are associated with energy balance regulation.
The peptide YY (PYY) can also be found among intestinal hormones which act to control food intake. Obesity is also associated with a decrease in PYY concentrations in fasting and postprandial states.8 PYY has consequently been identified as having potential as treatment for weight control.
Insulin has a vital function in the central nervous system in terms of inciting satiety, increasing energy expenditure and regulating the action of leptin.9 Plasma insulin levels, like those of leptin, are proportional to the changes in adiposity; they increase in times of positive energy balance and decrease when the balance is negative.9
Taking all the above into consideration, manipulation of the intensity and type of physical exercise (understanding this term as a variety of planned, structured, repetitive body movements aimed at maintaining or improving physical fitness and health) may alter appetite. In support of that idea, a recent study reported that ad libitum intake of energy or food at a lunch and an evening meal was reduced after a high-intensity cycling session (75% VO2 max), compared to a low-intensity session (40% VO2 max), in obese adolescents.10
The objective of this review is therefore to determine how physical exercise affects the levels of certain hormones (ghrelin, leptin, insulin and PYY) and the perception of appetite (physiological or psychological sensation experienced by a subject which induces him/her to eat), how certain regions of the brain are affected after a period of exercise and how this varies according to the intensity or type of physical exercise, and its influence on individuals’ feelings of hunger and satiety (understood as the length of time the feeling of satisfaction lasts before the hunger pangs return).
Materials and methodDesign and participantsThis is a descriptive study, involving the review of articles published in different bibliographic sources; 80% of the articles were extracted from Web of Science, with the remaining information being obtained from PubMed, Dialnet and Google Scholar databases. In accordance with the proposed objectives, the search was carried out using different keywords such as appetite, exercise, satiety, exercise intensity, physical activity, energy consumed, leptin, ghrelin, appetite hormones, brain, food, neuronal responses and obesity.
During the literature review, a total of 237 articles were found, 138 of which were excluded because of the title, 39 because of the abstract, 21 because of the content and 15 because of the publication dates. Lastly, a total of 24 articles have been used for this study, the most relevant of which have been included in the results section.
In order to optimise the methodological quality in this study, the Jadad scale was used to improve the psychometric properties (validity and reliability) and to obtain greater scientific rigour in the research.
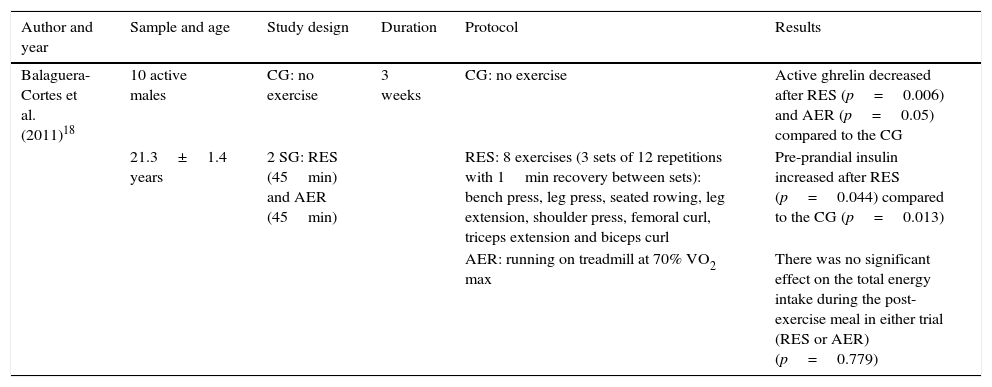
ResultsTable 1 shows the changes produced in ghrelin and insulin levels and food intake after physical exercise. The aspects taken into account for the analysis of this study appear in the table header. As can be seen from the results, active ghrelin decreased significantly after aerobic and resistance exercise compared to the control group; this difference was greater in the resistance exercise group. Similarly, there are significant differences in pre-prandial insulin, which increased in the resistance exercise group compared to the control. In addition, we see that there were no significant differences between the two study groups in energy intake after exercise.
