Conventional treatment for obesity with diet, exercise and bariatric surgery has limitations; thus, it is necessary to have pharmacological tools. In the past, different drugs were marketed that were withdrawn due to safety problems. There are currently 3 drugs approved by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) for obesity therapy (orlistat, combination of bupropion and delayed-release naltrexone and liraglutide) and two more only authorized by FDA (lorcaserin and the combination of phentermine and extended release topiramate). It is recommended to use as a second therapeutic line and its choice should be individualized taking into account multiple aspects such as expected weight loss, route of administration, safety profile and cost. Currently there are several drugs under development that act on different therapeutic targets.
El tratamiento convencional de la obesidad con dieta y ejercicio así como la cirugía bariátrica tienen sus limitaciones, por lo que es necesario disponer de fármacos para su tratamiento. En el pasado se comercializaron diferentes fármacos que fueron retirados por problemas de seguridad. Actualmente existen 3 fármacos aprobados por la Agencia Europea del Medicamento (EMA) y la Food and Drug Administration (FDA) para el tratamiento de la obesidad (orlistat, combinación de bupropión y naltrexona de liberación retardada y liraglutida) y 2 más solo autorizados por la FDA (lorcaserina y la combinación de fentermina y topiramato de liberación prolongada). Se aconseja su uso como segunda línea terapéutica y su elección debe individualizarse teniendo en cuenta múltiples aspectos como la pérdida de peso esperada, la vía de administración, su perfil de seguridad y el coste. Por otra parte, actualmente existen varios fármacos en vías de desarrollo que actúan sobre diferentes dianas terapéuticas.
In recent years, the incidence of obesity has been increasing at an alarming rate all over the world, to such an extent that it is currently considered to be the epidemic of the 21st century.1 In Spain, according to the ENRICA study, 16.5% of the population is overweight (body mass index [BMI] 25–30kg/m2), 21.7% present with slight or moderate obesity (BMI 30–40kg/m2), and 1.2% with severe or morbid obesity (BMI >40kg/m2). Moreover, this is a disease which is associated with a greater risk of comorbidities, such as high blood pressure, dyslipidaemia and diabetes mellitus type 2, and a reduction in life expectancy.2–4
The classic therapeutic approach consists of establishing changes in lifestyle for the majority of subjects suffering from obesity, limiting bariatric surgery to the most serious cases. Both treatments have their pros and cons. On one hand, the principal drawback of conventional treatment with diet and exercise is the scant effectiveness in the short-term, with a significant loss of effectiveness in the long-term. Thus, in the LOOK Ahead study—a paradigm of nutritional intervention not assumable in daily practice as it entails weekly interventions for the initial 6-month period—only 46% of subjects succeeded in losing more than 5% of their body weight per year. Subsequently, 60% of these patients with a good initial response regained the weight lost or exceeded their initial weight during the following 3 years.5 On the other hand, bariatric surgery is the most effective way for treating obesity, obtaining weight losses which may exceed 30%, and which are sustained in the long-term. In a high percentage of cases, it can also achieve the remission of comorbidities associated with obesity, and is associated with a reduction in mortality.6,7 Despite this, it must be taken into account that bariatric surgery is not without potential complications. Currently, with laparoscopic techniques, perioperative mortality is low, but perioperative complications—such as bleeding, infections and suture failures, as well as subsequent complications such as dumping8 syndrome—are common. On the other hand, it is important to consider that it is not indicated for all individuals suffering from obesity. In 1991, the National Institutes of Health limited the indication of bariatric surgery to those subjects between the ages of 18 and 60 with severe obesity (BMI >40kg/m2) or moderate obesity (BMI >35kg/m2) with associated comorbidities.9
More recently, in 2016, two new drugs for treating obesity were marketed in Spain: liraglutide and the combination of extended-release naltrexone/bupropion (Nal/Bup). Both drugs were already being marketed in the United States along with lorcaserin and the combination of prolonged-release phentermine and topiramate (Phen/Top). Taking the limitations of conventional treatment and of bariatric surgery into account, pharmacotherapy may play an important role as a treatment for obesity. In this context, we have considered it timely and appropriate to review the drugs classically used in the treatment of obesity, those currently available, and those being developed.
