Abdominal aortic aneurysm (AAA) is a vascular pathology with a high rate of morbidity and mortality and a prevalence that, in men over 65 years, can reach around 8%. In this disease, usually asymptomatic, there is a progressive dilatation of the vascular wall that can lead to its rupture, a fatal phenomenon in more than 80% of cases. The treatment of patients with asymptomatic aneurysms is limited to periodic monitoring with imaging tests, control of cardiovascular risk factors and treatment with statins and antiplatelet therapy. There is no effective pharmacological treatment capable of limiting AAA progression or avoiding their rupture. At present, the aortic diameter is the only marker of risk of rupture and determines the need for surgical repair when it reaches values greater than 5.5cm. This review addresses the main aspects related to epidemiology, risk factors, diagnosis and clinical management of AAA, exposes the difficulties to have good biomarkers of this pathology and describes the strategies for the identification of new therapeutic targets and biomarkers in AAA.
El aneurisma de aorta abdominal (AAA) es una patología vascular con una elevada tasa de morbimortalidad y una prevalencia que, en varones de más de 65 años, puede alcanzar el 8%. En esta enfermedad, habitualmente asintomática, se produce una dilatación progresiva de la pared vascular que puede llevar a su rotura, un fenómeno mortal en más de un 80% de los casos. El tratamiento de los pacientes con aneurismas asintomáticos se limita al seguimiento periódico con pruebas de imagen, el control de los factores de riesgo cardiovascular y un tratamiento con terapia antiagregante y estatinas, si bien actualmente no existe ningún tratamiento farmacológico efectivo capaz de limitar su progresión o evitar su rotura. En la actualidad el diámetro aórtico es el único marcador de riesgo de rotura y determina la necesidad de reparación quirúrgica cuando alcanza valores superiores a 5,5cm. En esta revisión se tratan los principales aspectos relacionados con la epidemiología, los factores de riesgo, el diagnóstico y el manejo terapéutico del AAA, se exponen las dificultades para disponer de buenos biomarcadores de esta enfermedad y se describen las estrategias para la identificación de nuevas dianas terapéuticas y biomarcadores en el AAA.
An abdominal aortic aneurysm (AAA) is a vascular pathology which consists of a localised and permanent dilation of the aorta due to a weakening of the vascular wall. Generally, it manifests in the infrarenal portion of the aorta, a region which is subjected to significant haemodynamic forces. An aneurysm is defined as a dilation greater than 50% of the normal diameter of the vessel in adjacent areas, meaning that, in the abdominal aorta, a diameter greater than or equal to 3cm is considered pathological. The natural development of an AAA supposes a progressive dilation, characterised by the proteolysis of the structural components of the vascular wall, the loss of vascular smooth muscle cells (VSMCs) and a chronic immunoinflammatory response. Vascular dilation in an AAA is progressive, but not linear, with alternating phases of acceleration and periods of stability, making monitoring of the patient difficult, and, therefore, worsening the prognosis of the disease. Vascular dilation can culminate in the rupture of the vascular wall, the most severe complication of this disease associated with 80% mortality.1,2 Recent research suggests that an AAA is a local display of a systemic vascular disease, with involvement of the patient's entire vascular tree existing in most cases. In addition, in patients with an AAA, a significant link has been demonstrated with coronary heart disease and with peripheral arterial disease. In this review, the main aspects related to the epidemiology, diagnosis and therapeutic management, risk factors and difficulties in having good biomarkers for this disease are gathered.
EpidemiologyMen are six times more likely than women to have an AAA. It affects more than 5–8% of the male population over the age of 65, and 2% of the female population in the same age range.1 The mean annual incidence of new diagnoses of AAA in Western populations ranges between 0.4 and 0.67%.3 In recent years, the incidence of ruptured aneurysm has been growing, accounting for 1–2% of all deaths.4 One possible explanation of this fact is the increase in the population's life expectancy, which would allow the progressive growth of the aneurysm in patients with an AAA until it ruptures. This would be mainly in those not diagnosed. In any case, the extent of the mortality related to the rupture of an abdominal aortic aneurysm is underestimated, in particular if the low number of cases in which a post-mortem examination is carried out is taken into account.1
PathophysiologyThe pathophysiology of an AAA is a complex and dynamic process which culminates in the irreversible remodelling of the connective tissue.5 There are four main processes which take place in an AAA: proteolysis, oxidative stress, inflammatory immune response and VSMC apoptosis (Fig. 1), processes which cause the loss of elasticity and resistance of the artery wall and impede the recovery of the normal artery diameter after each pulsation.
