Vascular cells and their interaction with inflammatory cells and the immune system play a key role in pathological vascular remodeling. A large number of genes and proteins regulated in a coordinated manner by a small number of transcription factors are involved in this process. In recent years, research on a small subfamily of transcription factors, the NR4A subfamily, has had a major impact on our understanding of vascular biology. The NR4A1 (Nur77), NR4A2 (Nurr1) and NR4A3 (NOR-1) receptors are products of early response genes whose expression is induced by multiple pathophysiological and physical stimuli. Their wide distribution in different tissues and cells places them in the control of numerous processes such as cell differentiation, proliferation, survival and apoptosis, as well as inflammation and the metabolism of lipids and carbohydrates. This review analyzes the role of these receptors, particularly NOR-1, in pathological vascular remodeling associated with atherosclerosis, abdominal aortic aneurysm and pulmonary arterial hypertension.
En el remodelado vascular patológico juegan un papel clave las células vasculares y su interacción con las células inflamatorias y del sistema inmune. En este proceso intervienen una gran cantidad de genes y proteínas regulados de forma coordinada por un reducido número de factores de transcripción. En los últimos años las investigaciones sobre una pequeña subfamilia de factores de transcripción, la subfamilia NR4A, han tenido un gran impacto sobre nuestra comprensión de la biología vascular. Los receptores NR4A1 (Nur77), NR4A2 (Nurr1) y NR4A3 (NOR-1) son productos de genes de respuesta temprana cuya expresión es inducida por múltiples estímulos fisiopatológicos y físicos. Su amplia distribución en los diferentes tejidos y células los sitúan en el control de numerosos procesos como la diferenciación, la proliferación, la supervivencia y la apoptosis celular, así como la inflamación y el metabolismo de lípidos y carbohidratos. Esta revisión analiza el papel de estos receptores, particularmente de NOR-1, en el remodelado vascular patológico asociado a la aterosclerosis, el aneurisma de aorta abdominal y la hipertensión arterial pulmonar.
Cardiovascular disease (CVD) causes 18.6 million deaths per year (33.6% of global mortality), a figure that is increasing year on year and makes it the leading cause of death worldwide.1 If this trend is not halted or reversed, more than one billion people will die of CVD in the first half of the 21st century. Among these diseases are those with an ischaemic component, such as ischaemic heart disease, peripheral arterial disease and cerebrovascular disease, the common cause of which is atherosclerosis.
Atherosclerosis is a chronic inflammatory process that progresses asymptomatically, favoured by risk factors such as hyperlipidaemia, hypertension and diabetes, and is characterised by the progressive accumulation in the intima of lipids, inflammatory cells, vascular smooth muscle cells (VSMCs), extracellular matrix (ECM) proteins and calcium deposits that form atherosclerotic lesions or plaques.2,3 Low-density lipoproteins (LDL)4 play a key role in the genesis, progression and complication of atherosclerosis.4 In early stages, endothelial dysfunction, caused by risk factors such as dyslipidaemia or hypertension, increases vascular permeability which facilitates LDL to accumulate in the intima, retained by ECM. The classically accepted hypothesis considers that oxidation of LDL in the subendothelium increases its atherogenicity and enhances the infiltration of inflammatory cells such as monocytes, favoured by increased expression of adhesion molecules and production of cytokines with chemotactic activity. In the vascular wall, monocytes differentiate into macrophages that capture oxidised LDL (LDLox) and transform into foam cells, which secrete cytokines and chemokines, and generate reactive oxygen species (ROS) that contribute to LDL oxidation. However, in recent years a role has also been attributed to native LDL as a trigger for the adaptive immune response. Thus, the pro-atherogenic mechanisms promoted by LDL would go beyond the oxidative hypothesis.5 As discussed below, in addition to monocytes/macrophages, other inflammatory cells such as T-lymphocytes, mast cells or neutrophils are involved in atherosclerosis. Moreover, cytokines and growth factors released in the inflamed arterial wall activate VSMCs, which migrate, proliferate and synthesise ECM, forming a fibrous cap that contributes to the growth and stabilisation of lesions.2,3 Indeed, the fibrous cap covers the lipid core composed mainly of foam cells and extracellular lipids, which when it contains an excess of dead cell debris forms a necrotic core. Accumulation of LDLox and oxidative stress can cause the death of VSMCs by apoptosis, which weakens the fibrous cap and makes the plaques vulnerable to rupture, increasing the risk of clinical complications.2,3
Another pathology that shares pathophysiological mechanisms with atherosclerosis, such as inflammation, oxidative stress, neovascularisation, apoptosis, and vascular calcification, is abdominal aortic aneurysm (AAA).6,7 As in atherosclerosis, AAA also involves the active participation of various inflammatory and immune system cells.8 AAA is a chronic degenerative disease that progresses asymptomatically and is characterised by a localised and permanent dilatation of the abdominal aorta. A dilatation of more than 50% of the normal diameter of the abdominal aorta is considered an aneurysm. The loss of ECM components, due to increased proteolytic activity, and of VMLC, due to apoptosis, weakens the vascular wall, which may rupture causing death.6,7 AAA affects about 6 times more men than women (incidence in men over 65 years of age is 5%–8%), and currently the only therapeutic option is surgery to repair high-risk aneurysms.6,7
Intense vascular remodelling also occurs in other pathologies for which the therapeutic arsenal is limited and treatment is only symptomatic, such as pulmonary arterial hypertension (PAH), in which an excess proliferation of VSMCs (hyperplasia) increases the resistance of the pulmonary vasculature and raises pulmonary artery pressure, leading to right ventricular failure and even death.9 Hyperplasia is also the main characteristic of "accelerated atherosclerosis" or restenosis, a complication responsible for the failure of a significant percentage of revascularisation interventions based on angioplasty and stenting.
These pathologies involve intense vascular remodelling involving vascular cells and a variety of inflammatory cell types and subtypes, and activate multiple signalling pathways that ultimately alter the expression of a large number of genes. A small number of genes encoding transcription factors responsible for coordinating gene expression and determining cellular responses play a critical role in these processes. Our group identified the nuclear receptor NOR-1 (Neuron-derived Orphan Receptor-1) as a novel transcription factor involved in atherosclerosis and restenosis10, and more recently in AAA.11–13 In this review we will discuss the role of NR4A (Nuclear Receptor subfamily 4 group A) receptors, mainly NOR-1, in vascular remodelling associated with diseases such as atherosclerosis, PAH and AAA.
