Triglycerides are the initiators of the metabolic changes that lead to atherogenic dyslipidemia (AD). The APOA5 and APOA1 genes are involved in the response and metabolism of serum lipids and lipoproteins, where single nucleotide polymorphisms (SNP) rs662799 (promoter region) and rs5070 (intronic region) have been associated with the susceptibility to dyslipidemia. Until now, few studies evaluate the association of these polymorphisms with the presentation of hypertriglyceridemia and AD among Mexican children. Therefore, the objective was to determine the association between rs662799 and rs5070 with hypertriglyceridemia and AD in a pediatric population of southeastern Mexico.
Materials and methodsA case–control analysis was performed including 268 infants aged 2–16 years, anthropometric, clinical variables, and serum lipid profiles were analyzed. DNA was extracted from blood samples and genotyping of polymorphisms was executed with the TaqMan SNP genotyping assay. Allele and genotypic frequencies were calculated. For genetic association analysis, logistic regression models were fitted according to models of inheritance.
ResultsThe SNP rs662799 (C) was significantly associated with hypertriglyceridemia in the overdominant model (OR=3.89, p=0.001) and AD in the dominant model (OR=4.01, p=0.001). The SNP rs5070 (T) has a protective effect against hypertriglyceridemia in the additive risk model (OR=0.68, p=0.03).
ConclusionPolymorphism rs662799 was significantly associated with cases of hypertriglyceridemia and AD in minors in southeastern Mexico. On the other hand, rs5070 polymorphism was not associated with cases of hypertriglyceridemia or AD.
Los triglicéridos son los iniciadores de los cambios metabólicos que conducen a la dislipidemia aterogénica (DA). Los genes APOA5 y APOA1 están implicados en la respuesta y metabolismo de lípidos séricos y lipoproteínas, donde los polimorfismos de nucleótido único (SNP) rs662799 (región promotora) y rs5070 (región intrónica) se han asociado con la susceptibilidad a la dislipidemia. Hasta ahora, pocos estudios evalúan la relación de estos polimorfismos con la presentación de hipertrigliceridemia y DA entre los niños mexicanos. Por lo tanto, el objetivo fue determinar la asociación entre rs662799 y rs5070 con hipertrigliceridemia y DA en una población pediátrica del sureste de México.
Materiales y métodosSe realizó un análisis de casos y controles con 268 niños de 2 a 16 años, se analizaron variables antropométricas, clínicas y perfiles de lípidos séricos. Se extrajo ADN de muestras de sangre y se realizó genotipado de polimorfismos con el ensayo de genotipado TaqMan SNP. Se calcularon las frecuencias alélicas y genotípicas. Para el análisis de asociación genética, los modelos de regresión logística se ajustaron según los modelos de herencia.
ResultadosEl SNP rs662799 se asoció significativamente con hipertrigliceridemia en el modelo sobredosis (OR=3,89, p=0,001) y DA en el modelo dominante (OR=4,01, p=0,001). El SNP rs5070 tiene un efecto protector contra la hipertrigliceridemia en el modelo de riesgo aditivo (OR=0,68, p=0,03).
ConclusiónEl polimorfismo rs662799 se asoció significativamente con casos de hipertrigliceridemia y DA en menores del sureste de México. Por otro lado, el polimorfismo rs5070 no se asoció con casos de hipertrigliceridemia o DA en esta población.