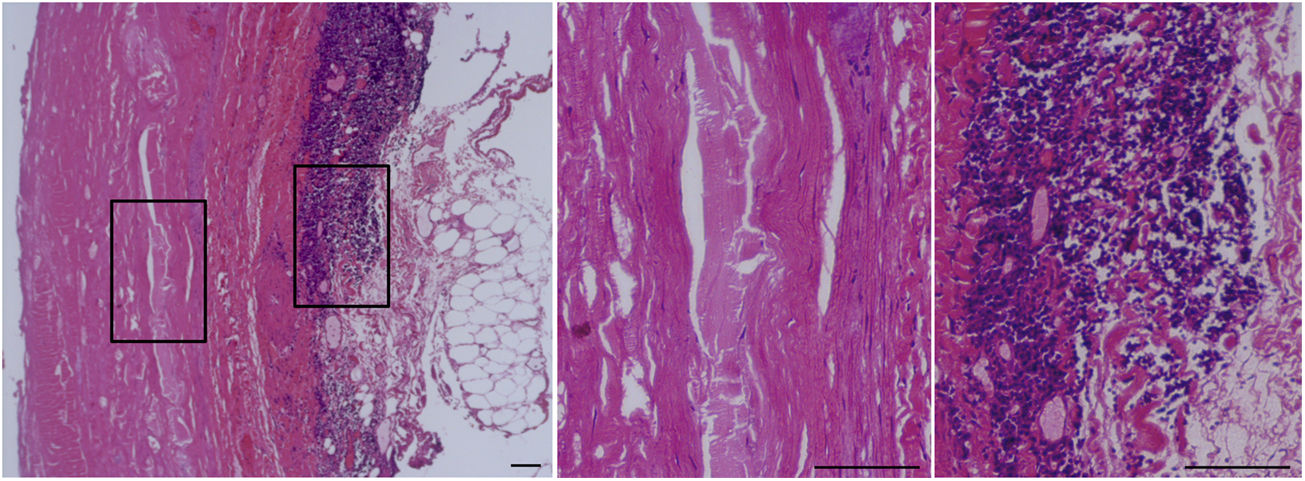
Histopathological characteristics of an abdominal aortic aneurysm (AAA).
Haematoxylin–eosin stain of the abdominal aorta of a patient with an AAA. The framed area is shown on the right at a higher magnification. The central panel shows the absence of cellularity (VSMC) in the middle layer of the vascular wall of these patients. Significant immunoinflammatory infiltrate characteristic of the adventitia of an AAA can be seen in the panel on the right. Bars: 150μm.
An AAA is characterised by a degradation of the connective tissue, mainly of the elastin fibres, through the activation of various proteases such as plasmin, elastase, cathepsins and matrix metalloproteinases (MMPs), along with a reduction in the expression of elastogenic proteins such as fibulin-5.6,7 All of this causes the loss of elastic properties of the artery wall and artery dilation and alters the homeostasis of the vascular cells. In addition, the artery wall shows a significant inflammatory infiltrate consisting of T- and B-cells, neutrophils and macrophages.8,9 Although the mechanism which triggers this inflammatory process is unknown, it is likely that the soluble peptides derived from the degradation of the components of the extracellular matrix act as chemotactic agents promoting the infiltration of macrophages. The increase in the levels of interleukin (IL)-8, MCP-1 and RANTES would in turn facilitate leucocyte recruitment.