Therapeutic targetsThe pathophysiology of obesity is highly complex, and a number of factors play a role therein. In a simplistic way, it can be explained using the analogy of scales. Whenever energy intake is greater than caloric expenditure, there is a positive energy balance which inhibits lipolysis and activates the accumulation of triglycerides in adipocytes (lipogenesis). Sustained over a number of years, this imbalance may lead to obesity. If we analyze the pathophysiology of obesity in greater detail, a number of factors may have a bearing on each side of the scales. On one hand, the regulation of intake at the level of the central nervous system and factors related to diet may lead to an increase in calorie intake. On the other hand, energy expenditure is conditioned by basal metabolism, thermogenesis in brown adipose tissue and physical exercise. Of these five factors, neither environmental factors related to nourishment and physical exercise, nor basal metabolism are potentially modifiable with pharmacological treatments. Accordingly, the therapeutic targets can be focused fundamentally on the regulation of intake and on the activation of thermogenesis in brown adipose tissue.
It should also be taken into consideration that the pathophysiology of obesity is even more complex. Roles are played therein by genetic or epigenetic factors, as well as by different organs and tissues, such as the intestine. It is in the digestive tract where the absorption of lipids and other nutrients takes place. Moreover, an entire range of hormones are produced, such as glucagon-like peptide 1 (GLP-1), gastric inhibitory peptide (GIP), peptide YY (PYY) and ghrelin, all of which have an important effect in regulating intake.10 Lastly, the gut microbiota may play a prominent role in the development of obesity.11
The stimulus of brown adipose tissue is an attractive therapeutic target for the treatment of obesity. This is capable of producing heat and consuming energy through the expression of uncoupling protein-1 (UCP-1).12 Despite this, none of the drugs currently marketed act on this tissue. The main therapeutic target is the regulation of intake through the central nervous system, which is brought about mainly in the arcuate nucleus of the hypothalamus. There are two types of neurons which are key in regulating intake. On one hand, neurons which express the agouti-related peptide (AgRP) and neuropeptide Y (NPY) which stimulate intake; and, on the other hand, other cells which express pro-opiomelanocortin (POMC) and which inhibit calorie intake. The activity of these neuronal populations is regulated by multiple stimuli, such as cerebral neurotransmitters or a number of peripheral stimuli indicative of the energy balance, such as glucose, insulin, leptin or the aforementioned intestine hormones.13
History of pharmacotherapy of obesityPrior to 1990Throughout the 20th century, a number of different drugs were used to treat obesity, with no studies to endorse their efficacy. A number of these were withdrawn from the market owing to their serious side effects; these included dinitrophenol, a decoupler of the respiratory chains which resulted in fatal hyperthermia,14 and amphetamines, which, in addition to addiction, can result in acute intoxication, psychotic disorders or cardiovascular toxicity. In turn, in a number of clinical trials, the combination of fenfluramine (a serotonin reuptake inhibitor) and phentermine (a sympathomimetic agent) was shown to result in sustained weight losses.15 Nonetheless, it was also withdrawn from the US market in 1997, owing to the increase in valvular heart diseases attributed to fenfluramine.16
1990–2010Between 1990 and 2010, three drugs were marketed in the United States and Europe to treat obesity: sibutramine in 1997, orlistat in 1999 and rimonabant in 2006. Sibutramine was a serotonin-norepinephrine reuptake inhibitor which resulted in a weight loss of 4.2% greater than placebo.17 It was withdrawn from the market in 2010 owing to an increase in adverse cardiovascular effects, which occurred mainly in patients with a high cardiovascular risk.18 Rimonabant was an inverse agonist and an antagonist of the cannabinoid receptor 1, with a placebo-subtracted weight loss of 4.7%.17 In this case, marketing thereof was suspended in 2008 owing to an increase in psychiatric disorders and risk of suicide.19 Of these, the sole survivor is orlistat.
OrlistatOrlistat is an intestinal lipase inhibitor which reduces the absorption of lipids by up to 30%. In order to obtain this effect, the recommended dose is 120mg immediately before, during or after the three main meals. In a meta-analysis which included 10,631 subjects with a mean BMI of 36.3kg/m2, orlistat was associated with a weight loss of 2.9kg more than placebo.20 In addition to weight loss, there is an improvement in the cardio-metabolic parameters. In this regard, in the XENDOS study, treatment with orlistat reduced the incidence of type 2 diabetes by 37.3% after 4 years.21
Although this drug has no serious adverse effects, its principal drawback is its low tolerability, which means that many patients drop out from treatment. The adverse effects of blocking fat absorption are greasy stools, diarrhoea, flatulence and the urgent need to defecate. These adverse effects appear in 15–30% of subjects, and are more prevalent in patients continuing to consume a high-fat diet.20 It can also result in a reduction in the absorption of liposoluble vitamins, owing to which the supplementation thereof is recommended.