Dyslipidemia is determined by excessive or deficient production of serum lipids and lipoproteins.1 In Mexico, about 70% of children with obesity and 50% without obesity present dyslipidemia, hypertriglyceridemia being the most frequent.2 Triglyceride (TG) levels are not only important because they are a risk factor for atherosclerosis, but also because they are the precursors of metabolic disorders that induce an atherogenic lipoprotein profile.3 Elevated plasma TG levels have been shown to modulate the size and number of some lipoproteins, developing an imbalance that causes the increase of small and dense low-density lipoprotein cholesterol (LDL-C) particles (sd LDL-C) and the decrease of high-density lipoprotein cholesterol (HDL-C) molecules,3 resulting AD. Elements of AD are a crucial risk factor for cardiovascular disease (CVD), and are part of the leading cause of morbidity and mortality in developed countries.4 These abnormalities have been extensively investigated in adults,5 due to a combination of associated risk factors such as excessive alcohol intake, uncontrolled diabetes mellitus, smoking, and obesity.6 Several studies indicate that components of AD are present from infancy,7,8 which justifies the search for these abnormalities in the childhood population and early identification of CVD in children and adolescents.9
Population-based genetic association research has described several genes that could have a direct effect on lipid concentrations,10 most notably the APOA5 and APOA1 genes. The APOA5 gene gives rise to Apolipoprotein A5 (ApoA5),11 ApoA5 has an important function in the regulation of TG levels.12 On the other hand, the APOA1 gene produces Apolipoprotein A1 (ApoA1),13 which is the main protein element of cholesterol high-density lipoproteins (C-HDL) and has an important role in reverse cholesterol transport.14 Studies have shown that SNP's in APOA5 gene promoter region rs662799,15 and in APOA1 gene intronic region rs5070,16 are significantly associated with lipid concentrations,5,17 hypertension,18 obesity or overweight,19 MS,15 and CVD.20 Generally, the genetic association of these polymorphisms has been performed in adult populations, however, the contribution of genetic susceptibility in pediatric patients is less evident.5 Therefore, the purpose of this study was to determine the association between the presence of the genetic polymorphisms rs662799 and rs5070 and the prevalence of hypertriglyceridemia and atherogenic dyslipidemia in a population of pediatric patients insured by the Institute of Social Security and Services for State Workers (ISSSTE, acronym in Spanish) Comitán de Domínguez, Chiapas and Villahermosa, Tabasco.
Materials and methodsCase–control studyThis study included minors aged 2–16 years who attended the ISSSTE in the city of Comitán de Domínguez, Chiapas, and Clinic of Family Medicine Casa Blanca Villahermosa, Tabasco, México who participated in the project “Factors associated with dyslipidemias in pediatric patients in the south-southeast region of Mexico”. The case group was defined as children who had the criteria for hypertriglyceridemia (described below), and the control group was defined as apparently healthy minors who did not meet the criteria. Minors aged 2–16 years, who meet the criteria for cases or controls and were members of families in which three or more of their generations were born in Mexico, were incorporated into the study,5 children who did not meet the criteria were discarded.
The sample size calculation was performed for analysis of unpaired cases and controls, for both polymorphisms a confidence level of 95%, a power of 80%, and a 1:1 ratio were considered, for the SNP rs662799 a prevalence of 45.2% in the cases group5 and 22.8% in the control group,21 obtaining a desirable sample of 142 minors. For the SNP rs5070, a prevalence of 29.6% in the case group17 and 79.3% in the control group,22 with a sizable sample of 32 individuals. For this study, a final sample of 268 children and adolescents was included.
Anthropometric, clinical, and biochemical profile evaluationWeight (kg), height (cm), BFP (body fat percent), and WHR (waist-hip ratio) were measured by a professional nutritionist. BFP was determined with the Siri formula (Nutrimind Software, Mexico). The WHR and BMI (Body Mass Index) (kg/m2) were calculated and classified according to World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) criteria for children and adolescents. Systolic (SBP) and Diastolic Blood Pressure (DBP) were taken three times with a sphygmomanometer (BEURER BC-32) with 10-min periods between measurements and averaged, categorizations were determined with the blood pressure percentile calculator for minors.23
Five milliliters of blood samples were extracted after 12h of fasting for analysis of TG, Total Cholesterol (TC), HDL-C, LDL-C by enzymatic colorimetric assay (Spinreact reagents, Girona, Spain) and for determining Apolipoprotein A1 (ApoA1) and Apolipoprotein B (ApoB) by immunoturbidimetry (DiaSys reagents, Diagnostic Systems GmbH, Germany) following the manufacturer's specifications, using a spectrophotometer (Thermo Fisher Multiskan plate reader).