Furthermore, the weakening of the vascular wall is linked to the significant reduction in the content of VSMCs which die due to apoptosis. As a compensatory effect, a large collagen deposition occurs, the content of which increases with the progression of the AAA. The fragmentation of the middle layer means that the adventitia has to support unusual centrifugal forces, to which it responds by generating inflammatory, fibrotic and angiogenic responses, complicating the development of the condition even further.10
In recent years, special attention has been paid to the intraluminal thrombus (ILT), present in 75% of AAAs. The ILT is a biologically active tissue formed in the luminal area by a fresh blood layer with fibrin fibres, while an active fibrinolysis is produced in the abluminal side. Furthermore, the presence of erythrocytes and neutrophils in the luminal area contributes to oxidative stress through the release of iron and myeloperoxidase. The thickness of this thrombus reduces the bioavailability of oxygen in the artery wall, which could increase the reactivity of neutrophils and their production of elastase. The presence of the ILT is associated with a reduced thickness of the artery wall, greater elastolysis, lower VSMC content in the middle layer and a higher level of inflammatory immune response in the adventitia.10 Recently, the protein levels retained in the ILT have been associated with greater growth of an AAA.11
Diagnosis and managementAn AAA is usually asymptomatic, meaning that the diagnosis tends to occur as an incidental finding after an imaging test (ultrasonography, computed axial tomography [CAT] or magnetic resonance imaging) performed due to a concomitant condition of the patient. In some countries, screening programmes aimed at certain risk groups are carried out using an ultrasound scan. These programmes have proven to be effective and cost-effective.12 Currently, the management of an AAA is conservative when its diameter is maintained between 3 and 5cm. It is based on a regular follow-up with imaging tests, an adequate control of cardiovascular risk factors and a medical treatment with antiplatelet therapy and statins, although currently there is no effective pharmacological treatment capable of limiting its progression or preventing its rupture. This type of management will be adequate as long as the AAA does not reach 5.5cm in diameter. In these circumstances, in which the risk of rupture exceeds the risk of surgical intervention, the therapeutic option tends to be surgery, a decision that can be brought forward if symptoms are present or there is a high rate of growth of the AAA (greater than 1cm/year). Depending on the patient's characteristics, the morphology and location of the AAA, the surgical intervention may be intravascular or will consist of open surgery.13 The indication that determines whether a patient is a candidate for one type of treatment or another will depend on the diameter of the aorta, the growth rate and the patient's potential surgical risk. For years it has been argued that the intravascular intervention has an improved perioperative survival, although several studies have shown that long-term survival is similar for both types of interventions.14,15 However, the incidence of delayed ruptures is higher after intravascular intervention, which continues to be a reason for concern that will require new studies and improvement strategies.14,15
Risk factorsThe pathogenesis of an AAA is complex and multifactorial and, although the aetiology is confusing, it shares several risk factors associated with atherosclerotic disease, such as: age, smoking, male gender, genetic susceptibility, hypertension, centripetal obesity, reduced levels of high-density lipoprotein (HDL) cholesterol, coronary heart disease and intermittent claudication. The fact that diabetes mellitus (DM), a risk factor for atherosclerosis, is, on the contrary, a protective factor for AAA should be highlighted.13
AgeThe incidence of an AAA increases with age. Deaths due to a ruptured aneurysm rarely occur in patients under the age of 65, and, from this point on, the risk increases by 40% every five years.16 The number of very elderly individuals (in their eighties and nineties) with an AAA is increasingly higher and there is no clear recommendation for them with respect to the indication of surgical intervention in the event that the diameter exceeds 5.5cm. The decision should be personalised and will vary depending on clinical, ethical and economic factors and factors related to the health system of the country in question.17
GenderAs has been mentioned previously, the risk in males is much higher than that in women, and in women it develops approximately 10 years later, which is probably due to hormonal and genetic factors and to the different exposure to risk factors.18,19 There is a large amount of information about the prevalence and risk factors for AAA in men, but few studies have focused specifically on the aneurysmal pathology in women.20 Consequently, the information about associated risk factors, indications for treatment and the results after the repair of an AAA in this group of patients is limited. The benefit of screening by ultrasound scan for an AAA in males over the age of 65 has been demonstrated. It is a procedure which has made it possible to reduce mortality related to AAA. On the contrary, this procedure is not recommended in women despite the fact that the disease takes on a more severe form: faster growth, greater risk of rupture with smaller diameters and greater hospital mortality associated with rupture and intervention.21 However, there is a subgroup of women with a substantially higher risk of aneurysm disease: those who are elderly (>65), with a history of smoking and heart disease, who could probably benefit from AAA screening.22
Family historyThere is an increased prevalence of AAA among first-degree relatives with AAA, reaching 15–19%,23 which has led to it being considered that there is a genetic component in this pathology. Positive family history for AAA in first-degree relatives increases the risk of a person suffering from it up to 10-fold, and at younger ages.24 Similarly, in recent years several studies have revealed the importance of epigenetic regulation in this pathology.7,25