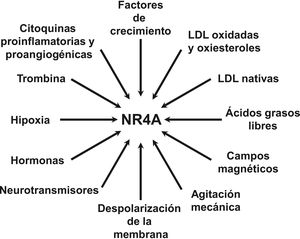
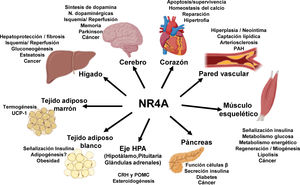
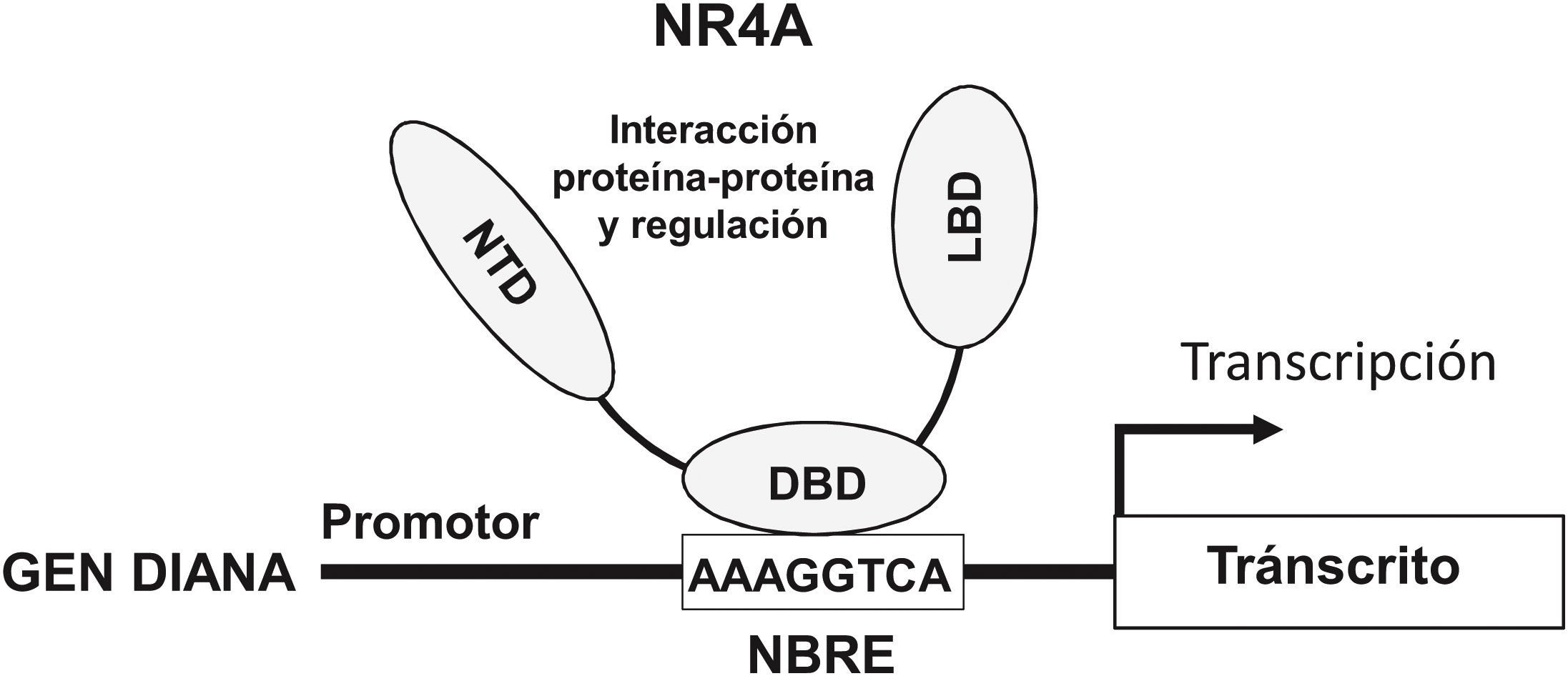
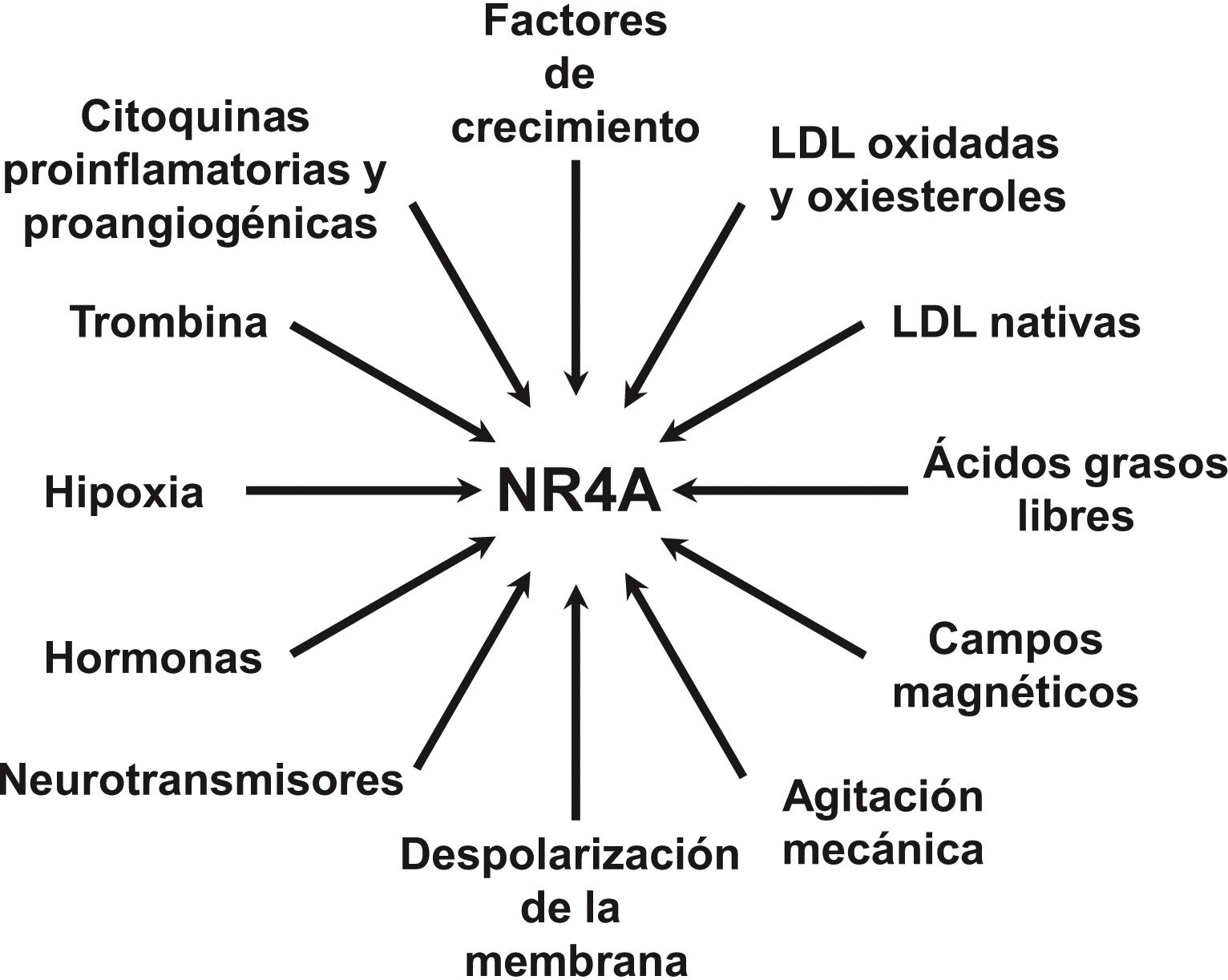
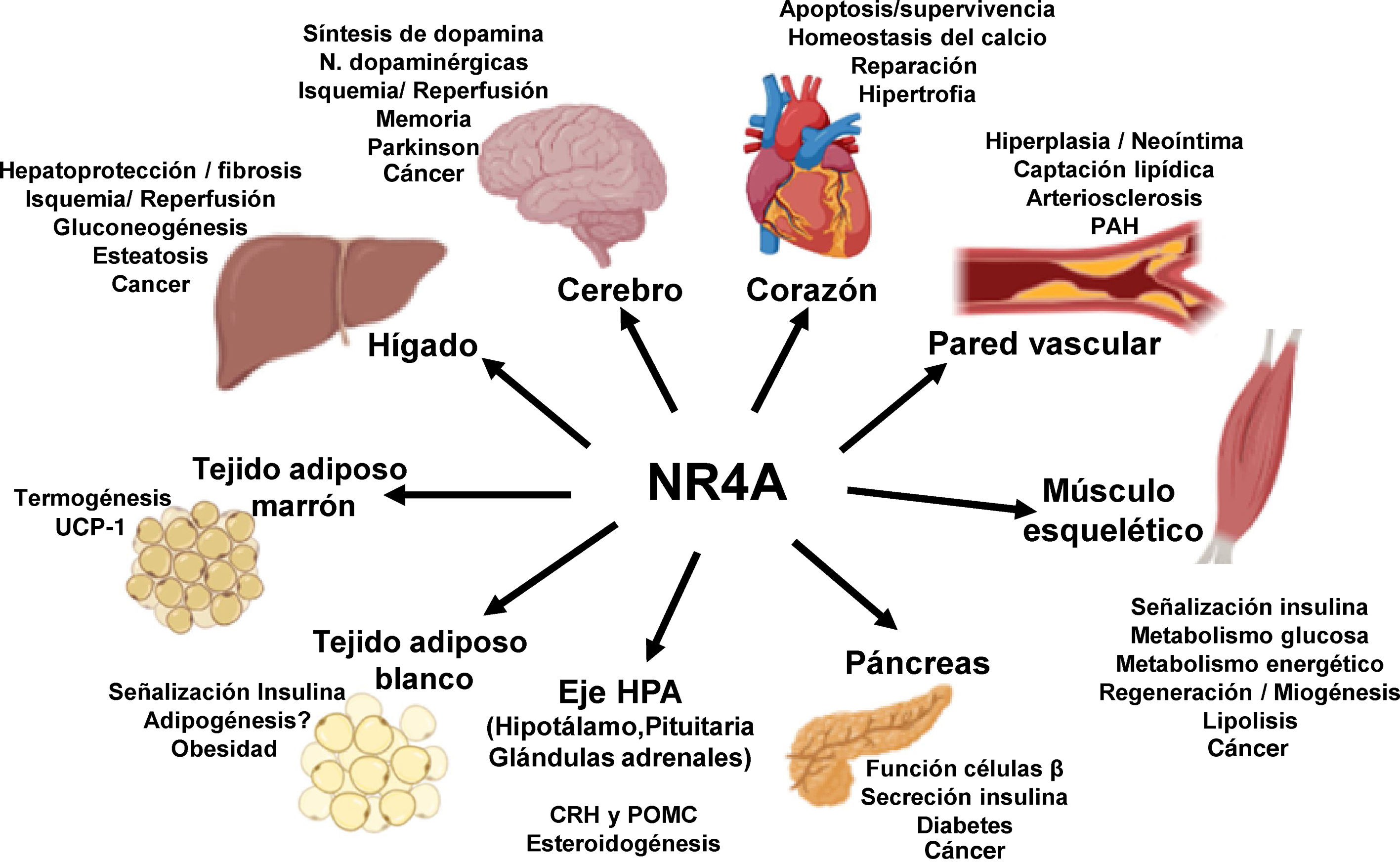
NOR-1: structure and functionNOR-1 (systematic name NR4A3 [NR4A member 3], also called MINOR) is one of the three members of the NR4A subfamily of nuclear receptors, to which Nur77 (NR4A1) and Nurr1 (NR4A2) also belong. All three members have the typical nuclear receptor structure with high homology (94% of identical amino acids) in the DNA-binding domain (DBD), moderate homology in the ligand-binding domain (LBD) and low homology in the N-terminal region. Terminal.14 Despite the structural similarities, the three receptors are not functionally equivalent. Although they can regulate cellular functions redundantly, they sometimes involve all three receptors, and in some cases exert antagonistic effects. NR4A receptors are transcription factors that bind to specific response elements present in the promoters of their target genes, thereby regulating their expression. They can bind to DNA as monomers or dimers. As monomers they bind to an octameric NBRE (Nerve growth Factor [NGFI-B] Response Element) whose consensus sequence is AAAGGTCA (Fig.1). In the form of homodimers, they bind to the NuRE (Nur-Response Element) element formed by two inverted sequences similar to NBRE.14 NOR-1 has a lower affinity for NuRE, and therefore a lower capacity to activate transcription.15 In addition, Nurr1 and Nur77 (but not NOR-1) can form heterodimers with RXR. They can also regulate gene expression indirectly by antagonising other transcription factors through different mechanisms.16,17 Although they share this typical nuclear receptor structure, they have some particularities. The main one concerns the LBD, which is essential for the recruitment of small lipophilic molecules (ligands) that act as activators. Crystallographic studies have shown that the LBD of NR4A is atypical, as it contains hydrophobic amino acids whose large radicals occupy the space that should be free for the ligand “pocket”, making binding impossible.18,19. Despite this, later studies suggested that cytosporone B (an antifungal isolated from Cytospora species) binds with high affinity to the LBD region of NR4A1, inducing its transcriptional activity.20 Cytosporone B would be the first known ligand of this family, which has increased interest in these receptors as potential drug targets.21 Subsequent studies have suggested that some fatty acids may also bind to the NR4A NR4A1.22 However, it seems unlikely that NR4A receptors are regulated by physiological ligands, so they are considered orphan receptors that behave as constitutively active transcription factors. Moreover, they are early response genes whose expression increases rapidly and highly accentuated in response to different stimuli,14 and are activated through various post-translational modifications.23–25 NR4A receptors are expressed in metabolically active tissues such as brain, heart, skeletal muscle, adipose tissue, kidney and liver.10,26 Furthermore, in tissues or cells where their basal expression is low, they are induced in response to pathophysiological stimuli. Stimuli capable of inducing their expression include hormones,27 growth factors,10,28 proangiogenic and proinflammatory cytokines,16,29,30 native LDL and LDLox,16,31,32 thrombin,33,34 oxygen deprivation (hypoxia) (hipoxia),11,35,36 physical factors such as