LDL-C concentrations were determined by Friedewald's formula only when TG levels <400mg/dL (4.52mmol/L)24; the case of TG ≥400mg/dL (4.52mmol/L), they were directly quantified. Non-HDL cholesterol (non-HDL-C) levels were obtained with the formula non-HDL−C=TC−HDL−C, the sd LDL-C was calculated with the ratio (LDL-C/ApoB).25
Classification of hypertriglyceridemia and atherogenic dyslipidemiaCases with hypertriglyceridemia were determined in accordance with the guidelines established by the National Cholesterol Education Program (NCEP) for children and adolescents ages 0–19 years as TG≥75mg/dL (0.84mmol/L) (0–9 years) and ≥90mg/dL (1.01mmol/L) (10–19 years).26
The AD was defined as high TG levels; 75mg/dL (0.84mmol/L) (0–9 years) and ≥90mg/dL (1.01mmol/L) (10–19 years), low HDL-C levels; <40mg/dL (1.03mmol/L)26 and the presence of sd LDL-C; <1.3.27
DNA extraction and genotyping of SNPsGenomic DNA was isolated from peripheral blood (DNAzol® GENOMIC DNA ISOLATION REAGENT, MRC brand). DNA concentration and purity were measured on a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific Corporation). DNA integrity was observed by electrophoresis on agarose gels (0.8%), stained with SYBR Safe.
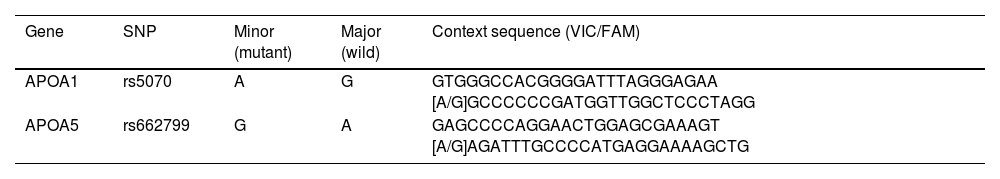
Genotyping of rs662799 and rs5070 SNPs was carried out by Real-Time Polymerase Chain reaction (RT-PCR) technique, with the TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, USA) (catalog number 4351379; rs662799 (C_2310403_10) and rs5070 (C_2679584_10)), in a thermocycler (Rotor-Gene Q, QIAGEN brand), according to manufacturer specifications. Fluorescence signals were examined in Rotor-Gene Q series software, version 2.1.0.9 for Windows. Sequence contexts of TaqMan probes for each polymorphism are presented in Table 1.
Evaluation of inheritance modelsFor the association analysis of polymorphisms with hypertriglyceridemia and AD, logistic regression models were fitted according to inheritance models; codominant, dominant, recessive, overdominant, and additive risk, to select the best fitting inheritance model Akaike's information criterion (AIC) was applied. The descriptive analysis of the SNPs and their association with the study conditions was carried out with the SNPStats program (https://www.snpstats.net/start.htm).
Statistical analysisDescriptive statistics were performed for all variables, using frequencies and proportions for qualitative variables and means and standard deviations (SD) for quantitative variables. For the analysis of the normal distribution of the data, the Kolmogorov–Smirnov test was used to compare between groups; for quantitative variables, the student's t-test was used for data with normal distribution and the U-Mann Whitney test for those without normal distribution; for qualitative variables, the Chi2 test was used. Allelic and genotypic frequencies were calculated. Hardy–Weinberg equilibrium (HWE) was evaluated. The magnitude of association was measured with OR with a confidence interval (CI) of 95%, the categorical variables of sex, age (2–4 years: preschool, 5–9 years: school, and 10–19 years: adolescent), and BMI grouped as normal (normal/underweight) and overweight (overweight/obese) were adjusted. Results were taken as significant when a p-value <0.05 was obtained. Data were analyzed with the statistical package IBM SPSS Statistics for Windows, 2013 (Armonk, NY; IBM Corp).
Ethical considerationsStudy participants and parents were provided with the letter of assent and/or informed consent. This research followed the Helsinki declaration of 1975 and the General Health Law on Research for Health (Ley General de Salud en Materia de Investigación Para La Salud) with category II – research with minimal risk, was approved by COBIOETICA (09-CEI-019-20160729), the Institutional Biosafety Committee (Comité Institucional de Bioseguridad) COFEPRIS (16 CB 09 012022) and the Ethics Committee of The College of the South Border (El Colegio de la Frontera Sur) (Ref. 2697).
ResultsAnthropometric, clinical, and biochemical parameters of the study populationTwo hundred sixty-eight minors were studied, 134 children had high triglyceride levels (47.8% women and 52.2% men) with a mean age of 8.32±3.98, and 134 children with normal triglyceride levels (46.2% women and 53.8% men) at a mean age of 8.86±4.03.