Pharmacotherapy of obesity at presentOver the last decade, four new pharmacological alternatives have emerged for the treatment of obesity. Two of these were approved in the United States by the Food and Drug Administration (FDA) in 2012, but have yet to be approved by the European Medicines Agency (EMA): lorcaserin and Phen/Top. The other two, liraglutide and Nal/Bup, were approved by the FDA in 2014 and subsequently by the EMA in 2015, and are currently available in Spain.
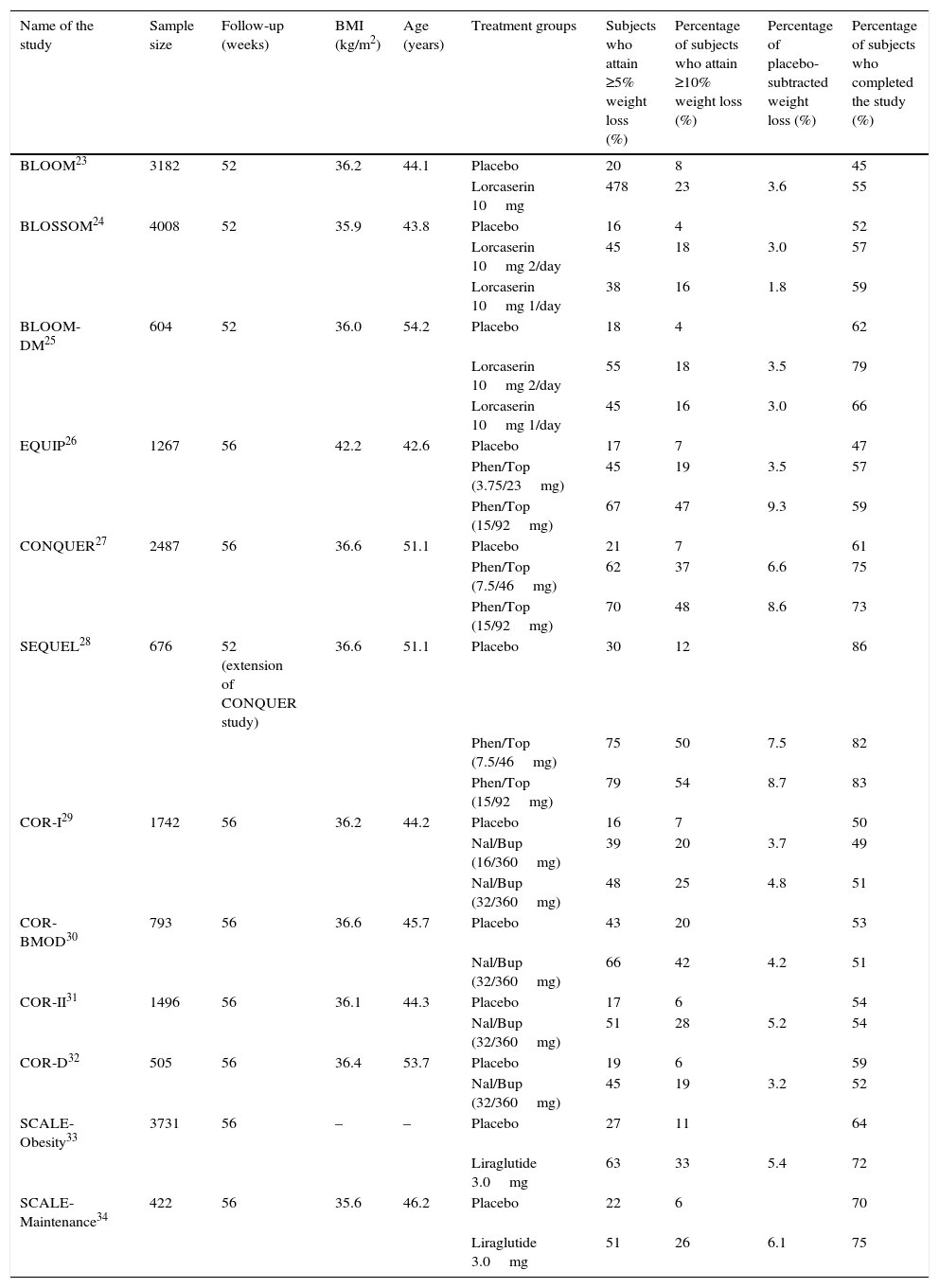
LorcaserinLorcaserin is a selective serotonin 2C receptor agonist which acts in the hypothalamus by increasing satiety.22 It is administered at a dose of 10mg twice daily. Its approval by the FDA was based on three phase III clinical studies: BLOOM, BLOSSOM and BLOOM-DM.23–25 Weight loss after one year, and at the recommended dose, is 3–3.5% higher than for placebo (Table 1). In the BLOOM-DM study, which included patients with diabetes mellitus type 2, treatment with lorcaserin resulted in a decrease in HbA1c of 0.5% relative to placebo.25 Generally speaking, it is a well-tolerated drug, as can be seen from the fact that more patients finished the studies in the intervention group than in the placebo group. The main adverse effects are usually serotonergic symptoms, such as headaches, nausea, dry mouth, asthenia or constipation.35 Its use is contraindicated with drugs with serotonergic action, such as selective serotonin reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors, owing to the risk of causing a serotonin syndrome. Unlike fenfluramine, which had no selective action on serotonin, no increase in valvular defects have been detected in patients treated with lorcaserin.36
Clinical efficacy of drugs approved for the treatment of obesity in phase III studies.
| Name of the study | Sample size | Follow-up (weeks) | BMI (kg/m2) | Age (years) | Treatment groups | Subjects who attain ≥5% weight loss (%) | Percentage of subjects who attain ≥10% weight loss (%) | Percentage of placebo-subtracted weight loss (%) | Percentage of subjects who completed the study (%) |