mechanical agitation and magnetic fields,37,38 and plasma membrane depolarisation39 (Fig. 2). Nuclear receptors regulate virtually every aspect of metazoan biology. The NR4A subfamily perfectly exemplifies the plurality of functions of these transcription factors. Indeed, the response to this wide variety of stimuli and their broad distribution in different tissues and cells place this subfamily in control of multiple processes such as differentiation, proliferation, cell survival and apoptosis, inflammation, embryonic development, and lipid and glucose metabolism.40–42 This complexity explains why these receptors are implicated in high-incidence human diseases such as CVD,14,21 diabetes,41 obesity43,44 or cancer.45,46 (Fig. 3)
Structure and function of NR4A receptors. NR4A receptors activate transcription through binding by their DNA Binding Domain (DBD) to specific sequences in the promoter of their target genes. One of the most common is the octameric NBRE (Nerve Growth Factor-Induced clone B [NGFI-B] Response Element) whose consensus sequence is AAAGGTCA. The NTD (N-terminal Domain, amino-terminal domain) is important for the regulation of the activity of these transcription factors, e.g., by post-translational modifications such as phosphorylation, and for the interaction with cofactors and other transcription factors. The LBD (Ligand Binding Domain) is also a multifunctional domain necessary for dimerisation and interaction with other proteins.
The generation of genetically modified animal models has helped to clarify the pathophysiological role of these nuclear receptors. With regard to NOR-1, however, the information provided by its deletion has been somewhat controversial. Two independent groups generated mice deficient for NOR-1 almost simultaneously. While one determined that deletion of NOR-1 affected embryonic development and was lethal, letal,47 the other produced viable animals that phenotypically had only inner ear defects and vestibular abnormalities. Vestibulares.48 As discussed below, animals deficient in NOR-1 have been used in studies demonstrating its role in vascular remodelling.
NOR-1 in atherosclerosis and restenosisOur group demonstrated for the first time that NOR-1 is overexpressed in atherosclerotic plaques in patients with ischaemic heart disease and in vascular lesions induced in the porcine model by the administration of an atherogenic diet or by mechanical damage caused by percutaneous transluminal coronary angioplasty.10,49 In addition, all three NR4A receptors are strongly induced in response to proatherogenic stimuli in the different cell types involved in atherosclerosis.14,30–36 However, the results of early experimental studies on the role of NOR-1 in vascular remodelling generated some controversy, due to the increased thickening of the intima observed in a murine model of carotid artery ligation in which the transcriptional activity of all three receptors was suppressed.50 Subsequent studies, however, clarified that these receptors are not redundant, and that they exert opposite effects on endothelial cell proliferation and VSMCs. While NOR-1 regulates genes required for cell cycle progression and its overexpression increases vascular cell proliferation,10,28,31,40 as discussed in detail below, Nur77 is a negative regulator of cell proliferation.51–53 The experimental evidence that has allowed us to understand the role of NOR-1 in the different cell types involved in vascular remodelling is detailed below.