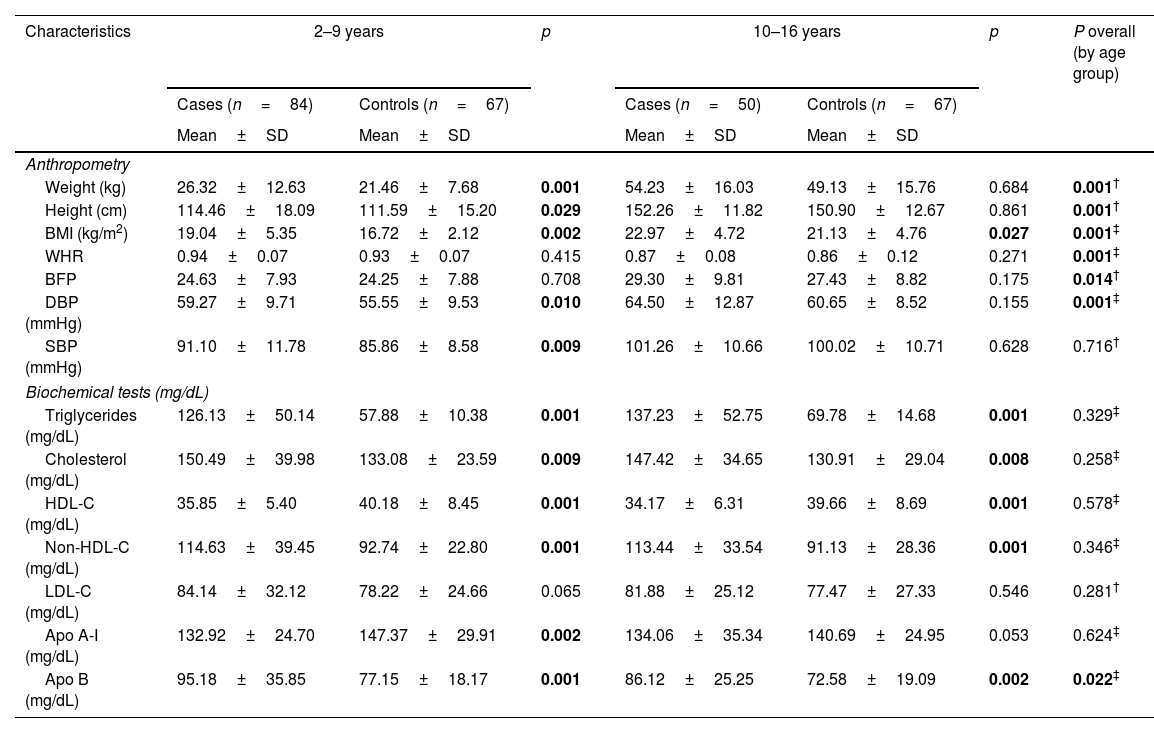
In the analysis of anthropometric variables (height, weight, BMI) by age groups, we found statistical differences between the groups, as part of the phenomenon of natural growth of minors (Table 2). For the biochemical analysis by age group, only differences were found in serum ApoB concentrations (p=0.022) (Table 2). In both age groups, the cases present higher values in cholesterol total, Non-HDL-C and low levels of HDL-c.
Anthropometric characteristics, and biochemical analysis of the minor's with hypertriglyceridemia (cases) by age groups.