Tobacco consumptionSmoking increases the relative risk (RR) of AAA 7.6-fold.26 Males who smoke more than 25 cigarettes/day present a risk of AAA 15 times higher than that of men who have never smoked.27 The number of cigarettes per day is important, but the duration of the smoking habit is more important. Every year of smoking increases the RR of AAA by 4%.26 Once tobacco consumption has ceased, the risk of AAA is maintained for up to 10 years. The mechanisms through which smoking would trigger the formation of an AAA are still unknown. The involvement of the inhibition in collagen synthesis, the alteration in the expression of metalloproteinases and the possible relationship with oxidative stress are considered28; and recent studies in AAA models associate exposure to tobacco with an alteration of the function of inflammatory cells.29
HypertensionHypertension (HTN) is associated with an increased risk of AAA formation and its rupture. However, antihypertensive treatment has not proven to be effective at limiting the progression of an AAA.30
Heart disease and peripheral arterial diseaseThe incidence of coronary heart disease and peripheral arterial disease, both a reflection of the presence of atherosclerosis, is very high in patients with an AAA and, in fact, both conditions frequently coexist. Cardiovascular events are the main cause of death in patients with an AAA.31 The risk of having a cardiovascular event in patients with an AAA is 52% higher compared to those patients without aortic aneurysm pathology.32
Lipid levelsThe link between lipid levels in plasma and an AAA is not simple. There are contradictory results with regard to whether serum cholesterol levels above 240mg/dl are associated with a greater risk of AAA.33,34 The possible benefit that statins could present with regard to the stabilisation of an AAA has also been analysed. However, the studies are not very conclusive and trials of a prospective nature and with a greater number of patients are required in order to be able to establish solid conclusions. In recent years, it has been revealed that HDLs are the most relevant lipoproteins when it comes to predicting the risk of developing an AAA. In a study directed by Golledge et al.35 it was observed that the serum concentrations of HDL are associated independently with a reduced risk of AAA in males who are not undergoing lipid-lowering therapy. On the contrary, in this study there was no link between the presence of an AAA and triglyceride or LDL cholesterol levels. Therefore, these results suggest that the reduction in the concentration of HDL could be considered a risk factor for the development of the disease.35 Other lines of research indicate that lipid alterations in an AAA could be related to the presence of abnormal LDLs, which are small and with a phenotype that can be called atherogenic.36 This could mean that the traditional determination of cholesterol and triglycerides would not be sufficient to evaluate the contribution of dyslipidaemia to the disease.
Diabetes mellitusIn contrast with its role as a risk factor for atherosclerotic disease, DM has been described as a protective factor against the development of an AAA.37 DM is associated with a low prevalence of AAA and, if it does exist, to a slower growth of it. The mechanism which underlies this negative relationship is unknown, but it could be related, among others, to an increase in the synthesis of extracellular matrix due to the presence of advanced glycation end products (AGEs) in diabetic patients. AGEs promote the proliferation of VSMCs and increase the content of matrix proteins in the vessel wall. Furthermore, DM suppresses plasmin, an MMP activator, which would reduce the levels and activity of MMPs 2 and 9 and would increase resistance to the proteolysis of collagen.38 Finally, DM would be associated with an increase in the extracellular matrix of the aneurysmal aortic wall, which would counteract the loss of matrix and susceptibility to the formation of an AAA.
Biomarkers of abdominal aortic aneurysmA biomarker is a biological characteristic that can be measured objectively and be evaluated as an indicator of a normal or pathological biological process, or of a response to a therapeutic intervention. Biomarkers include genes, proteins, peptides, genetic variations, lipids or metabolic products of different origin, such as body fluids or tissues.39 Although the term biomarker is relatively recent, biomarkers have been used in preclinical research and in clinical diagnosis for a considerable period of time.40 The ideal biomarker should comply with a series of characteristics such as: presenting a causal relationship with the disease, being specific for that particular disease, being involved in the pathophysiological pathways of this disease and being able to reflect the severity and progression of the disease.41 The research and discovery of new biomarkers also contribute to a better knowledge and understanding of the pathophysiology of the disease.
The identification of circulating biomarkers with diagnostic and prognostic value in an AAA is a challenge.42,43 Currently, no biochemical marker is known for this condition with sufficient specificity and sensitivity in order to be able to use it in clinical practice. The only prognostic factors in this disease are established through imaging techniques and are limited to aortic diameter and speed of aneurysm expansion. Therefore, biological markers which make it possible to diagnose the disease, assess its progression and risk of aortic rupture, and to determine patients’ response to treatment are required.43 In accordance with the pathophysiology of an AAA, circulating biomarkers can be classified in accordance with their relationship with prothrombin activity, degradation of the extracellular matrix of the vascular wall or the immunoinflammatory response.