Ghrelin and insulin level status and food intake after physical exercise.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Balaguera-Cortes et al. (2011)18 | 10 active males | CG: no exercise | 3 weeks | CG: no exercise | Active ghrelin decreased after RES (p=0.006) and AER (p=0.05) compared to the CG |
| 21.3±1.4 years | 2 SG: RES (45min) and AER (45min) | RES: 8 exercises (3 sets of 12 repetitions with 1min recovery between sets): bench press, leg press, seated rowing, leg extension, shoulder press, femoral curl, triceps extension and biceps curl | Pre-prandial insulin increased after RES (p=0.044) compared to the CG (p=0.013) | ||
| AER: running on treadmill at 70% VO2 max | There was no significant effect on the total energy intake during the post-exercise meal in either trial (RES or AER) (p=0.779) |
AER: aerobic exercise; CG: control group; SG: study group; RES: resistance exercise.
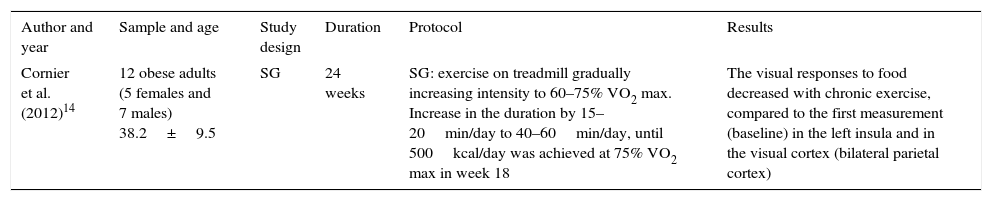
Table 2 shows the effect of physical exercise on the neuronal response after looking at different food-related images. The table header shows the aspects that were taken into account for the analysis of this study. The results reveal that exercising on a treadmill gradually increasing the intensity produces a decrease in visual responses to food in certain regions of the brain such as the left insula and the visual cortex.
Effect of exercise on neuronal response after looking at images of food.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Cornier et al. (2012)14 | 12 obese adults (5 females and 7 males) 38.2±9.5 | SG | 24 weeks | SG: exercise on treadmill gradually increasing intensity to 60–75% VO2 max. Increase in the duration by 15–20min/day to 40–60min/day, until 500kcal/day was achieved at 75% VO2 max in week 18 | The visual responses to food decreased with chronic exercise, compared to the first measurement (baseline) in the left insula and in the visual cortex (bilateral parietal cortex) |
SG: study group.
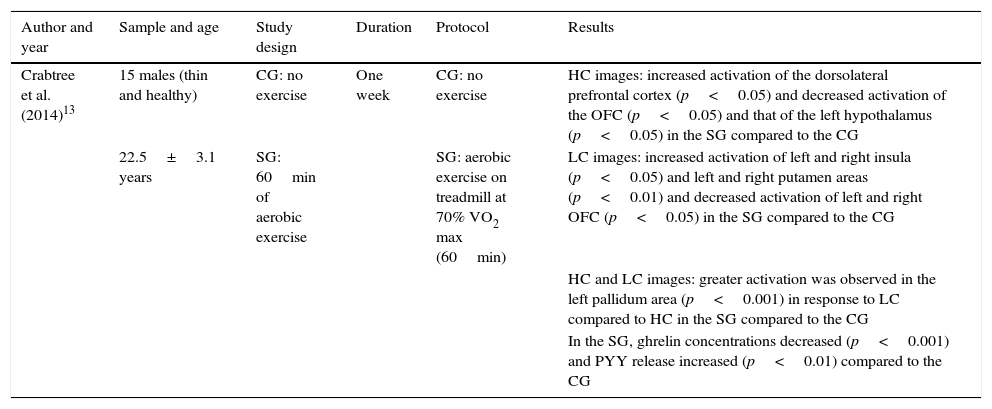
Table 3 shows the neuronal responses after looking at different images with high and low calorific value after exercise. The aspects taken into account for the analysis of this study appear in the table header. The results show how aerobic exercise on a treadmill influenced the neuronal responses when viewing images of foods with low and high calorific value. When these foods were high in calories, significant differences were found; there was an increase in activation of the dorsolateral prefrontal cortex and decrease in activation of the orbitofrontal cortex (OFC). When the images were of low-calorie foods, activation of the insula and the putamen increased and activation of the OFC decreased, with the differences being significant. Significant differences were also found in terms of increased activation in the left palladium region when viewing images of low-calorie foods compared to high-calorie foods after aerobic exercise. Moreover, high-intensity exercise reduced ghrelin concentrations and increased plasma PYY concentrations, with significant differences.