| Characteristics | 2–9 years | p | 10–16 years | p | P overall (by age group) | ||
|---|---|---|---|---|---|---|---|
| Cases (n=84) | Controls (n=67) | Cases (n=50) | Controls (n=67) | ||||
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Anthropometry | |||||||
| Weight (kg) | 26.32±12.63 | 21.46±7.68 | 0.001 | 54.23±16.03 | 49.13±15.76 | 0.684 | 0.001† |
| Height (cm) | 114.46±18.09 | 111.59±15.20 | 0.029 | 152.26±11.82 | 150.90±12.67 | 0.861 | 0.001† |
| BMI (kg/m2) | 19.04±5.35 | 16.72±2.12 | 0.002 | 22.97±4.72 | 21.13±4.76 | 0.027 | 0.001‡ |
| WHR | 0.94±0.07 | 0.93±0.07 | 0.415 | 0.87±0.08 | 0.86±0.12 | 0.271 | 0.001‡ |
| BFP | 24.63±7.93 | 24.25±7.88 | 0.708 | 29.30±9.81 | 27.43±8.82 | 0.175 | 0.014† |
| DBP (mmHg) | 59.27±9.71 | 55.55±9.53 | 0.010 | 64.50±12.87 | 60.65±8.52 | 0.155 | 0.001‡ |
| SBP (mmHg) | 91.10±11.78 | 85.86±8.58 | 0.009 | 101.26±10.66 | 100.02±10.71 | 0.628 | 0.716† |
| Biochemical tests (mg/dL) | |||||||
| Triglycerides (mg/dL) | 126.13±50.14 | 57.88±10.38 | 0.001 | 137.23±52.75 | 69.78±14.68 | 0.001 | 0.329‡ |
| Cholesterol (mg/dL) | 150.49±39.98 | 133.08±23.59 | 0.009 | 147.42±34.65 | 130.91±29.04 | 0.008 | 0.258‡ |
| HDL-C (mg/dL) | 35.85±5.40 | 40.18±8.45 | 0.001 | 34.17±6.31 | 39.66±8.69 | 0.001 | 0.578‡ |
| Non-HDL-C (mg/dL) | 114.63±39.45 | 92.74±22.80 | 0.001 | 113.44±33.54 | 91.13±28.36 | 0.001 | 0.346‡ |
| LDL-C (mg/dL) | 84.14±32.12 | 78.22±24.66 | 0.065 | 81.88±25.12 | 77.47±27.33 | 0.546 | 0.281† |
| Apo A-I (mg/dL) | 132.92±24.70 | 147.37±29.91 | 0.002 | 134.06±35.34 | 140.69±24.95 | 0.053 | 0.624‡ |
| Apo B (mg/dL) | 95.18±35.85 | 77.15±18.17 | 0.001 | 86.12±25.25 | 72.58±19.09 | 0.002 | 0.022‡ |