Markers related to the degradation of the extracellular matrixThe first biomarkers to be studied were related directly to the proteolysis of the extracellular matrix. It has been demonstrated that elastin peptides and the amino-terminal propeptide of type III procollagen are high in the serum of patients with an AAA. However, they present a low sensitivity and specificity. The usefulness of the direct measurement of proteolytic enzymes has also been determined. Several studies aimed at evaluating the possible role of circulating levels of MMP-9 as a marker of aneurysm expansion have been carried out. However, the study with a greater sample size did not find a correlation between the levels of this MMP and AAA expansion.44 Other authors have demonstrated high levels of MMP-1 and MMP-9 in patients with a ruptured aneurysm compared to patients awaiting repair surgery, although these results should be ratified in studies including a larger number of patients.45
Markers related to immunoinflammatory responseGiven that neutrophils infiltrate the ILT of an AAA, circulating markers related to their activation, such as the α1 antitrypsin-elastase, myeloperoxidase or α-defensin complexes, can be found. Levels of the NGAL protein, released by circulating polymorphonuclear neutrophils and by the ILT itself, are correlated with the presence of an AAA.46 Likewise, biomarkers related to inflammation, specifically to the adaptive immune response, are also high in patients with an AAA, demonstrating the evidence of an inflammatory process associated with the development of an AAA. These include: cytokines, tumour necrosis factor alpha (TNF-α), IL-6 and C-reactive protein (CRP). There is also a clear elevation of IgG4 and IgE.47 Proteomic studies have been useful for the identification of possible new candidate biomarkers. These include chemokine CCL20, the levels of which increase in patients with an AAA in comparison with healthy individuals48 and which has recently been demonstrated as a high-sensitivity biomarker of AAA.49
Markers related to prothrombin activityIn an AAA there is increased platelet activation, such as thrombin-antithrombin complexes circulating in plasma. The simultaneous activation of markers related to clotting and fibrinolysis shows evidence of the biological activity of the ILT. Plasmin–antiplasmin complexes have a moderate sensitivity in an AAA, whether or not they are associated with fibrin degradation products. Therefore, it has been demonstrated that plasmin–antiplasmin complexes and D-dimer are correlated with the aortic diameter, with ILT thickness, with aneurysm growth and also with a worsening of lung function in patients with an AAA.50
The most recent evidence, based on a prospective study with a large sample size, supports the fact that these patients, who combine a higher number of biomarkers related to AAA, are significantly related, after adjustment for risk factors, with an increase in the incidence of AAA. Alterations in the circulating concentrations of several biomarkers related to AAA are therefore capable of identifying a group of patients with a high risk of developing an AAA.51
Limited knowledge of the pathophysiology of an AAA, and the absence of reliable biomarkers of expansion and rupture, mean that invasive intervention is the only therapeutic option that can be offered to patients at present. Currently, the only determining parameters of the need for this intervention are the diameter of the AAA, its growth rate and the patient's surgical risk. Therefore, it is fundamental to be able to deepen and expand our knowledge about the pathophysiology of AAA, as well as to identify biomarkers of the presence of AAA, of its size, growth and eventual rupture. The identification of a useful biomarker in this condition is complicated, given its multifactorial nature and complex pathophysiology. Various circulating molecules, the levels of which are high in patients with an AAA compared to control subjects, potential biomarkers of the disease, including lipids, cytokines, elements that make up the extracellular matrix, clotting factors and proteases, have been identified, but none of them has a direct application in clinical practice.
Medical treatment of an abdominal aortic aneurysmCurrent medical treatment of an AAA is based on the adequate control of the risk factors, through the use of antiplatelet drugs and statins. However, there is no medical treatment capable of modulating aneurysm growth. This is a highly significant issue, given that it does not make it possible to offer any effective treatment to patients with an AAA measuring less than 5.5cm. It has even been suggested that the quality of life of patients with an AAA measuring less than 5.5cm deteriorates during the follow-up with aneurysm growth.52,53 Furthermore, if a future medical treatment was able to postpone, or even avoid the surgical treatment of these patients, the medical cost would be substantially reduced.
Therefore, work has been carried out in this field for years, and there has been an attempt to identify drugs capable of modulating aneurysm progression. Research has also been conducted into experimental therapies, based on the pathophysiology of the disease, which may have a clinical application in the future. With that in mind, we will first focus on drugs which have already been marketed for other purposes and, secondly, on possible treatments in a preclinical phase.
A list of the drugs currently used for the treatment of other conditions, which have had a positive impact in their transition to aneurysm conditions, is then presented.