NOR-1 in vascular endothelial proliferation, survival and inflammationNOR-1 is expressed in the vascular endothelium and its expression is induced by stimuli that promote endothelial cell migration, proliferation and survival as well as angiogenesis (Fig. 4). Indeed, in endothelial cells, NOR-1 expression increases transiently and markedly when exposed to increasing concentrations of vascular endothelial growth factor (VEGF)30 or thrombina.33,34 Stimuli that through their receptors, VEGFR-2 (VEGF receptor-2) and PAR-1 (Protease-Activated Receptor 1), respectively, activate Ca2++-dependent signalling pathways, PKC (Protein Kinase C) and mitogen-activated protein kinases (ERK1/2 [Extracellular Signal-Regulated Kinase 1/2] and p38 MAPK [Mitogen-Activated Protein Kinase]).30,33,34 Thus, inhibiting NOR-1 expression prevents the migratory and proliferative response to these stimuli.30,33,34 NOR-1 is also induced in endothelial cells exposed to hypoxic stress, by direct regulation of HIF-1 (Hypoxia-Inducible Factor 1.35,36 In this case, its inhibition increases the proportion of cells that die by apoptosis, while its overexpression limits apoptosis and promotes cell survival.35,36 In this prosurvival effect of NOR-1, the anti-apoptotic protein cIAP2 (cellular Inhibitor of Apoptosis 2)11,35 which NOR-1 regulates at the transcriptional level, seems to play a key role. In relation to angiogenesis, it has been suggested that NOR-1 regulates the expression of endothelin 1 (ET-1), a vasoconstrictor peptide with proangiogenic activity,54 while its inhibition by the CRISPR/Cas9 technique reduces cell migration and the formation of HBMEC (Human Brain Micro Endothelial Cells) angiotubes.55
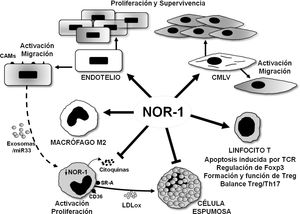
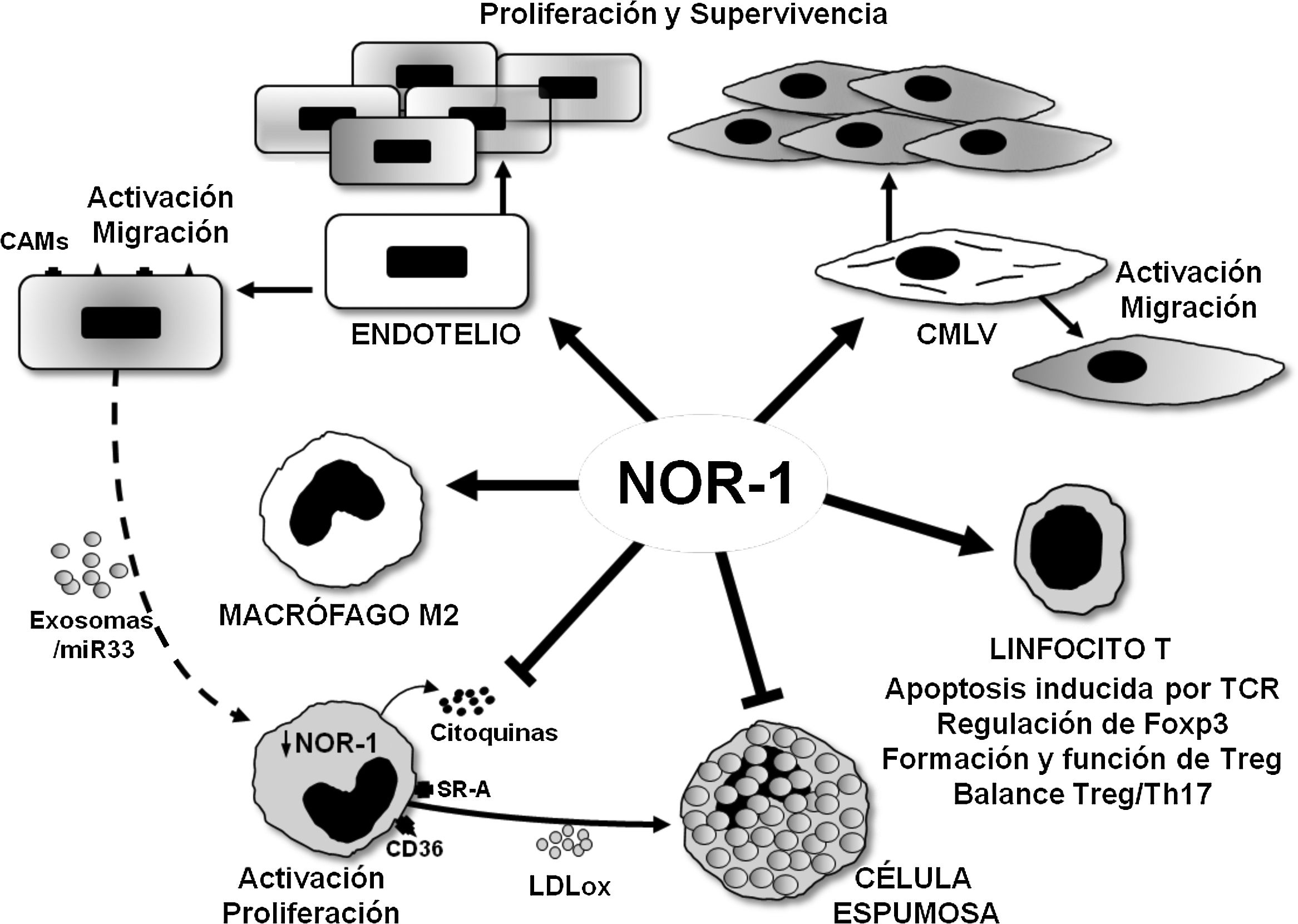
Schematic representation of the effects of the nuclear receptor NOR-1 on vascular cells and inflammatory cells/immune system. In vascular cells, NOR-1 induction leads to activation and increased expression of cell adhesion molecules (CAMs) and regulates cell migration, proliferation and survival. In macrophages, NOR-1 favours polarisation to the anti-inflammatory M2 phenotype, and limits their activation and proliferation, as well as the secretion of cytokines and the expression of receptors involved in the uptake of modified lipoproteins, thus preventing their transformation into foam cells. Possible indirect regulation of NOR-1 expression in macrophages via miR33-loaded exosomes is illustrated. In T lymphocytes, it modulates TCR (T Cell Receptor)-induced apoptosis and regulates the expression of Foxp3 transcription factor considered the “master gene” that controls the formation and function of regulatory T lymphocytes (Treg).
Endothelial expression of NOR-1 also increases in response to inflammatory stimuli and is related to the induction of VCAM-1 (Vascular Cell Adhesion Molecule 1),56 in the regulation of which miR-17 and miR-20a, two microRNAs that modulate NOR-157 levels, are indirectly involved. Furthermore, in studies of experimental atherosclerosis, NOR-1 deficiency, and the consequent reduction of endothelial VCAM-1 expression, decreases the macrophage content of atherosclerotic lesions and atherosclerosis in ApoE-/- mice fed an atherogenic diet.56 NOR-1 expression is increased in atherosclerotic lesions of diabetic patients, and appears to mediate the proinflammatory and proatherogenic effects of glycated apolipoprotein A-IV (g-ApoA-IV).58. Indeed, g-ApoA-IV induces the expression of this nuclear receptor, and silencing NOR-1 prevents the inflammatory responses produced by g-ApoA-IV. Furthermore, in ApoE-/-/ NOR-1-/- animals, atherosclerosis induced by g-ApoA-IV administration is significantly reduced.55 Finally, NOR-1 may exert a dual effect on endothelial inflammation as it may also have anti-inflammatory effects through the regulation of thrombomodulin,59 a protein on the luminal surface of the endothelium that has potent anticoagulant, antifibrinolytic and anti-inflammatory activity.