| n (%) | n (%) | P | n (%) | n (%) | P | P overall (by age group) | |
|---|---|---|---|---|---|---|---|
| Nutritional status | |||||||
| Normal/underweight | 46 (54.80) | 52 (77.60) | 19 (38.0) | 36 (53.70) | |||
| Overweight | 17 (20.20) | 9 (13.40) | 0.009 | 15 (30.0) | 18 (26.90) | 0.179 | 0.012* |
| Obese | 21 (25.0) | 6 (9.0) | 16 (32.0) | 13 (19.40) | |||
Most characteristics are presented as mean and standard deviation (M ± SD); nutritional status is presented as frequencies and percentage n (%).
BMI: body mass index; WHR: waist-hip ratio; BFP: body fat percentage; DBP: diastolic blood pressure; SBP: systolic blood pressure; C-HDL: high-density lipoprotein cholesterol; Non-HDL-C: non-high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Apo A-I: apolipoprotein A-I; Apo B: apolipoprotein B; n: number of pediatric patients: p: significant (p < 0. 05); tests: † t Student; ‡ U-Mann Whitney; * Chi2.
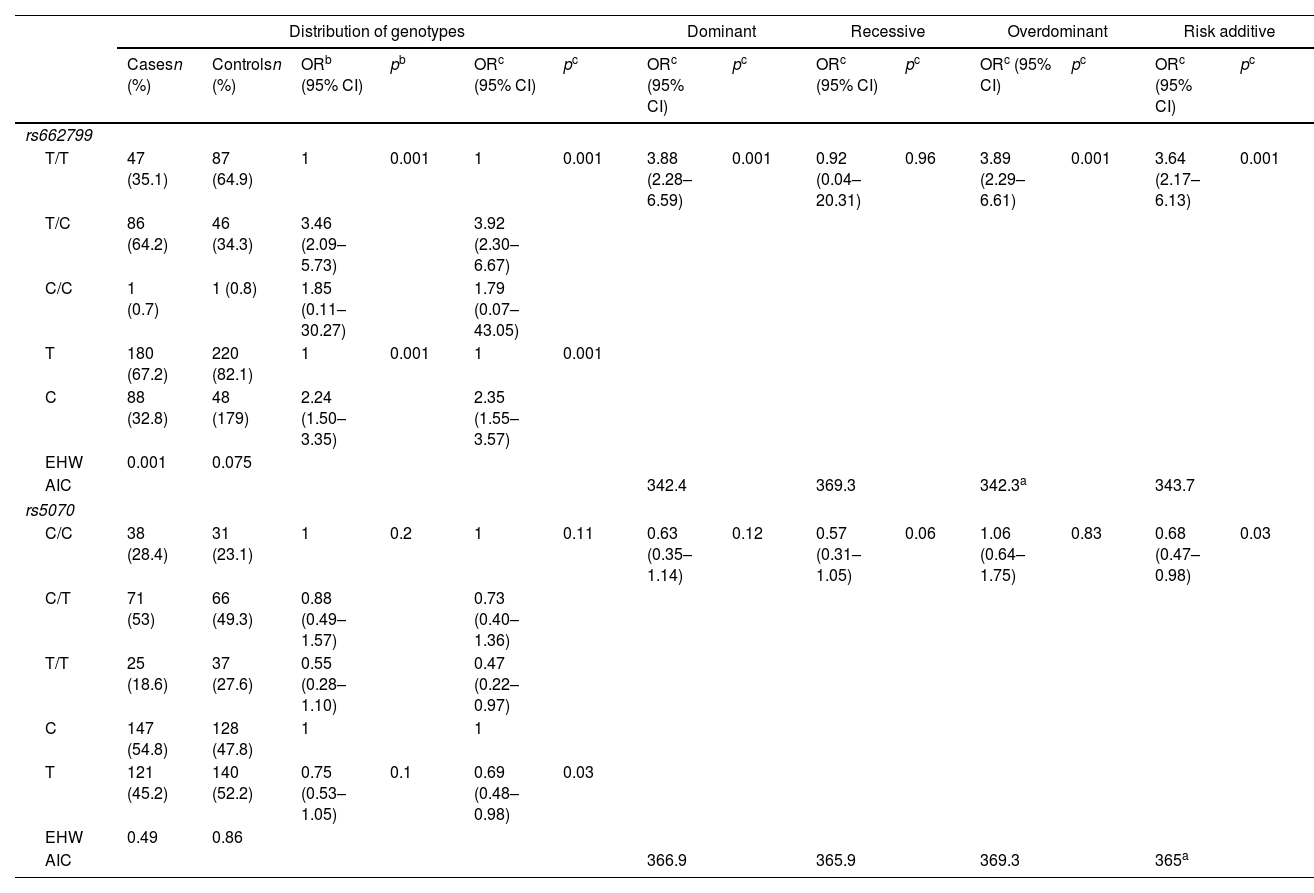
Allelic frequency analysis found that both SNP‘s were under the HWE rs662799 (p=0.075) and rs5070 (p=0.86). The rs662799 (C) polymorphism presented a frequency of 32.8% in hypertriglyceridemia cases, and 17.9% in controls, it was found that carriers of the T/C genotype had a higher risk of hypertriglyceridemia (OR=3.92, 95% CI: 2.30–6.67, p=0.001) (Table 3). The over-dominant was the inheritance model that best fitted with cases of hypertriglyceridemia, carriers of the T/C genotype had a higher risk of hypertriglyceridemia than those who had T/T+C/C genotype (OR=3.89, 95% CI: 2.29–6.61, p=0.001) (Table 3).
Genotypic association of rs662799 and rs5070 polymorphisms with hypertriglyceridemia as a function of inheritance model.