Neuronal responses after looking at images with high and low calorific value after exercise.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Crabtree et al. (2014)13 | 15 males (thin and healthy) | CG: no exercise | One week | CG: no exercise | HC images: increased activation of the dorsolateral prefrontal cortex (p<0.05) and decreased activation of the OFC (p<0.05) and that of the left hypothalamus (p<0.05) in the SG compared to the CG |
| 22.5±3.1 years | SG: 60min of aerobic exercise | SG: aerobic exercise on treadmill at 70% VO2 max (60min) | LC images: increased activation of left and right insula (p<0.05) and left and right putamen areas (p<0.01) and decreased activation of left and right OFC (p<0.05) in the SG compared to the CG | ||
| HC and LC images: greater activation was observed in the left pallidum area (p<0.001) in response to LC compared to HC in the SG compared to the CG | |||||
| In the SG, ghrelin concentrations decreased (p<0.001) and PYY release increased (p<0.01) compared to the CG |
CG: control group; SG: study group; HC: foods with a high calorific value; LC: foods with a low calorific value; OFC: orbitofrontal cortex.
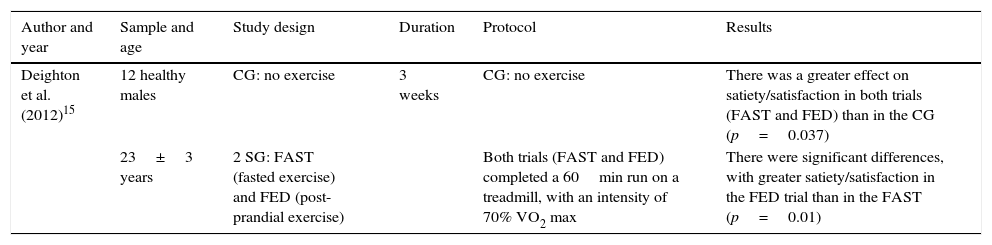
Table 4 shows satiety/satisfaction after physical exercise on an empty stomach and after breakfast. The table header shows the aspects that were taken into account for the analysis of this study. The results show greater satiety in the groups that exercised (running on treadmill) on an empty stomach and post-prandial compared to the control group, with significant differences. By contrast, after exercise, there were clear differences in relation to satiety/satisfaction between exercising on an empty stomach and after eating. Subjects felt more satiated after postprandial exercise, compared to exercising on an empty stomach.
Satiety/satisfaction after physical exercise on an empty stomach and after breakfast.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Deighton et al. (2012)15 | 12 healthy males | CG: no exercise | 3 weeks | CG: no exercise | There was a greater effect on satiety/satisfaction in both trials (FAST and FED) than in the CG (p=0.037) |
| 23±3 years | 2 SG: FAST (fasted exercise) and FED (post-prandial exercise) | Both trials (FAST and FED) completed a 60min run on a treadmill, with an intensity of 70% VO2 max | There were significant differences, with greater satiety/satisfaction in the FED trial than in the FAST (p=0.01) |
CG: Control group; SG: Study group.
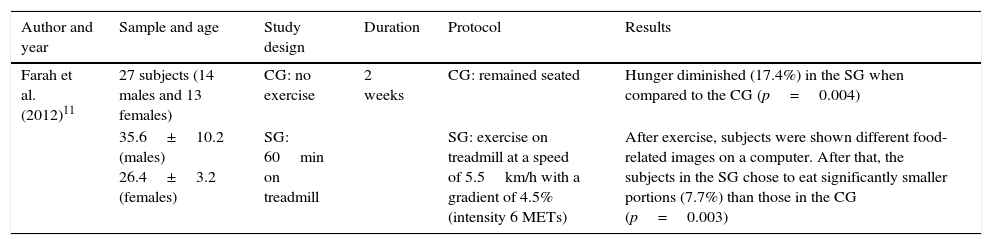
Table 5 shows the effects of physical exercise on hunger. The aspects that were considered relevant for the analysis of this study appear in the table header. As can be seen from the results, there were significant differences, with a decrease in hunger in the group that had exercised compared to the control group. Both the study group, after exercise, and the control group were shown food-related images, with the study group choosing significantly smaller portions of food than the control group.