|---|---|---|---|---|---|---|---|---|---|
| BLOOM23 | 3182 | 52 | 36.2 | 44.1 | Placebo | 20 | 8 | 45 | |
| Lorcaserin 10mg | 478 | 23 | 3.6 | 55 | |||||
| BLOSSOM24 | 4008 | 52 | 35.9 | 43.8 | Placebo | 16 | 4 | 52 | |
| Lorcaserin 10mg 2/day | 45 | 18 | 3.0 | 57 | |||||
| Lorcaserin 10mg 1/day | 38 | 16 | 1.8 | 59 | |||||
| BLOOM-DM25 | 604 | 52 | 36.0 | 54.2 | Placebo | 18 | 4 | 62 | |
| Lorcaserin 10mg 2/day | 55 | 18 | 3.5 | 79 | |||||
| Lorcaserin 10mg 1/day | 45 | 16 | 3.0 | 66 | |||||
| EQUIP26 | 1267 | 56 | 42.2 | 42.6 | Placebo | 17 | 7 | 47 | |
| Phen/Top (3.75/23mg) | 45 | 19 | 3.5 | 57 | |||||
| Phen/Top (15/92mg) | 67 | 47 | 9.3 | 59 | |||||
| CONQUER27 | 2487 | 56 | 36.6 | 51.1 | Placebo | 21 | 7 | 61 | |
| Phen/Top (7.5/46mg) | 62 | 37 | 6.6 | 75 | |||||
| Phen/Top (15/92mg) | 70 | 48 | 8.6 | 73 | |||||
| SEQUEL28 | 676 | 52 (extension of CONQUER study) | 36.6 | 51.1 | Placebo | 30 | 12 | 86 | |
| Phen/Top (7.5/46mg) | 75 | 50 | 7.5 | 82 | |||||
| Phen/Top (15/92mg) | 79 | 54 | 8.7 | 83 | |||||
| COR-I29 | 1742 | 56 | 36.2 | 44.2 | Placebo | 16 | 7 | 50 | |
| Nal/Bup (16/360mg) | 39 | 20 | 3.7 | 49 | |||||
| Nal/Bup (32/360mg) | 48 | 25 | 4.8 | 51 | |||||
| COR-BMOD30 | 793 | 56 | 36.6 | 45.7 | Placebo | 43 | 20 | 53 | |
| Nal/Bup (32/360mg) | 66 | 42 | 4.2 | 51 | |||||
| COR-II31 | 1496 | 56 | 36.1 | 44.3 | Placebo | 17 | 6 | 54 | |
| Nal/Bup (32/360mg) | 51 | 28 | 5.2 | 54 | |||||
| COR-D32 | 505 | 56 | 36.4 | 53.7 | Placebo | 19 | 6 | 59 | |
| Nal/Bup (32/360mg) | 45 | 19 | 3.2 | 52 | |||||
| SCALE-Obesity33 | 3731 | 56 | – | – | Placebo | 27 | 11 | 64 | |
| Liraglutide 3.0mg | 63 | 33 | 5.4 | 72 | |||||
| SCALE-Maintenance34 | 422 | 56 | 35.6 | 46.2 | Placebo | 22 | 6 | 70 | |
| Liraglutide 3.0mg | 51 | 26 | 6.1 | 75 |
BMI, body mass index; Nal/Bup, combination of naltrexone and bupropion; Phen/Top, combination of phentermine and topiramate.