| Distribution of genotypes | Dominant | Recessive | Overdominant | Risk additive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casesn (%) | Controlsn (%) | ORb (95% CI) | pb | ORc (95% CI) | pc | ORc (95% CI) | pc | ORc (95% CI) | pc | ORc (95% CI) | pc | ORc (95% CI) | pc | |
| rs662799 | ||||||||||||||
| T/T | 47 (35.1) | 87 (64.9) | 1 | 0.001 | 1 | 0.001 | 3.88 (2.28–6.59) | 0.001 | 0.92 (0.04–20.31) | 0.96 | 3.89 (2.29–6.61) | 0.001 | 3.64 (2.17–6.13) | 0.001 |
| T/C | 86 (64.2) | 46 (34.3) | 3.46 (2.09–5.73) | 3.92 (2.30–6.67) | ||||||||||
| C/C | 1 (0.7) | 1 (0.8) | 1.85 (0.11–30.27) | 1.79 (0.07–43.05) | ||||||||||
| T | 180 (67.2) | 220 (82.1) | 1 | 0.001 | 1 | 0.001 | ||||||||
| C | 88 (32.8) | 48 (179) | 2.24 (1.50–3.35) | 2.35 (1.55–3.57) | ||||||||||
| EHW | 0.001 | 0.075 | ||||||||||||
| AIC | 342.4 | 369.3 | 342.3a | 343.7 | ||||||||||
| rs5070 | ||||||||||||||
| C/C | 38 (28.4) | 31 (23.1) | 1 | 0.2 | 1 | 0.11 | 0.63 (0.35–1.14) | 0.12 | 0.57 (0.31–1.05) | 0.06 | 1.06 (0.64–1.75) | 0.83 | 0.68 (0.47–0.98) | 0.03 |
| C/T | 71 (53) | 66 (49.3) | 0.88 (0.49–1.57) | 0.73 (0.40–1.36) | ||||||||||
| T/T | 25 (18.6) | 37 (27.6) | 0.55 (0.28–1.10) | 0.47 (0.22–0.97) | ||||||||||
| C | 147 (54.8) | 128 (47.8) | 1 | 1 | ||||||||||
| T | 121 (45.2) | 140 (52.2) | 0.75 (0.53–1.05) | 0.1 | 0.69 (0.48–0.98) | 0.03 | ||||||||
| EHW | 0.49 | 0.86 | ||||||||||||
| AIC | 366.9 | 365.9 | 369.3 | 365a | ||||||||||
OR: odds ratio; CI: 95% confidence interval; p: p-value, significant (<0.05). AIC: Akaike's information criterion.
The rs5070 (T) polymorphism showed a prevalence of 45.2% in cases, and 52.2% in the control group, carriers of the T/T genotype have a lower risk of developing hypertriglyceridemia (OR=0.47, 95% CI: 0.22–0.97, p=0.11) (Table 3). The association analysis based on the inheritance models showed significant protection in the additive risk model, carriers of the T/T genotype were less likely to have hypertriglyceridemia in children (OR=0.68, 95% CI: 0.47–0.99, p=0.03) (Table 3).