Recently, it has been observed that an important post-translational regulation of NOR-1 occurs at the endothelial level in a ROS-dependent manner. Thus, re-oxygenation of endothelial cells previously deprived of oxygen and glucose leads to a decrease in protein levels of NOR—1.60 The effect is prevented by treatment with the antioxidant TRIOL, such that in cynomolgus monkeys (Macaca fascicularis) treated with this compound there is a recovery of NOR-1 levels that is associated with an improvement in hypobaric hypoxia-induced hyperpermeability of the endothelial barrier. This regulation of NOR-1 protein levels depends on the interaction with SMARCB1 (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1) through its DBD.60
NOR-1 in the proliferation of the VSMCs and hyperplasia of the intimaNOR-1 expression in non-proliferative (quiescent) VSMCs is very low, but is strongly induced in response to different agents such as growth factors (PDGF [Platelet-Derived Growth Factor], EGF [Epidermal growth factor]), thrombin, native LDL and LDLox and cytokines, and participates in the molecular mechanisms that regulate the migratory and proliferative activity of these cells and their inflammatory response10,16,28,31,49 (Fig. 4). As in endothelial cells, multiple signalling pathways dependent on Ca2+ mobilisation, PKC and mitogen-activated protein kinases (ERK1/2 and p38 MAPK), among others, are involved in the induction of NOR-1 by these agonists, and inhibition of their expression reduces VSMC migration and proliferation.10,28,31 The strong dependence of VSMCs on NOR-1 for proliferation is also evident in studies that have analysed the impact of cell shape on proliferation. Using microarray arrays that force VSMCs to acquire more elongated versus more epitheloid/rhomboid forms, a differential expression pattern of NOR-1 related to cell proliferation has been observed.61
Key to the study of NOR-1 function in the vascular wall has been the use of genetically modified animal models. Our group developed a transgenic mouse model that overexpresses the human NOR-1 receptor in VSMC.62 In these animals, when vascular injury was induced by carotid artery ligation, we observed exacerbated remodelling leading to increased thickening of the neointima.62,63 The VSMCs of the transgenic animals are more proliferative, and exhibit less differentiated VSMC characteristics such as increased expression of the SMemb (embryonic Smooth Muscle myosin heavy chain) marker.62,63 Subsequently, other authors associated the pro-proliferative effect of NOR-1 to its ability to regulate at the transcriptional level the expression of cyclin D1, whose activity is necessary for the transition from the G1 to the S phase of the cell cycle in which DNA replication takes place.64
NOR-1 also regulates the expression of the gene coding for SKP2 (S phase kinase-associated protein 2),65 which is a limiting component for the activity of the SCF (Skp1/Cul1/Fbox) complex that degrades p27, a necessary condition for the transition to S phase of the cell cycle celular.66 In human aortic VSMC, NOR-1 has been shown to be phosphorylated by DNA-PK (DNA-dependent Protein Kinase), which prevents its degradation by ubiquitination. ubiquitinación25 In fact, NOR-1 and DNA-PK are predominantly detected in the intima of human atherosclerotic plaques, and inhibition of DNA-PK attenuates neointima formation in the mouse femoral artery lesion induction model.25 On the other hand, drugs such as simvastatin, which reduces VSMC proliferation and neointima formation, decreases LDL-induced NOR-1 expression in VSMC and aorta of pigs fed an atherogenic diet. aterogénica49 Similarly, exendin 4, a GLP1R (Glucagon-Like Peptide-1 Receptor) agonist that reduces the proportion of proliferative VSMCs and neointima formation in the femoral artery injury model in diabetic and non-diabetic animals, inhibits ERK1/2 signalling and NOR-1 expression.67
In addition to the regulation of proteins directly or indirectly involved in cell proliferation, NOR-1 regulates the expression of other structural genes that code for proteins with a relevant role in vascular remodelling. Of note is the regulation of the expression and activity of metalloproteinases (MMPs) through several mechanisms, including the induction of alpha-2-macroglobulin (A2M), a pan-inhibitor of MMPs.68 Also noteworthy is the transcriptional regulation of the expression of vitronectin (VTN), a multifunctional glycoprotein present in plasma, platelets and ECM that plays an important role in cell adhesion, migration and proliferation as well as in thrombosis and fibrinolysis,69 which co-localises with NOR-1 in human atherosclerotic lesions.70 Finally, overexpression of NOR-1 in human VSMCs increases ROS production concomitantly with increased levels of the NADPH oxidase NOX1.71 Regulation of NOX1 by NOR-1 was confirmed by silencing NOR-1 by siRNA and by colocalisation of both proteins in human atherosclerotic lesions. Positive regulation of superoxide dismutase 1 (SOD1) and SOD3 was also observed, and conversely negative regulation of NOX4 and SOD2, the latter as a consequence of NOR-1 antagonism with NFκB.71 Thus, NOR-1 appears to be involved in multiple ways in the intricate network of genes that regulate redox homeostasis and oxidative stress in the vasculature. In addition, a long noncoding RNA (lncRNA) has recently been identified that is induced in AngII-stimulated VSMCs via NOR-1.72 Like microRNAs (18−22 nt), lncRNAs are longer non-coding RNAs (>200 nt) that regulate genes associated with diverse biological processes and have emerged in recent years as important in CVD.73 Coordinated regulation of NOR-1 and Lnc-Ang164 by AngII and other growth factors such as PDGF is observed, and silencing of Lnc-Ang164 inhibits both AngII-induced oxidative stress and proliferation induced by AngII.73
NOR-1 in vascular inflammation and immune responseIn VSMCS, NOR-1 is induced by various inflammatory stimuli such as LPS, LDLox and cytokines such as IL-1 and TNF and acts as a negative modulator of these stimuli.16 Indeed, lentiviral overexpression of NOR-1 reduces the basal expression of cytokines and chemokines (IL-1, IL-6, IL-8, MCP-1 and CCL20) whose expression is increased by silencing NOR-1.16 Furthermore, NOR-1 reduces the expression of these mediators induced by stimuli such as LPS, TNF or LDLox in cultured VSMC, while in animals transgenic for NOR-1, an attenuation of the acute inflammatory response of the aorta in response to LPS16 administration is observed. This effect appears to be a consequence of the attenuation of mitogen-activated kinase-dependent signalling pathways (ERK1/2, p38 MAPK and Jun N-terminal kinase), which are less activated in response to LPS in the vascular wall of NOR-1 transgenic animals. This results in reduced phosphorylation and degradation of IB, reduced phosphorylation and translocation of p65 to the nucleus and consequently reduced activation of the NFB pathway and NFκB-dependent inflammatory genes.16 This anti-inflammatory effect of NR4A receptors by antagonism to the NFB pathway has been observed in other cell types, and occurs through different mechanisms such as low affinity binding to NFκB response elements,74 increased IB expression (NFκB inhibitor)75 or physical interaction with p65.76
In addition to this role in VSMCs, a number of inflammatory and immune system cells are involved in atherosclerosis in which NOR-1 appears to play an important role, although, as discussed in the following sections, the extent to which it is relevant in lesion formation and progression is largely unknown.
NOR-1 in monocyte/macrophage functionAs mentioned above, a prominent role in atherosclerosis is played by monocytes/macrophages that capture LDLox and contribute to the formation of the lipid core of the lesions.3. Early studies on NR4A receptors in THP-1 and RAW264.7 cell lines reported their induction by inflammatory stimuli and oxidised lipids (LDLox, 25-hydroxycholesterol and 7β-hydroxycholesterol) through an NFB29-dependent mechanism. These results seemed to suggest that NR4A receptors played a proinflammatory role, because, in addition, their overexpression in RAW264.7 and J774 macrophages increased the expression of genes involved in cell cycle, apoptosis and inflammation.77 However, subsequent studies suggest that these receptors exert primarily anti-inflammatory functions.