Antihypertensive drugsBeta-blockersBeta-adrenoceptor blocking agents are the most studied drugs. Both observational, mainly cohort studies54,55 and clinical trials56–58 have been performed. Different types of beta-blockers which, in many cases, were not specified, were used in the cohort studies. These studies revealed a difference in terms of aneurysm growth speed among the groups depending on whether or not they received the treatment. However, this difference was statistically significant only in less than half of the cases.55,59 Similarly, clinical trials carried out with propranolol did not present significant results in terms of speed of AAA growth.56,57
Furthermore, bisoprolol has demonstrated a reduction in mortality due to cardiac cause after AAA surgery. It is not confirmed that this effect occurs in the case of other beta-blockers, but the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines grant a class I recommendation (level of evidence A) during the perioperative period of an AAA in terms of the reduced RR of morbidity due to cardiac adverse events associated with this period.60
It is interesting to note that, to begin with, the usefulness of beta-blockers was considered to come from their capacity to reduce blood pressure and heart rate. However, more recent studies have led to the belief that the effectiveness of these drugs as a cardioprotective therapy is not only related to their effects as beta-adrenoceptor blocking agents, but due to presenting a direct antiatherosclerotic effect, both on an experimental and human level.61 Furthermore, the evidence supports the theory that the therapeutic target of metoprolol as a cardioprotective drug comes from its interaction with the inflammatory response and, more specifically, due to its involvement in the response of macrophages and neutrophils.62
Angiotensin-converting enzyme (ACE) inhibitorsIt has been observed that the activation of the renin–angiotensin system is involved in the development of an AAA.63 Cohort studies have obtained favourable, although not significant, results in the group treated with angiotensin-converting enzyme (ACE) inhibitors.30 More recent studies, with a recruitment of almost 10,000 patients, have demonstrated that the administration of ACE inhibitors is linked to a reduction in mortality and in the risk of surgical intervention in patients with an AAA.64 However, two publications from 2010 not only obtained positive results, but also observed an increase in the growth rate as well as in the perioperative mortality rate in those patients who received ACE inhibitor treatment.65,66 In addition, the first and only clinical trial conducted so far with these drugs, in 2016, specifically with perindopril, did not obtain favourable results.67
Calcium channel blockersThe potential role of calcium channel blockers in an AAA is controversial. On the one hand, they have shown beneficial effects which are not directly related to their blood-pressure lowering function. Therefore, nifedipine has been able to inhibit the progression of aneurysms through the suppression of the activity of NF-kB and MMP-9,68 while other drugs have been related to an increase in the rigidity of the aortic wall.69 Furthermore, the cohort studies performed have demonstrated a difference in favour of the group treated, although this difference has never been statistically significant.30 On the other hand, calcium channel blockers seem to promote the formation of an AAA in its initial stages, which could be in relation to its role in the metabolism of elastin. In the case of amlodipine, it has been determined in an animal model that this drug strengthens elastase activity.54
Antiplatelet therapyWe know that the ILT plays a fundamental role in the progression and risk of rupture of an AAA.70 Experimental studies conducted with antiplatelet therapy suggest the beneficial effect of this treatment.71 Several observational studies supported these results, while other studies were contradictory.72 Perhaps clinical trials with another type of antiplatelet, such as ticagrelor, already under way, might clarify all these doubts. In any case, despite the fact that there is no conclusive evidence, it is recommended that patients with an AAA receive low doses of aspirin.60
Anti-inflammatoriesIt is necessary to highlight a series of drugs, such as statins and certain antibiotics, whose effects which have enabled them to be used in aneurysmal diseases, even though their anti-inflammatory effects are not their main function.
Practically all experimental studies support statins as being able to reduce aneurysm growth.73 As we have mentioned, their anti-inflammatory function is added to their lipid-lowering ability. Within the observational studies, some have shown a difference in terms of growth rate,74 but not all.75 Overall, the meta-analyses which exist in this regard support the ability of statins to modulate the growth of an AAA, but the evidence is of low quality.76 In any case, we can confirm that the information that we have nowadays supports the fact that statins are recommended to patients with an AAA.