Physical exercise and its effects on hunger.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Farah et al. (2012)11 | 27 subjects (14 males and 13 females) | CG: no exercise | 2 weeks | CG: remained seated | Hunger diminished (17.4%) in the SG when compared to the CG (p=0.004) |
| 35.6±10.2 (males) 26.4±3.2 (females) | SG: 60min on treadmill | SG: exercise on treadmill at a speed of 5.5km/h with a gradient of 4.5% (intensity 6 METs) | After exercise, subjects were shown different food-related images on a computer. After that, the subjects in the SG chose to eat significantly smaller portions (7.7%) than those in the CG (p=0.003) |
CG: Control group; SG: Study group; MET: metabolic equivalent of task.
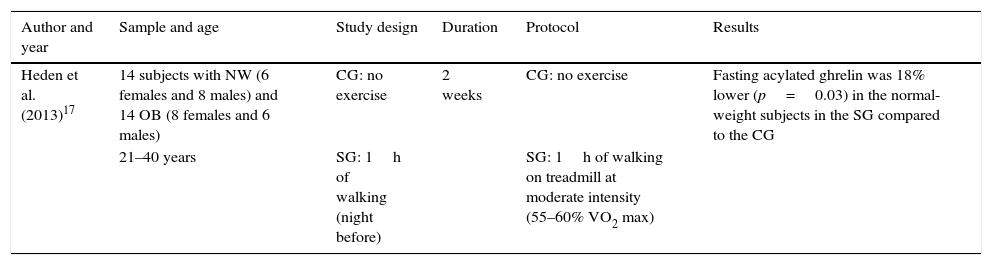
Table 6 shows the results for acylated (active) ghrelin and satiety after physical exercise. The aspects that were considered relevant for the analysis of this study are shown in the table header. The results show a significant decrease in fasting acylated ghrelin in the normal-weight subjects belonging to the study group compared to the control group.
Acylated ghrelin and satiety after physical exercise.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Heden et al. (2013)17 | 14 subjects with NW (6 females and 8 males) and 14 OB (8 females and 6 males) | CG: no exercise | 2 weeks | CG: no exercise | Fasting acylated ghrelin was 18% lower (p=0.03) in the normal-weight subjects in the SG compared to the CG |
| 21–40 years | SG: 1h of walking (night before) | SG: 1h of walking on treadmill at moderate intensity (55–60% VO2 max) |
CG: control group; SG: study group; OB: obese: NW: normal weight.
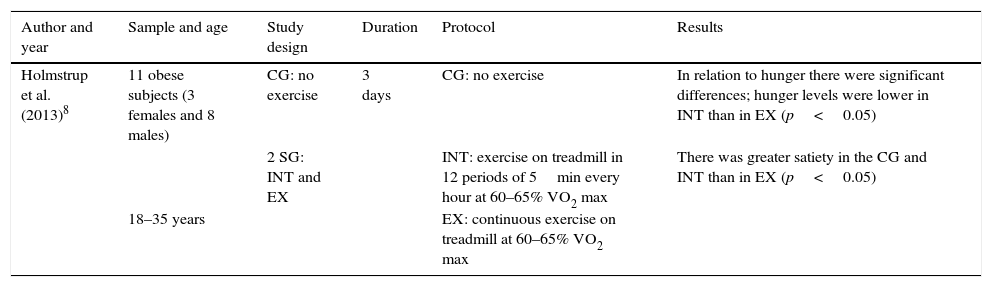
Table 7 shows the effects of physical exercise on individuals’ hunger and satiety. The table header shows the aspects that were deemed to be relevant for the analysis of this study. The results revealed significant differences, with less hunger after intermittent exercise than continuous exercise or no exercise (control group). Significant differences were also found in terms of satiety; there was greater satiety in the intermittent exercise group and the control group compared to the continuous exercise group.
Physical exercise and its effects on hunger and satiety.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Holmstrup et al. (2013)8 | 11 obese subjects (3 females and 8 males) | CG: no exercise | 3 days | CG: no exercise | In relation to hunger there were significant differences; hunger levels were lower in INT than in EX (p<0.05) |
| 2 SG: INT and EX | INT: exercise on treadmill in 12 periods of 5min every hour at 60–65% VO2 max | There was greater satiety in the CG and INT than in EX (p<0.05) | |||
| 18–35 years | EX: continuous exercise on treadmill at 60–65% VO2 max |
EX: continuous exercise; CG: control group; SG: study group; INT: intermittent exercise.