All three receptors (NOR-1, Nur77 and Nurr1) are expressed in macrophages in human atherosclerotic lesionss78 and in gain- and loss-of-function experiments in cultured cells reduce macrophage activation and proinflammatory activity. Thus, they decrease the production of cytokines and chemokines (IL1, IL8, MIP-1 [Macrophage Inflammatory Protein-1], MIP-1 and MCP-1), as well as the expression of SR-A (Scavenger receptor type A) and CD36, which limits LDLox uptake and foam cell formation78 (Fig. 4). These results, however, contrast with those discussed above in endothelial cells, where NFB-dependent NOR-1 induction increases the expression of adhesion molecules, and with the reduced macrophage infiltration and atherosclerotic plaque formation in NOR-156 -deficient ApoE-/- mice.56
The anti-inflammatory role of NOR-1 in monocytes/macrophages is also supported by studies linking it to M2/anti-inflammatory macrophage polarisation (Fig. 4). Indeed, in vitro polarisation of primary cultures of human monocytes to M2 macrophages by IL4 treatment increases NOR-1 expression.79 Moreover, in human atherosclerotic lesions, NOR-1 expression is higher in areas enriched in CD68+ MR+ macrophages, and its silencing in human macrophages reduces the expression of several M2 markers, such as IL-1 receptor antagonist (IL-1Ra, Interleukin-1 Receptor antagonist) and IL-10, which possess potential NOR-1 response elements in their promoter regions. Recently, the proatherogenic effect of endothelin-1 (ET-1),80 a potent vasoconstrictor peptide, expressed primarily by endothelial cells but also by VSMCs and macrophages, which plays an important role in vascular homeostasis,81 has been related to the negative regulation that endothelial cells would exert on the expression of NR4A receptors in macrophages.82 Conditioned culture media of ET-1-overexpressing endothelial cells treated with LDLox would release miR-33-loaded exosomes that upon uptake by macrophages would attenuate the expression of NR4A receptors and M2 phenotype markers (Arg-1, PPARg, Mrc1 and IL-10), thereby favouring macrophage activation and induction of proinflammatory genes82 (Fig. 4). Through this intercellular communication mechanism the miR-33/NR4A axis would contribute to the exacerbated atherosclerosis observed in ApoE-/- animals overexpressing ET-1 specifically in the endothelium.82 Studies on the function of NR4A receptors on monocytes/macrophages in other pathologies seem to confirm their role in modulating macrophage alternative polarisation and their anti-inflammatory effect.83–85 In fact, most studies support an anti-inflammatory and anti-atherogenic role of NOR-1 in monocytes/macrophages. In this regard, specific NOR-1 deficiency in haematopoietic stem cells accelerates atherosclerosis.86 In irradiated ApoE-/- animals, whose bone marrow was reconstituted with haematopoietic stem cells from NOR-1-deficient (NOR-1-/-) mice and fed a diet enriched in saturated fat, macrophage recruitment was accelerated and atherosclerosis increased.86 In turn, NOR-1 expression was increased in marrow progenitor cells treated with IL-3 or GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor), stimuli that promote proliferation and differentiation to myeloid lineage. Furthermore, deletion of NOR-1 caused an increase in macrophage and dendritic progenitor cells (MDP) in the bone marrow, induced splenomegaly and Ly6C+ monocytosis, and at the vascular level increased the rate of macrophage replication in atherosclerotic lesions and foam cell formation.86 Thus, NOR-1 function in haematopoietic stem cells would protect against atherosclerosis by negatively modulating myelopoiesis and preventing proatherogenic macrophage activity.
As mentioned, NR4A receptors are regulators of metabolismo,40–42 which, together with their fine regulation in monocytic lineage cells, has led NOR-1 to be proposed as a marker of metabolic syndrome, since its expression is significantly decreased in peripheral blood mononuclear cells.87 Indeed, Random Forest Analysis found that both NOR-1 and PPAR expression was specifically decreased in CD14+ cells (mainly monocytes) from patients with metabolic syndrome.88
NOR-1 in T- and B-lymphocyte functionNR4A receptors play an important role in the development and activation of T cells.85 In fact, both NOR-1 and Nur77 regulate TCR (T cell receptor)-induced apoptosis in negative selection during T cell differentiation in the thymus.89,90 Beyond their role as transcription factors that regulate gene expression at the transcriptional level, this process appears to be largely dependent on extranuclear activities, as both Nur77 and NOR-1 are phosphorylated by PKC and translocate to the mitochondria where they interact with Bcl2 inducing exposure of the proapoptotic BH3.91,92 NR4A receptors are also essential for the development of regulatory T cells (Treg), a critical lineage for tolerance. Treg lineage specification and function is controlled by the transcription factor Foxp3, whose expression is regulated by NR4A receptors, particularly Nur77 and NOR-193 (Fig. 4). Animals lacking NR4A receptors on lymphocytes do not produce Treg cells and die prematurely from systemic autoimmunity. They are therefore key to determining the fate of CD4+ T cells in the thymus and to immune homeostasis. Moreover, these receptors, whose expression on Treg is higher than on any other T cell subtype, are essential in the regulation of gene expression for the maintenance and functionality of these cells.94
In recent years, the impact of immunity on atherosclerosis and the importance of the balance between Th17, Treg and their cytokines in the development of atherosclerosis and in the rupture of vulnerable plaques has been highlighted.95,96 In this context, our group has recently characterised CD69 as a novel LDLox receptor on T cells required for NR4A receptor induction and differentiation to Treg.32 Administration of atherogenic diet to animals lacking CD69 in the lymphoid compartment increases the Th17/Treg ratio in peripheral blood and exacerbates atherosclerotic plaque formation. Through binding to CD69, LDLox activates the expression of NR4A receptors with anti-inflammatory activity, in particular NOR-1, and inhibits the formation of Th17 lymphocytes. Furthermore, in peripheral blood leukocytes from participants in the PESA (Progression of Early Subclinical Atherosclerosis) study, individuals with subclinical atherosclerosis, a lower expression of CD69 and NR4A receptors was observed, which allows them to be proposed as early markers of atherosclerosis.32
Regarding B lymphocytes, through the production of antibodies and cytokines, different subtypes are involved in the regulation of atherosclerosis with both atheroprotective and proatherogenic functions.97 Recently, it has been shown that NOR-1 and Nur77 are rapidly induced by antigen receptor stimulation of B cells, and act in a partially redundant manner to restrict the B cell response to antigens when co-stimulation by T cells is limited or absent. 98 They do this in part by repressing the expression of the transcription factor BATF (Basic leucine zip Transcription Factor ATF-like), as well as the chemokines CCL3 and CCL4 and ICAM-1.95 So far, no studies have examined whether this NOR-1 activity in B cells impacts on the development of atherosclerosis. In contrast, a recent study in LDLR-/-/Nur77-/ animals fed an atherogenic diet shows that loss of Nur77 function in B lymphocytes, or specifically in marginal zone B cells, increases atherosclerosis.99
NOR-1 in mast cells, neutrophils and dendritic cellsProteins and lipid mediators released by mast cells are recognised to play an important role in the progression of vascular remodelling associated with atherosclerosis and AAA. Mastocitos.8,100 Activated mast cells contribute to ECM degradation, apoptosis, and increased recruitment of inflammatory cells to the vascular wall through the release of proteases such as chymase and tryptase, growth factors, histamine, and chemokines.101 It has been observed that mast cell activation leads to the induction of NOR-1, in some cases in a clearly differential manner compared to the other two members of the NR4A family whose expression does not change significantly.102 In fact, NOR-1 is the most frequently induced gene in the entire mast cell genome in response to certain infections.103 and is also the main NR4A receptor induced in mast cells when exposed to LPS.102 NOR-1 appears to regulate cytokine/chemokine secretion by mast cells;104 however, the role of NOR-1 in the regulation of vasodilation, vascular homeostasis, angiogenesis and innate and adaptive immune responses involving these cells has not been investigated in detail.105
Other cells involved in vascular remodelling include neutrophils8,106 and dendritic cells. dendríticas8,107 Neutrophils are the body's first line of defence and play a key role in innate immunity. Low neutrophil numbers or low survival of this cell population are often associated with haematopoietic disorders and chronic inflammatory diseases. In addition, neutrophils are essential components of the so-called neutrophil extracellular traps (NETs) that are involved in the pathogenesis of thrombosis, atherosclerosis and AAA.106,108 NOR-1 is one of the genes that is activated to a greater extent in these cells in response to protein kinase A (PKA, Protein Kinase A), its expression is increased at sites of inflammation where neutrophils accumulate, and it has been observed that silencing this receptor reduces apoptosis and lengthens the half-life of these cells.109 Dendritic cells are involved in each stage of atherosclerosis and AAA through a myriad of functions in the immune response, ranging from lipid uptake, efferocytosis and antigen presentation to secretion of pro- and anti-inflammatory cytokines. Antiinflamatorias.8,107 NOR-1 is involved in the activation and induction of TLR (Toll-Like Receptors)110 mediated gene expression, and the NOR-1/FOXO1/CCR7 axis appears critical for the migration of these cells.111 More recently, NOR-1 has been shown to be essential for directing the differentiation of monocytes into dendritic cells, a transformation that is activated in response to microbial stimulation.112 However, despite a growing number of studies describing the important role of NOR-1 in mast cells and dendritic cells, no specific studies have yet been performed to examine whether this receptor modulates vascular remodelling through its function in these cells.