On the other hand, it was also suggested that an infectious agent, Chlamydia pneumoniae could be related as an initiating agent or accelerator in the process of aneurysm formation and expansion.77–79 Doxycycline, an antibiotic capable of inhibiting MMPs, has had favourable results in experimental models,80,81 as well as in clinical trials conducted a few years ago.70 Furthermore, it has been observed that in patients with an AAA treated with doxycycline, plasma levels of MMP-9, as well as the mRNA level of this MMP in the abdominal aorta, were reduced.82,83 However, more recent clinical trials have obtained contradictory results, contrary to what the pathophysiological evidence indicates.84 In terms of roxithromycin, clinical trials which have demonstrated a difference between the treated and non-treated group have been performed, but this difference was not statistically significant either.79
With regard to anti-inflammatory drugs per se, cohort studies have also been performed for non-steroidal anti-inflammatory drugs (NSAIDs). As with statins, in this case there was improvement in the treated group, but this difference was not statistically significant.74
New therapeutic targets for the treatment of an abdominal aortic aneurysmKnowledge of the pathogenesis of an AAA will make it possible to determine new therapeutic molecular targets with the potential for being transferred to routine clinical practice in the future.
The studies performed in this regard are diverse. We will therefore focus on the more recent ones which we consider may be representative of how the pathophysiology may help to identify new therapeutic targets for the treatment of an AAA. Specifically, we will give examples of the line to which most efforts have probably been dedicated, such as the modulation of the immunoinflammatory response. Similarly, we will outline other possible newer lines of research, but for which there is less information, such as post-transcriptional regulators and cell therapy.
Regulation of the immunoinflammatory responseAs mentioned above, an AAA is characterised by the presence of different cell types involved in the immunoinflammatory response, such as neutrophils, macrophages and B- and T-cells, as well as pro-inflammatory mediators such as cytokines and TNF-α. Different interleukins have been related to the progression of an AAA. Both on a clinical and experimental level, an elevation of IL-1β has been observed in these patients. Furthermore, the experimental absence of IL-1β and of the IL-1 receptor, as well as treatment with anakinra (an IL-1 receptor antagonist), reduced the aneurysm dilation, in turn generating a reduction in the infiltration of macrophages and neutrophils, and maintaining the integrity of the elastin.85 In this regard, recent results with an IL-1β-blocking antibody in the CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study), study performed in patients with a previous myocardial infarction, have shown its capacity to reduce cardiovascular events.86 In contrast, a recent publication shows that this treatment may have damaging effects on advanced atherosclerotic lesions,87 meaning that its possible application to other diseases such as AAA will need additional studies.
In terms of TNF-α, on an experimental level it has been observed how infliximab, a monoclonal antibody which inhibits the functional activity of this cytokine, has been able to limit aneurysm expansion.88 A recent publication highlights the contribution of TNF-α to the polarisation of macrophages, and concludes that blocking them in conditions such as AAA could be more effective than the blocking of IL-1β.89
Other members of the TNF-α superfamily have also been involved in the development of an AAA and in the underlying inflammatory response. Therefore, both the tumour necrosis factor-like weak inducer of apoptosis (TWEAK) protein and its functional receptor Fn14 are present in human aneurysm lesions.90 Gene deletion of these proteins in experimental aneurysm models in mice reduces the inflammatory response as well as the size and progression of an AAA.90 It is worth highlighting that circulating levels of soluble TWEAK have been associated with the expansion of an AAA in humans.91
The relationship between prostaglandin E2 (PGE2) and vascular inflammatory processes in an AAA is also known.92,93 Among the PGE2 receptors, EP-4 seems to have an important role in the development of an aneurysm.93 Therefore, the reduction of the expression of EP-4 and/or the administration of antagonists reduces aneurysm dilation and levels of IL-6, IL-17, MIP-1α, MMP-2 and MMP-9.94
The recruitment of inflammatory cells to the artery wall is enhanced by the synthesis and secretion of several mediators such as chemokines. Among them, we have recently demonstrated that the inhibition of galectin-3, which is chemotactic for monocytes, reduces the development of experimental AAA through the regulation of MCP-1, the main chemokine of macrophages.95 With regard to neutrophils, lipocalin-2 is a protein which acts as a neutrophil chemoattractant and whose circulating levels are significantly increased in the plasma of patients with an AAA and are correlated with the activity of the AAA.46 Recently, we have observed how, experimentally, the deficiency of lipocalin-2 was capable of limiting the expansion of an AAA in the elastase perfusion model in mice.96
Finally, the immune system, by means of the regulation of inflammation, but also by modulating the innate immune response, as well as the immunoglobulins, can alter the progression of an AAA. The regulation of the polarisation of T-cells (Th1/Th2 ratio) may be a line of research in the treatment of an AAA.97 B-cells have also been involved recently in an AAA. The most recent publications, based on experimental studies, support the possibility that B-cells, in an environment of AAA, may be damaged. This reason could explain the paradoxical result of how, on the one hand, the elimination of B-cells reduces the aneurysm progression, while, on the other hand, the insertion of B-cells from healthy animals generates the same effects.98
Epigenetic regulatorsAs in the majority of complex diseases, in cardiovascular diseases interactions occur between genetic and environmental factors in which epigenetic mechanisms intervene. Epigenetic regulation has important repercussions on the repertoire of genes expressed at the cellular level. Epigenetic mechanisms controlled by histone deacetylases (HDACs) and microRNA (miRNA) modulate processes such as inflammation, thrombosis, proliferation, apoptosis and vascular remodelling involved in the development of complex diseases such as AAA.