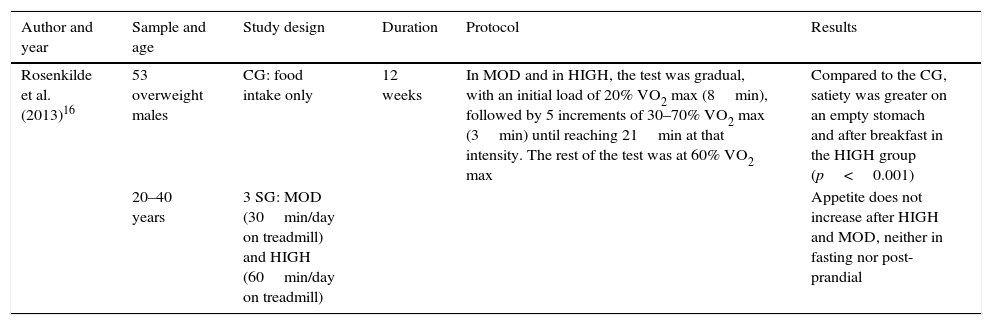
Table 8 shows levels of satiety and appetite in subjects after physical exercise on an empty stomach and after eating. The aspects taken into account for the analysis of this study appear in the table header. The results revealed significant differences; satiety was greater in the fasting state and after exercise when high-intensity exercise was carried out, compared to the group that did no exercise. Appetite was not found to increase with moderate or high-intensity exercise.
Satiety and appetite on an empty stomach and after breakfast after physical exercise.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Rosenkilde et al. (2013)16 | 53 overweight males | CG: food intake only | 12 weeks | In MOD and in HIGH, the test was gradual, with an initial load of 20% VO2 max (8min), followed by 5 increments of 30–70% VO2 max (3min) until reaching 21min at that intensity. The rest of the test was at 60% VO2 max | Compared to the CG, satiety was greater on an empty stomach and after breakfast in the HIGH group (p<0.001) |
| 20–40 years | 3 SG: MOD (30min/day on treadmill) and HIGH (60min/day on treadmill) | Appetite does not increase after HIGH and MOD, neither in fasting nor post-prandial |
CG: control group; SG: study group; HIGH: high-intensity exercise; MOD: moderate exercise.
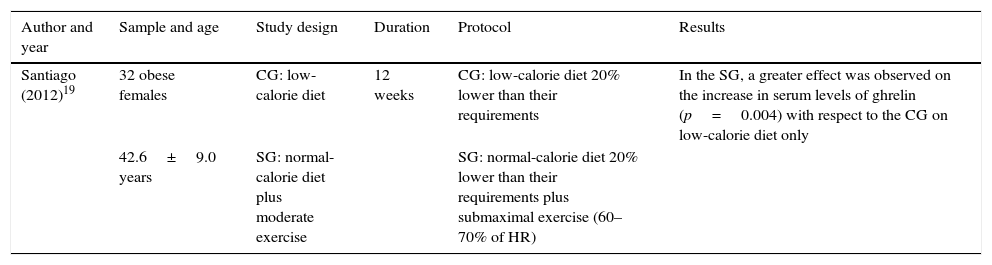
Table 9 shows the changes produced in ghrelin according to the type of diet and exercise. The table header shows the aspects most relevant for the analysis of this study. The results show significant differences in serum (blood serum) levels of ghrelin after submaximal exercise compared to the group that only followed a low-calorie diet.
Changes in ghrelin and association with type of diet and physical exercise.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Santiago (2012)19 | 32 obese females | CG: low-calorie diet | 12 weeks | CG: low-calorie diet 20% lower than their requirements | In the SG, a greater effect was observed on the increase in serum levels of ghrelin (p=0.004) with respect to the CG on low-calorie diet only |
| 42.6±9.0 years | SG: normal-calorie diet plus moderate exercise | SG: normal-calorie diet 20% lower than their requirements plus submaximal exercise (60–70% of HR) |
HR: heart rate; CG: control group; SG: study group.