Regulation of NOR-1 by microRNAs and role in pulmonary arterial hypertensionAs discussed, NOR-1 regulates the expression of several components essential for cell cycle progression, which would explain the major impact that experimental interventions that enhance or inhibit its expression have on cell proliferation and vascular remodelling. Furthermore, this critical role of NOR-1 in VSMC proliferation would explain why this receptor is a target of several relevant microRNAs in vascular hyperplasia and remodelling. Indeed, in VSMCs, NOR-1 messenger RNA levels are regulated by the combined action of multiple microRNAs. A microarray performed in PDGF-stimulated human aortic VSMCs identified miR638, a microRNA highly expressed in VSMCs whose expression significantly decreases in response to PDGF, as a modulator of NOR-1 and indirectly of PDGF-induced migration and proliferation113. Indeed, down-regulation of NOR-1 by miR638 would be responsible for the inhibitory effect of this microRNA on cyclin D1 induction by PDGF113. Similar regulation by miR638 has been observed in airway SMCs in which excessive remodelling (hyperplasia) may contribute to the pathogenesis of asthma114, and by miR-107 in human pulmonary artery VSMCs, which by reducing NOR-1 would act as a negative modulator of PDGF-induced migration and proliferation.115 Regulation of pulmonary artery VSMC proliferation has put the spotlight on NOR-1 in relation to PAH, a pathology characterised by exacerbated remodelling of the pulmonary artery intima.9 NOR-1 has emerged as a key transcription factor regulating pulmonary artery VSMC proliferation in response to mitogens and hypoxia-induced remodelling in chronic obstructive pulmonary disease (COPD).116,117 Several studies identify NOR-1 as a key gene targeted by several microRNAs, such as miR-106b-5p,118 508-3p119 or miR-638,120 to regulate pulmonary artery VSMC hyperplasia, placing this receptor at the centre of a crucial axis in the design of therapeutic strategies for PAH and acute pulmonary embolism. Indeed, the preventive effect of resveratrol on pulmonary vascular remodelling, which has attracted considerable interest as a potential therapeutic approach to PAH, has been linked to its ability to regulate miR-638 and thereby act on the NOR-1/cyclin D1 tandem.120,121 The opposite effect of Nur77 on VSMC proliferation to NOR-1 explains why, conversely, activation of Nur77 prevents PAH progression.122,123 It should be emphasised that PAH is a disease for which only symptomatic treatment is available, based on the administration of vasodilators that do not prevent progression of the pathology and that this disease is characterised by progressive remodelling of the pulmonary vasculature, elevated pulmonary artery pressure, and resistance of the pulmonary vasculature leading to right ventricular failure that can cause death.8
NOR-1 in abdominal aortic aneurysmNOR-1 expression is highly increased in human aneurysm samples from patients undergoing repair surgery.1 In these samples, immunohistochemical analyses detect stabilisation of HIF1α,11 which could be involved in the induction of NOR-1 whose expression is induced by hypoxia in vascular cells.11,35,36 Indeed, several studies point to hypoxia and HIF1 as important mediators in the regulation of gene expression in the medial layer of aneurysmal aortas.124 Moreover, in VSMCs of aneurysmal lesions, NOR-1 co-localises with cIAP2, a protein with anti-apoptotic functions that is induced by NOR-1 in response to hypoxia in both endothelial cells and VSMCs.11,35,36 Considering the anti-inflammatory properties of NOR-1 in macrophages78,79,86 and in VSMCs16 and the upregulation and colocalisation of NOR-1 with cIAP2 in VSMCs of the aneurysmal aortic wall, it was hypothesised that NOR-1 may have anti-inflammatory properties in macrophages and VSMCs, it was hypothesised that NOR-1 might regulate the expression of genes with vasoprotective functions and that the increased expression of NOR-1 in the aneurysmal wall might be part of a compensatory mechanism to stop or slow down the progressive damage to the vascular wall as this chronic disease progresses. In addition, as discussed above, NOR-1 deficiency in haematopoietic stem cells accelerated atherosclerosis.86 Against this background, Qing et al.125 examined whether the function of this receptor in the haematopoietic compartment could affect the development of AAA.125 To do this, animals deficient in the LDL receptor (LDLR−/−) were irradiated, their bone marrow was reconstituted with haematopoietic stem cells from NOR-1/ mice, and AAA was induced by AngII infusion and a diet rich in saturated fat. A large number of inflammation-related genes were found to be differentially regulated in macrophages from NOR-1 deficient animals compared to control animals (36 out of 184 genes analysed). However, no differences in the diameter of the adrenal aorta were observed, leading to the conclusion that NOR-1 function in haematopoietic lineage cells does not play a role in AAA formation, at least in this animal model.125
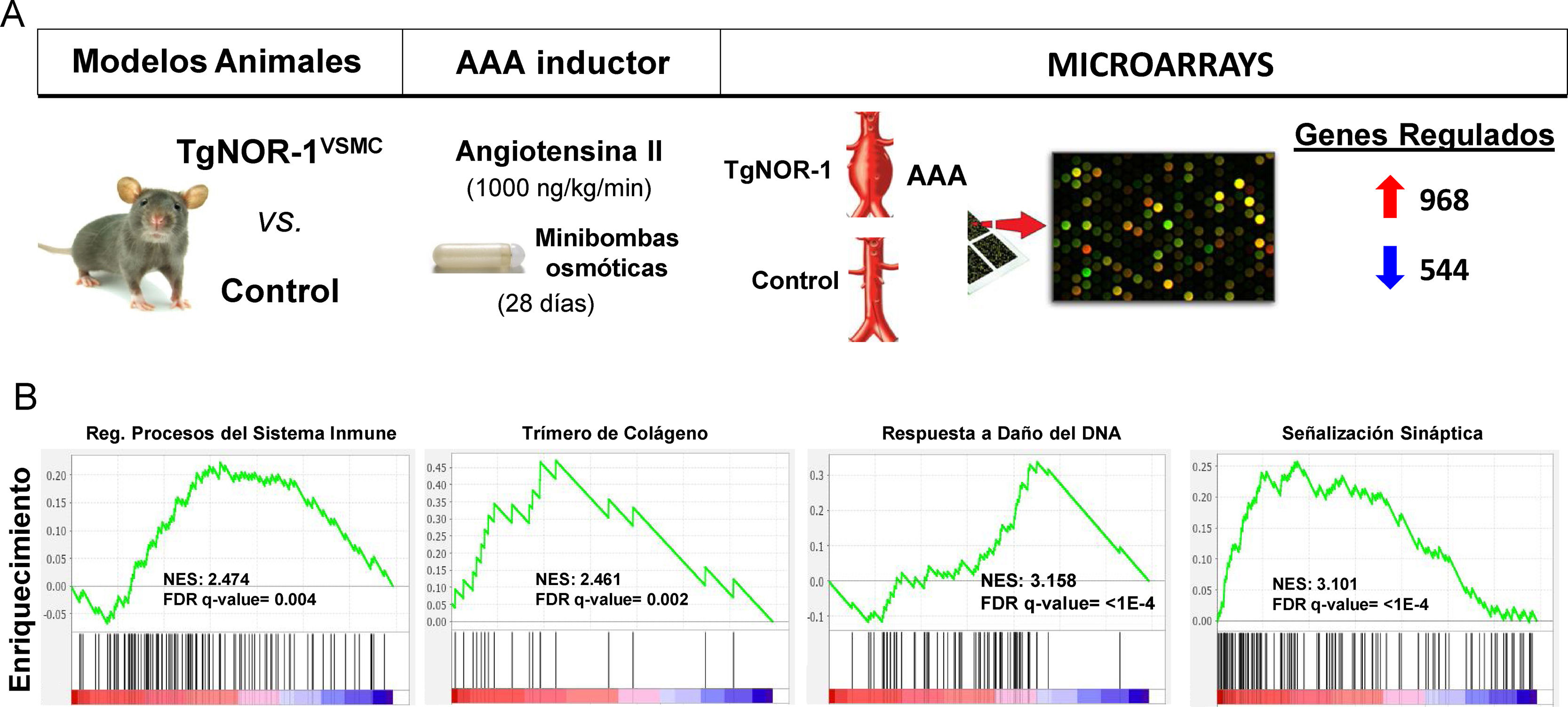
More recently, using animal models that overexpress NOR-1 in the vascular wall,62,126 a condition similar to that observed in the human aneurysmal wall where NOR-1 expression is increased, we were able to determine an increased susceptibility of these animals to AAA formation induced by AngII infusion.12 Although AngII increased blood pressure as in controls, in NOR-1 transgenic animals it caused more inflammation (increased production of cytokines and chemokines such as IL-1, IL6, MCP-1 and CXCL2), more oxidative stress (ROS production), increased expression and activity of MMP2 and MMP-12 and increased rupture of elastic laminae. Taken together, the combined action of these effects overcame the resistance of C57BL/6 animals, the genetic background of the NOR-1 transgenic animals, to develop AAA in response to AngII. Echocardiographic monitoring determined that transgenesis favours AngII-induced aortic dilatation, with a significant increase in luminal diameter from the first week, and AAA formation at 4 weeks. We conclude that increased NOR-1 expression in the mid-layer VSMCs is a necessary and sufficient condition for AAA development in response to AngII. Furthermore, we observed that doxycycline, a drug that reduces inflammation in human AAAs127 and whose clinical utility is under investigation,128 prevented AngII-induced AAA formation in NOR-1 transgenic animals.12 These results underline the relevance of NOR-1 in the pathophysiology of AAA and suggest the usefulness of NOR-1 transgenic animals for the study of the pathophysiological mechanisms of AAA and as preclinical models to evaluate potential therapies for this disease. In this regard, expression microarrays identified a large number of genes whose expression is induced or repressed (960 vs. 544, respectively) in the wall of NOR-1 transgenic animals (TgNOR-TgNOR-1VSMC) exposed to AngII.12 Using GSEA (Gene Set Enrichment Analysis) we identified groups of genes related