miRNAs are post-transcriptional gene regulators and are involved in various pathophysiological processes. In order to not make the article too long, readers are recommended to read a review in which the latest advances on miRNAs, their role in an AAA99 and their interest as therapeutic targets and/or biomarkers in this disease are detailed comprehensively. However, their transition to clinical practice would probably be accompanied by severe side effects. Similarly, there is recent interest surrounding the effects of long non-coding RNAs (lncRNAs) in chronic vascular remodelling processes such as an AAA.100,101
In relation to HDACs and their involvement in aneurysm pathology, recent studies have demonstrated the alteration of the expression pattern of HDACs of class I and IIa in the human aneurysm and how treatment with HDAC inhibitors limits the development of an AAA in an experimental model in mince, which suggests that these drugs could constitute an effective therapeutic strategy for the treatment and stabilisation of an AAA.25
Cell therapyMesenchymal stem cells are not only able to differentiate between different cell types, but they also present potent anti-inflammatory and immunoregulatory actions. In this way, the intravenous administration of this cell type in murine AAA models by means of perfusion of angiotensin II delayed the development of an AAA.102 There are various events which are related to this delay in the progression of aneurysms, including: the reduction of MMP-2, MMP-9, IL-6 and MCP-1 and the elevation of TIMP-1.103 It is worth highlighting that these treatments have also been analysed in more translational models such as pig models.104 Currently, there is a clinical trial in progress on AAA treatment (in small AAAs without surgical indication) using mesenchymal cells.105
The combination of the preclinical and clinical research is necessary to figure out the mechanisms which underlie the development of an AAA and, in this way, to validate the medical approaches. Current evidence of the medical treatment available for an AAA is of low quality. However, it is important to highlight that clinical trials conducted up to now are limited. They have also been carried out with a small number of patients, a low quality of the study and, lastly, the growth has been analysed, taking it as a surrogate marker of rupture, but without this being a direct marker, which could be of interest to analyse other markers. Furthermore, some possible therapeutic targets have been identified in preclinical models, mainly in mice, which need to be validated in more translational experimental models106 in order to potentially be transferred to the patient.
ConclusionsCurrently, knowledge of the pathophysiology of an AAA is still limited. Studying the different cellular and molecular mechanisms which modulate the pathological vascular remodelling will make it possible to determine with greater accuracy the processes which result in the formation, growth and rupture of an AAA. In turn, the identification of these mechanisms will make it possible to identify potential therapeutic targets to slow down the advance of this condition. Currently, different clinical trials are being conducted with antihypertensive, antiplatelet and anti-inflammatory drugs to clarify the suitability of these types of treatment in patients with aneurysms. Finally, the search and identification of circulating biomarkers of AAA is a major challenge as nowadays the diagnosis of an AAA tends to be causal and non-causal. These new biomarkers should allow for an early and improved treatment of this condition.
FundingThe laboratory work carried out by JLMV and CR was funded by the Instituto de Salud Carlos III [Carlos III Health Institute] (PI15/01016 and PI16/01419) and the Ministry of Economy, Industry and Competition (SAFSAF2015-64767-R and SAF2016-80843-R), with co-funding by the European Regional Development Fund (ERDF). The Biomedical Research Network Centres (CIBER) for Cardiovascular Diseases is an ISCIII initiative. LC received a grant from the funds for the training and contracting of new research staff (FI) programme of the Agència de Gestió d’Ajuts Universitaris i de Recerca [Agency for the Management of University and Research Grants] (2017FI_B_00175, Regional Government of Catalonia).
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors apologise to all those researchers whose work could not be cited due to length limitations.
Please cite this article as: Torres-Fonseca M, Galan M, Martinez-Lopez D, Cañes L, Roldan-Montero R, Alonso J, et al. Fisiopatología del aneurisma de aorta abdominal: biomarcadores y nuevas dianas terapéuticas. Clín Investig Arterioscler. 2019;31:166–177.