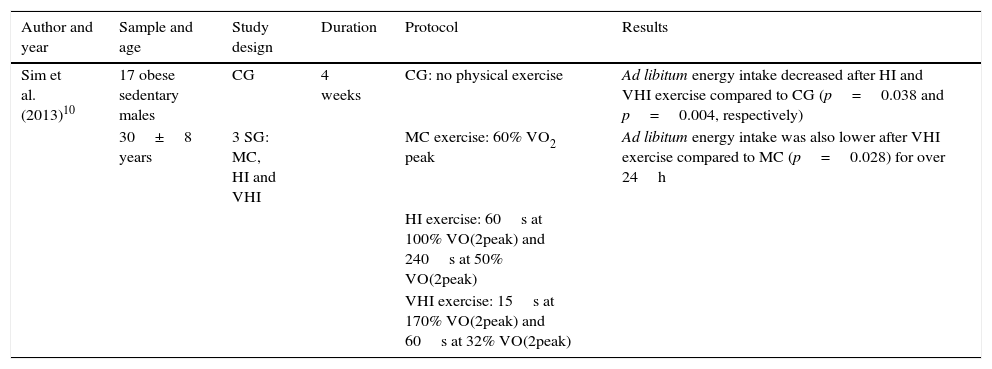
Table 10 shows ad libitum energy intake after individuals have engaged in physical exercise. Some of the aspects taken into account for the analysis of this study are shown in the table header. We see from the results that ad libitum energy intake (food intake) was lower after intermittent high-intensity and very-high-intensity exercise compared to the control group, with significant differences. Ad libitum energy intake was also lower with intermittent very-high-intensity exercise compared to continuous moderate exercise, again with significant differences.
Ad libitum energy intake after physical exercise.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Sim et al. (2013)10 | 17 obese sedentary males | CG | 4 weeks | CG: no physical exercise | Ad libitum energy intake decreased after HI and VHI exercise compared to CG (p=0.038 and p=0.004, respectively) |
| 30±8 years | 3 SG: MC, HI and VHI | MC exercise: 60% VO2 peak | Ad libitum energy intake was also lower after VHI exercise compared to MC (p=0.028) for over 24h | ||
| HI exercise: 60s at 100% VO(2peak) and 240s at 50% VO(2peak) | |||||
| VHI exercise: 15s at 170% VO(2peak) and 60s at 32% VO(2peak) |
CG: control group; SG: study group; HI: high-intensity intermittent; MC: continuous moderate; VHI: very high-intensity intermittent.
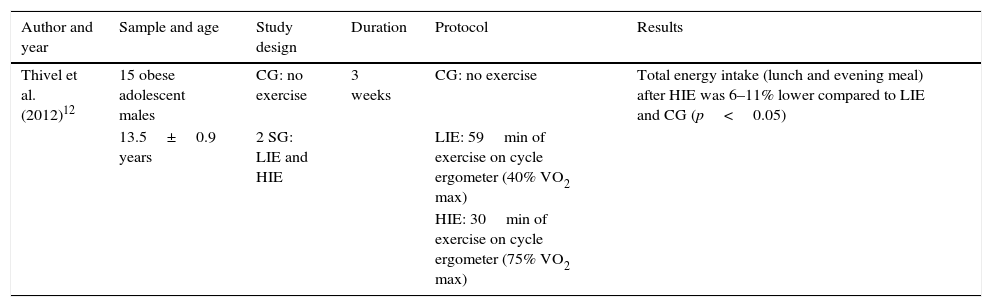
Table 11 shows total intake at lunch and evening meals after physical exercise. Other data relevant for the review of this study are also shown in the table header. As seen from the results, compared to moderate exercise, high-intensity exercise reduced energy intake at lunch and evening meal times, with significant differences.
Total intake at lunch and evening meals after physical exercise.
| Author and year | Sample and age | Study design | Duration | Protocol | Results |
|---|---|---|---|---|---|
| Thivel et al. (2012)12 | 15 obese adolescent males | CG: no exercise | 3 weeks | CG: no exercise | Total energy intake (lunch and evening meal) after HIE was 6–11% lower compared to LIE and CG (p<0.05) |
| 13.5±0.9 years | 2 SG: LIE and HIE | LIE: 59min of exercise on cycle ergometer (40% VO2 max) | |||
| HIE: 30min of exercise on cycle ergometer (75% VO2 max) |
CG: control group; SG: study group; HIE: high-intensity exercise; LIE: low-intensity exercise.
We should point out that, as reflected in the Material and Method section, the articles used in this review were selected according to their validity, reliability and scientific quality, by applying the Jadad scale.