Phentermine is a sympathomimetic amine with an appetite suppressant effect, and its action is similar to that of amphetamines.15 Topiramate is a neurostabiliser drug which has been used for a number of years to treat epilepsy and migraines; however, at higher doses than those used for the treatment of obesity.37 Its appetite suppressant effect is obtained through the inhibition of the orexigenic effect of glutamate on a central level.38 With the combination of both drugs, a synergistic effect is obtained in weight loss.39 The advantages of acting on different mechanisms may be those of, on one hand, avoiding compensatory mechanisms and, on the other, achieving a synergistic effect with lower doses, which in turn allow the improvement of its safety profile. There are currently four presentations of the Phen/Top combination. The initial dose is 3.75mg of phentermine and 23mg of topiramate, the usual maintenance dose being 7.5mg of phentermine and 46mg of topiramate, which can be increased to a maximum dose of 15mg of phentermine and 92mg of topiramate.
The phase III pre-marketing studies were EQUIP and CONQUER.26,27 In these, a placebo-subtracted weight loss of 6.6% was observed for the 7.5mg of phentermine/46mg of topiramate dose and of 8.4–8.6% for the maximum dose of 15mg of phentermine/92mg of topiramate (Table 1). Additionally, the SEQUEL trial (an extension of the CONQUER study) showed that the weight losses achieved after one year were sustained after two years.28 This is a drug with adherence rates in phase III studies which are higher than placebo. The most common side effects are paresthesia, dizziness, headaches, dysgeusia, insomnia, constipation and dry mouth; they are dose-dependent and improve after one year of treatment.35 Owing to the teratogenic effect of topiramate on orofacial cleft, the FDA requires a pregnancy test before commencing treatment, and on a monthly basis while treatment is maintained. It is classified by the FDA as a schedule IV drug, and is only available under supervision through the Risk Evaluation and Mitigation Strategy (REMS) programme.