mainly to inflammation/immune response, ECM remodelling, and VSMC differentiation, among others that could be involved in the differential response mediated by NOR-1. Of particular note is a group of genes related to sympathetic activity, which the GSEA analysis classified as synaptic signalling genes (Fig. 5). In particular, genes coding for enzymes of the catecholamine synthesis pathway, such as tyrosine hydroxylase (TH) and dopamine hydroxylase (DBH) as well as a noradrenaline transporter (SLC6A2)12 whose expression was significantly induced in NOR-1 transgenic animals infused with AngII. Increased expression of TH, DBH and SLC6A2 was also detected in AAA from the AngII-infused ApoE−/− mouse model, and importantly, these genes are highly induced in human AAA samples, where TH expression is more than 100-fold higher than in control aortas and correlates with NOR-1 expression.13 In both human aneurysmal aorta and animal models, HT expression was mainly localised in sympathetic nerves innervating the vasculature, in lymphocytes of the inflammatory infiltrate, and to a lesser extent in VMLCs dispersed in the media. Interestingly, AAA induction by AngII is associated with increased TH expression in the aorta of susceptible animals (NOR-1 transgenic and ApoE−/− deficient),13 and treatment with doxycycline normalises this expression along with the other parameters associated with vascular damage (inflammation, oxidative stress and elastic lamina rupture).13 Therefore, the catecholamine synthesis pathway, and in particular HT, the limiting enzyme and main point of regulation of this biosynthetic pathway, appears to be key in the pathophysiology of aortic aneurysms. So much so that treatment with a competitive inhibitor of HT (alpha-methyl-p-tyrosine, AMPT) was able to prevent aortic dilatation and AAA formation in both TgNOR-1VSMC and ApoE−/− animals infused with AngII.13 AMPT dramatically reduced the incidence and severity of aneurysms, and prevented vascular damage caused by angiotensin: reduced inflammatory cell infiltration (macrophages, lymphocytes and neutrophils), normalised vascular expression of inflammatory markers such as MCP-1, decreased the number of ruptures of the elastic laminae of the medial layer, and prevented increased expression and activity of ECM-degrading metalloproteinases such as MMP2 and oxidative stress.13 In conclusion, inhibiting the activity of an enzyme encoded by a gene regulated by NOR-1 prevented the development of AAA, underlining the role of NOR-1 in the pathophysiology of this disease.
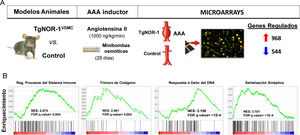
Animals overexpressing human NOR-1 in VSMCs (TgNOR-1VSMC) are more susceptible to develop angiotensin II (AngII)-induced abdominal aortic aneurysm (AAA). (A) Shown is the experimental design in which AAA formation and gene expression (by microarrays) in the aorta of TgNORVSMC and control animals were compared after infusing AngII (1000 ng/kg/min) via osmotic minipumps for 28 days. Differential expression analysis detected 968 and 544 genes whose expression was increased or decreased, respectively.11 B) Gene Set Enrichment Analysis (GSEA) pathway analysis identified clusters of differentially regulated genes related to, among other processes, inflammation/immune response, extracellular matrix remodelling, DNA damage response, and synaptic signalling, the latter including genes coding for enzymes of the catecholamine synthesis pathway such as tyrosine hydroxylase. FDR: False discovery rate; NES: Normalized enrichment score.
The nuclear receptor NOR-1 plays an important role as a regulator of vascular cells and inflammatory and immune system cells involved in vascular remodelling in atherosclerosis, PAH and AAA. Its contribution to VSMC migration and proliferation is highlighted by its anti-inflammatory activity on monocyte/macrophage lineage cells, and its role, together with the other NR4A receptors, in immune homeostasis through the regulation of Treg cell differentiation and maintenance of Treg cell function. However, it is still unknown, for example, whether its role in B-lymphocytes, mast cells, neutrophils or dendritic cells is relevant in vascular remodelling. In view of their potential therapeutic implications, further studies should be undertaken to elucidate and understand in detail the specific and distinct functions of each of the NR4A receptors in the different cell types involved in vascular remodelling, in order to determine their impact on the development, progression, or possible regression or stabilisation of pathological vascular remodelling.
FundingThis work has been carried out thanks to a 2019 research grant from the Fundación Española de Arteriosclerosis (FEA) - Sociedad Española de Arteriosclerosis (SEA), and projects funded by the Spanish Ministry of Science and Innovation (RTI2018-094727-B-100), the Instituto de Salud Carlos III (ISCIII) (PI18/0919), and the support received from the Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR) (2017-SGR-00333). C. B-S and L.P obtained a grant from the university teacher training programme (FPU) and the PFIS programme of the ISCII, respectively.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Ballester-Servera C, Cañes L, Alonso J, Puertas L, Taurón M, Rodríguez C, et al. El receptor nuclear NOR-1 (Neuron-derived Orphan Receptor-1) en el remodelado vascular patológico. Clin Investig Arterioscl. 2022;73:229–242.





![Structure and function of NR4A receptors. NR4A receptors activate transcription through binding by their DNA Binding Domain (DBD) to specific sequences in the promoter of their target genes. One of the most common is the octameric NBRE (Nerve Growth Factor-Induced clone B [NGFI-B] Response Element) whose consensus sequence is AAAGGTCA. The NTD (N-terminal Domain, amino-terminal domain) is important for the regulation of the activity of these transcription factors, e.g., by post-translational modifications such as phosphorylation, and for the interaction with cofactors and other transcription factors. The LBD (Ligand Binding Domain) is also a multifunctional domain necessary for dimerisation and interaction with other proteins. Structure and function of NR4A receptors. NR4A receptors activate transcription through binding by their DNA Binding Domain (DBD) to specific sequences in the promoter of their target genes. One of the most common is the octameric NBRE (Nerve Growth Factor-Induced clone B [NGFI-B] Response Element) whose consensus sequence is AAAGGTCA. The NTD (N-terminal Domain, amino-terminal domain) is important for the regulation of the activity of these transcription factors, e.g., by post-translational modifications such as phosphorylation, and for the interaction with cofactors and other transcription factors. The LBD (Ligand Binding Domain) is also a multifunctional domain necessary for dimerisation and interaction with other proteins.](https://static.elsevier.es/multimedia/25299123/0000003400000004/v2_202311240533/S2529912322000420/v2_202311240533/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)