DiscussionAfter reviewing all the studies, the results indicate that physical exercise reduces hunger, i.e. the physiological or psychological sensation that induces us to eat. One study found that after physical exercise, hunger diminishes and subjects prefer to eat smaller food portions.11 Another research study12 indicates that food intake after high-intensity exercise is lower than after low-intensity exercise or no exercise. Yet another study indicates that hunger is also reduced with intermittent exercise compared to continuous exercise.8 They show that after high-intensity and very-high-intensity intermittent exercise, ad libitum food intake is lower than after no exercise (control group).10Ad libitum energy intake is also lower with very-high-intensity intermittent exercise than with moderate continuous exercise. This effect lasts for over 24h.
Moreover, from the point of view of physical exercise inducing neuronal activation or altering neuronal responses, a number of different studies have produced similar results. In another study, when images of low-calorie foods were viewed, there was an increase in the responses of the reward systems (centres in the central nervous system that obey specific, natural stimuli, regulated by neurotransmitters, which allow the subject to develop learned behaviours in response to unpleasant or pleasant events), and suppression of these systems when viewing images with high calorific value.13 A further study establishes that exercise alters the neuronal response; the response was suppressed after viewing food-related images.14
Very similar results have also been found in different studies in relation to exercise and satiety. One study found that after physical exercise, whether fasted or post-prandial, individuals experienced greater satiation/satisfaction compared to others who did not exercise. Differences in satiety/satisfaction were also found depending on whether the exercise was on an empty stomach or after food; the subjects felt more satiated after post-prandial exercise than after exercising when fasted.15 Another study with similar results indicates that satiety is greater when exercise is intermittent rather than continuous.8 Yet another states that whether fasting or post-prandial, the level of satiety is higher with high-intensity exercise than no exercise.16 In addition, appetite does not increase in fasting or post-prandial subjects when they do either moderate or high-intensity exercise.
Several studies have reported similar findings with regard to changes in ghrelin levels as a result of physical exercise17; lower fasting acylated ghrelin values were found when subjects exercised the night before. Another study confirms these results, reporting a decrease in ghrelin after resistance and aerobic exercise. To add to that, significant differences have also been seen in relation to pre-prandial insulin, with higher levels found in the resistance exercise group compared to the control group. It therefore seems that exercise decreases ghrelin and increases insulin levels; both of these circumstances being associated with a reduction in dietary intake.18 Another study also reported a decrease in ghrelin concentrations and an increase in PYY after high-intensity exercise.13 However, according to another,19 ghrelin levels increase in obese subjects with a normal-calorie diet when they exercise, compared to other subjects treated only with a low-calorie diet.
In order to improve the study and as a proposal for future research, further studies should be conducted in this area to provide us with a better understanding of the influence of exercise on satiety and appetite according to intensity.
Although developed and validated to assess the quality of pain studies, the Jadad scale has also been used extensively in other clinical areas.12 Many clinical trials now include the Jadad scale items in their methodology with the aim of ensuring their study has a high level of methodological quality. Nevertheless, Herbison et al.13 concluded that the Jadad scale may not be sufficiently sensitive to distinguish between different levels of quality. Use of the Jadad scale and its validity therefore need to be re-assessed for different areas of research.
In this review, all the articles were on randomised studies, with 90% of them describing the randomisation sequence. All of the selected studies describe themselves as double-blind and all used a suitable method of blinding and applied scientific rigour. With regard to the description of the losses in the selected studies, only 90% make explicit reference to them.
We conclude that, by applying the Jadad scale in this scientific review, an RCT score of 4 points is obtained: this indicates an acceptable degree of methodological quality for this type of study.
In conclusion, the results of this study show that physical exercise is associated with a decrease in hunger, appetite and energy intake, mainly when it is moderate or high intensity. Physical exercise also leads to certain changes in the concentrations of hormones related to appetite regulation, with an increase in leptin and PYY and a decrease in ghrelin.22 Studying the influence of exercise on the activation of certain regions of the brain shows that, after physical exercise, the reward systems of the central nervous system are incremented when individuals view images of low-calorie foods. Lastly, physical exercise influences the neuronal responses, reducing them after viewing different foods and thus diminishing our appetite.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols implemented in their place of work regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThere are no conflicts of interest.
Please cite this article as: Gómez Escribano L, Gálvez Casas A, Escribá Fernández-Marcote AR, Tárraga López P, Tárraga Marcos L. Revisión y análisis del ejercicio físico a nivel hormonal, cerebral y su influencia en el apetito. Clin Invest Arterioscler. 2017;29:265–274.