Combination of sustained-release naltrexone/bupropionNaltrexone is an opioid receptor agonist which has been used for the treatment of alcohol and opioid dependence.40,41 Bupropion is a norepinephrine and dopamine reuptake inhibitor with a well-known anti-tobacco and anti-depressant effect.42,43 The daily dose of Nal/Bup is 8mg/90mg twice daily, with a progressive increase in the dose over four weeks. In the same way as for Phen/Top, a synergistic effect has been observed with their combination, along with the dose-dependent effect in terms of both appetite suppression and weight loss.44
The phase III pre-marketing studies were the COR-I, COR-BMOD and COR-II studies (Table 1).29–31 These were conducted on overweight or obese subjects and weight loss was 4–6% better than placebo after one year. On the other hand, the COR-D study conducted on subjects with diabetes mellitus type 232 showed that weight loss in diabetic subjects is probably slightly lower (3% higher than placebo). The treatment was also associated with a decrease in HbA1c of 0.5% relative to placebo. The most common adverse effects are nausea, headaches, dizziness, insomnia and vomiting. Although these are mild, and in some cases transitory, they do lead to some patients dropping out of treatment. In fact, this is the only one of the four treatments with drop-out rates higher than placebo in the phase III studies (Table 1). Treatment with Nal/Bup has been associated with an increase in the adverse effects related to blood pressure. Thus, in the COR-D study, the rate of adverse effects associated with blood pressure was higher than in the placebo group (12% vs 6.5%).32 This was the main reason for the FDA's refusal to approve it in 2011. Despite this, the preliminary results of the cardiovascular safety study (Light study) led to the marketing of the drug finally being approved in the United States.45 For this reason, blood pressure monitoring is required with this treatment. Apart from this, no increase in severe neuropsychiatric adverse effects has been detected, nor of the risk of suicide, which have been described for bupropion when used at higher doses for smoking cessation treatments.35
LiraglutideLiraglutide is a GLP-1 analogue, a family of drugs which has been used in the treatment of diabetes mellitus type 2 for a number of years.46 In addition to improving glycaemic control, these drugs result in greater weight loss than other diabetes therapies.47 This was the reason for investigation into its potential use as an obesity drug at doses higher than those used for diabetes. The weight loss mechanisms would seem to be related, on one hand, with the slowing down of gastric emptying, which gives rise to early satiety, and, on the other, with an anorectic effect on the hypothalamus.48 This drug is administered subcutaneously on a daily basis, with a progressive weekly increase in the dose up to 3mg/day.
The phase III studies which demonstrated the efficacy of 3mg of liraglutide in obese patients without diabetes were the SCALE-Obesity and the SCALE-Maintenance studies (Table 1).33,34 In these studies, weight loss was 5.4–6.1% greater than for placebo. In addition to weight loss, liraglutide was associated with a greater reduction in HbA1c levels without an increase in hypoglycaemia, and an improvement in other cardiovascular risk parameters, such as blood pressure or the lipid profile.
Liraglutide is a well-tolerated drug. Its most common adverse effects are nausea and vomiting, which are usually transitory and may actually contribute to weight loss.49 The possible association between GLP-1 analogues and the development of acute pancreatitis is currently not conclusive, as reported by the FDA and the EMA in 2014.50 The use of GLP-1 analogues is not recommended in subjects with personal or family histories of medullary thyroid cancer or multiple endocrine neoplasia type 2, owing to an increase in tumours in thyroid C cells having been observed in animal models in mice.51
Although there are no specific data on the possible cardiovascular benefits of liraglutide 3mg in subjects with obesity, an extensive study conducted on type 2 diabetes with the doses used in this disease (1.2mg) has demonstrated a reduction in cardiovascular episodes in high-risk cardiovascular patients.52
Usefulness in daily practiceAccording to the summary of product characteristics, the use of these drugs is indicated in combination with a low-calorie diet and an increase in physical activity in individuals with obesity (BMI >30kg/m2) or in overweight subjects (BMI 27–30kg/m2) who also present with concomitant diseases related to being overweight, such as type 2 diabetes, dyslipidaemia and high blood pressure. From this point on, the first question that we need to ask is at what point in the natural history of the disease are we going to indicate pharmacological treatment. In this regard, the Endocrine Society, in collaboration with the European Society of Endocrinology and The Obesity Society, recommends pharmacological treatment in those subjects with a history of inability to lose weight or maintain weight loss with conventional treatment, but never as an initial therapeutic option.53
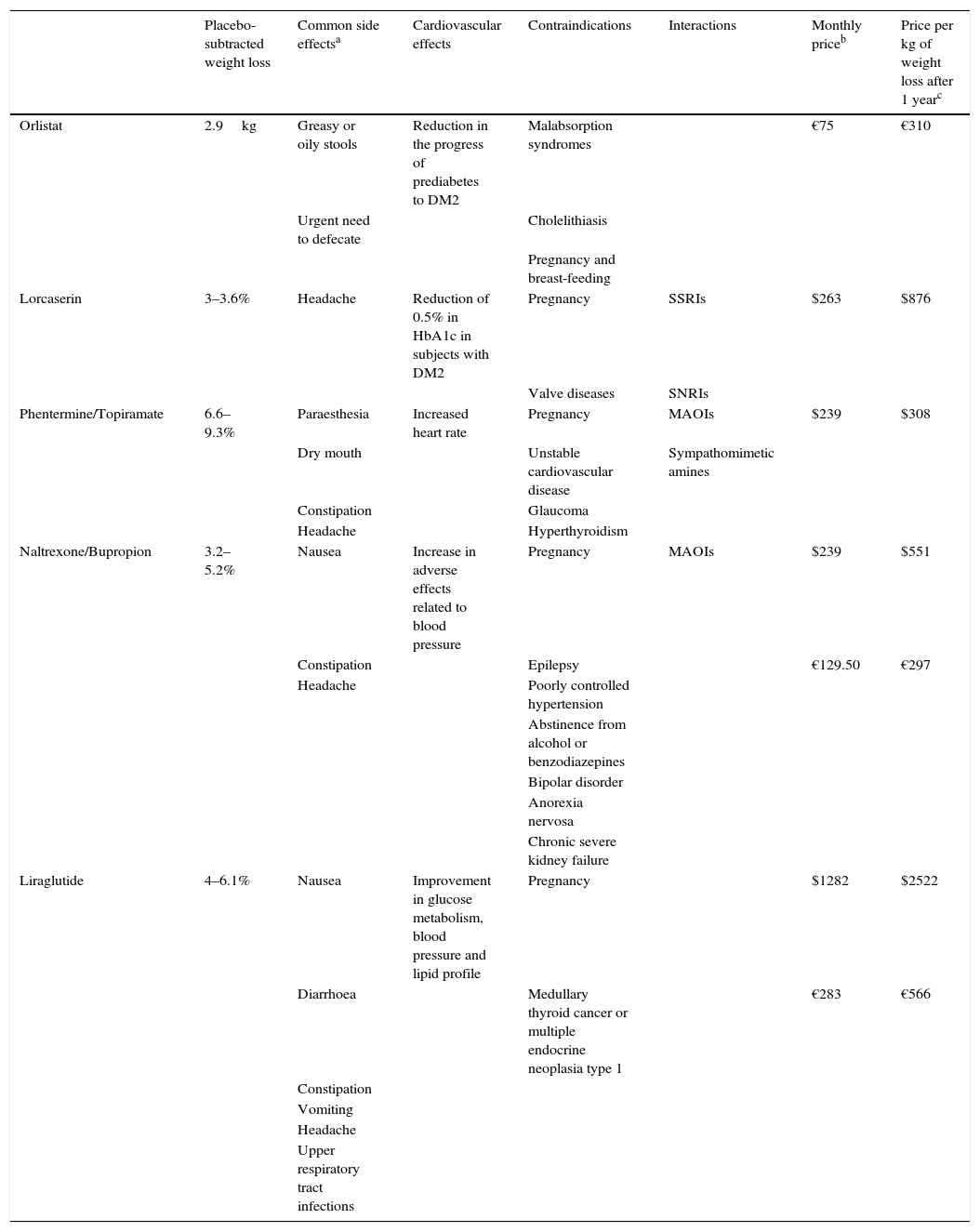
A further question to be posed is which of the five treatments we should select. When making comparisons between the drugs, a number of circumstances will need to be taken into account. First of all, there is the fact that there are no head-to-head studies between them, and comparisons in terms of weight loss and tolerability must be carried out with caution. Moreover, phase III studies include an intensive dietetic intervention, which means that weight loss in clinical practice is generally lower than that observed in other studies. Other circumstances to be taken into account are the desired weight loss, tolerability, administration pathway, the patient's comorbidities and cost (Table 2). The economic aspect becomes increasingly relevant as these drugs are not funded by the National Health Service.
Characteristics of drugs approved for the treatment of obesity.
| Placebo-subtracted weight loss | Common side effectsa | Cardiovascular effects | Contraindications | Interactions | Monthly priceb | Price per kg of weight loss after 1 yearc | |
|---|---|---|---|---|---|---|---|
| Orlistat | 2.9kg | Greasy or oily stools | Reduction in the progress of prediabetes to DM2 | Malabsorption syndromes | €75 | €310 | |
| Urgent need to defecate | Cholelithiasis | ||||||
| Pregnancy and breast-feeding | |||||||
| Lorcaserin | 3–3.6% | Headache | Reduction of 0.5% in HbA1c in subjects with DM2 | Pregnancy | SSRIs | $263 | $876 |
| Valve diseases | SNRIs | ||||||
| Phentermine/Topiramate | 6.6–9.3% | Paraesthesia | Increased heart rate | Pregnancy | MAOIs | $239 | $308 |
| Dry mouth | Unstable cardiovascular disease | Sympathomimetic amines | |||||
| Constipation | Glaucoma | ||||||
| Headache | Hyperthyroidism | ||||||
| Naltrexone/Bupropion | 3.2–5.2% | Nausea | Increase in adverse effects related to blood pressure | Pregnancy | MAOIs | $239 | $551 |
| Constipation | Epilepsy | €129.50 | €297 | ||||
| Headache | Poorly controlled hypertension | ||||||
| Abstinence from alcohol or benzodiazepines | |||||||
| Bipolar disorder | |||||||
| Anorexia nervosa | |||||||
| Chronic severe kidney failure | |||||||
| Liraglutide | 4–6.1% | Nausea | Improvement in glucose metabolism, blood pressure and lipid profile | Pregnancy | $1282 | $2522 | |
| Diarrhoea | Medullary thyroid cancer or multiple endocrine neoplasia type 1 | €283 | €566 | ||||
| Constipation | |||||||
| Vomiting | |||||||
| Headache | |||||||
| Upper respiratory tract infections |
DM2, diabetes mellitus type 2; MAOIs, monoamine oxidase inhibitors; SNRIs, serotonin norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors.
Of the five drugs, only three are currently available in Spain, which reduces the range of options. Of those not marketed in Spain, lorcaserin is a drug with a good tolerability profile, but it is probably one of the least effective in weight loss in conjunction with orlistat. On the other hand, Phen/Top is the drug with the greatest weight loss potential, and it is generally well tolerated. The main drawback is its teratogenicity and sympathomimetic effect, which render its use inadvisable in patients with cardiovascular diseases.
Of the drugs available in Spain, orlistat has been marketed longest. Its use in general practice is limited, owing mainly to its low tolerance and efficacy. The other two drugs, Nal/Bup and liraglutide, result in comparatively similar weight losses. Liraglutide presents good tolerability and may also have beneficial effects on cardiovascular risk factors, which could make it the alternative of choice in subjects with prediabetes, high blood pressure or hypercholesterolaemia, whereas the tolerability of Nal/Bup is lower, and it must be used with caution in subjects with high blood pressure. The main drawbacks of liraglutide are that it must be administered subcutaneously and it is more expensive.
The final question we need to ask is how these drugs should be used. The monitoring of weight and adverse effects on a monthly basis during the first three months, and subsequently every three months, is recommended. The use thereof should be interrupted if a weight loss of more than 5% is not attained during the first three months, or in the event of intolerance.53
The future of the pharmacological treatment of obesityThe epidemic of obesity makes research into the new targets for its treatment highly appealing for pharmaceutical companies. One attractive strategy could be that of combining liraglutide with one of the three drugs which act on a central level, owing to their different mechanisms of action. Another interesting target is drugs which could act by increasing energy expenditure. Mirabegron is a beta-3 agonist which was tested as a drug for the treatment of overactive bladder. This drug is capable of activating thermogenesis in brown adipose tissue, increasing energy expenditure by 40cal/day.54 Beloranib is an inhibitor of methionine aminopeptidase II, which reduces the hepatic synthesis of fatty acids and stimulates the breakdown thereof. It is an injected medicine, currently under investigation in subjects with Prader–Willi syndrome, in whom it results in weight losses of 1kg per week.55 Lastly, another potential therapeutic target is drugs which act on other gastrointestinal hormones, such as analogues of GIP, PYY and ghrelin inhibitors.
ConclusionsIn Spain, there are currently three drugs indicated for the treatment of obesity. In addition to orlistat, there is the combination of naltrexone with bupropion and liraglutide. The recent approval of these new pharmacological agents for the treatment of obesity has increased the options for managing this disease. These are indicated as second-line treatments in those overweight or obese subjects for whom conventional treatment has failed. The choice of treatment must be based on factors such as associated comorbidities, tolerability profile and cost.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
AuthorshipAll of the authors have collaborated in the writing of this review and have approved the final version to be published.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Benaiges D, Pedro-Botet J, Flores-Le Roux JA, Climent E, Goday A. Pasado, presente y futuro de la farmacoterapia para la obesidad. Clin Invest Arterioscler. 2017;29:256–264.