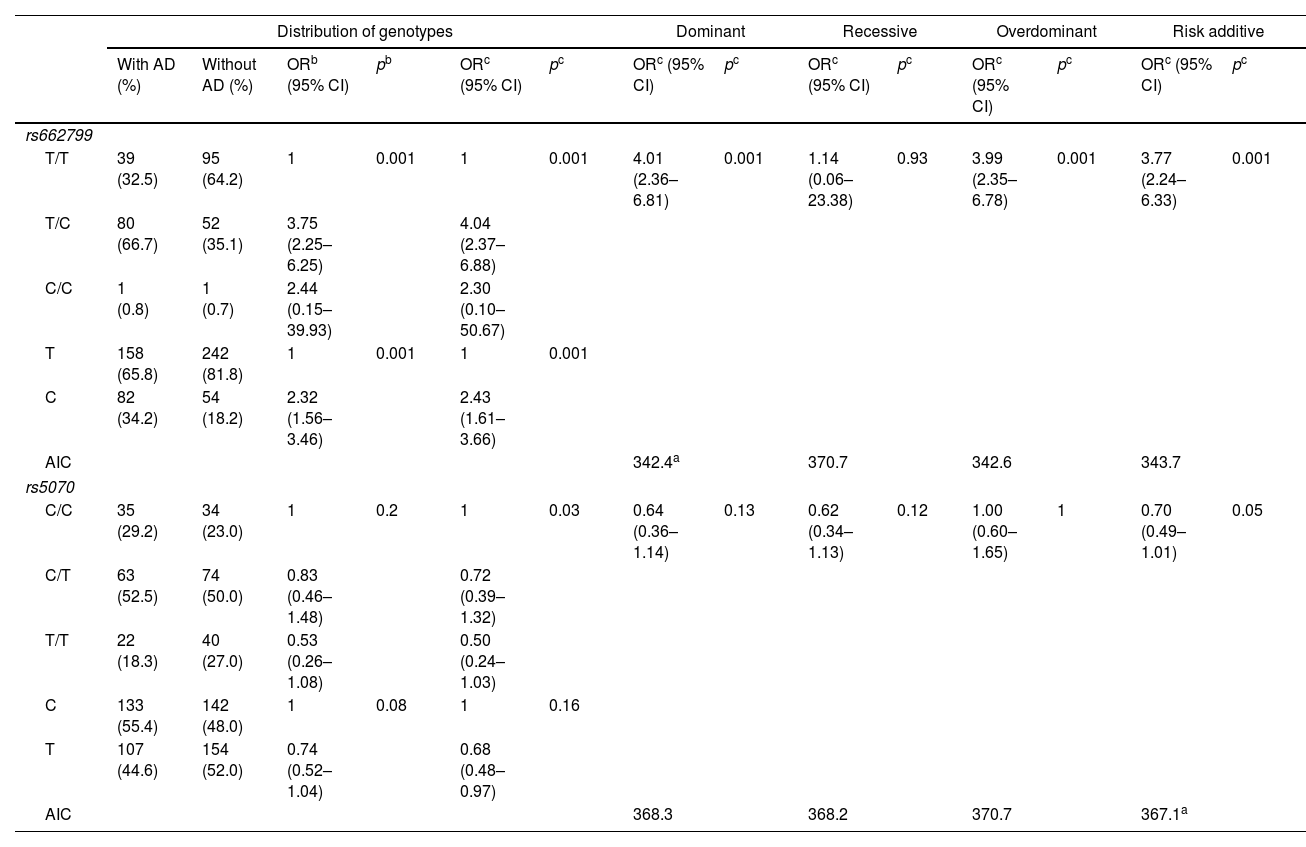
In this study, 120 individuals with AD, were identified. The rs662799 (C) polymorphism was present in 34.2% of minors with AD (Table 4). In relation to genotype frequencies, it was found that carriers of the T/C genotype had a higher risk of developing AD (OR=4.04, 95% CI: 2.37–6.88, p=0.001) (Table 4). Genotypic frequency analysis showed a significant risk association with AD in the dominant inheritance model in carriers of the T/C+C/C (OR=4.01, 95% CI: 2.36–6.81, p=0.001) (Table 4).
Genotypic association of rs662799 and rs5070 polymorphisms with AD as a function of inheritance model.
| Distribution of genotypes | Dominant | Recessive | Overdominant | Risk additive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With AD (%) | Without AD (%) | ORb (95% CI) | pb | ORc (95% CI) | pc | ORc (95% CI) | pc | ORc (95% CI) | pc | ORc (95% CI) | pc | ORc (95% CI) | pc | |
| rs662799 | ||||||||||||||
| T/T | 39 (32.5) | 95 (64.2) | 1 | 0.001 | 1 | 0.001 | 4.01 (2.36–6.81) | 0.001 | 1.14 (0.06–23.38) | 0.93 | 3.99 (2.35–6.78) | 0.001 | 3.77 (2.24–6.33) | 0.001 |
| T/C | 80 (66.7) | 52 (35.1) | 3.75 (2.25–6.25) | 4.04 (2.37–6.88) | ||||||||||
| C/C | 1 (0.8) | 1 (0.7) | 2.44 (0.15–39.93) | 2.30 (0.10–50.67) | ||||||||||
| T | 158 (65.8) | 242 (81.8) | 1 | 0.001 | 1 | 0.001 | ||||||||
| C | 82 (34.2) | 54 (18.2) | 2.32 (1.56–3.46) | 2.43 (1.61–3.66) | ||||||||||
| AIC | 342.4a | 370.7 | 342.6 | 343.7 | ||||||||||
| rs5070 | ||||||||||||||
| C/C | 35 (29.2) | 34 (23.0) | 1 | 0.2 | 1 | 0.03 | 0.64 (0.36–1.14) | 0.13 | 0.62 (0.34–1.13) | 0.12 | 1.00 (0.60–1.65) | 1 | 0.70 (0.49–1.01) | 0.05 |
| C/T | 63 (52.5) | 74 (50.0) | 0.83 (0.46–1.48) | 0.72 (0.39–1.32) | ||||||||||
| T/T | 22 (18.3) | 40 (27.0) | 0.53 (0.26–1.08) | 0.50 (0.24–1.03) | ||||||||||
| C | 133 (55.4) | 142 (48.0) | 1 | 0.08 | 1 | 0.16 | ||||||||
| T | 107 (44.6) | 154 (52.0) | 0.74 (0.52–1.04) | 0.68 (0.48–0.97) | ||||||||||
| AIC | 368.3 | 368.2 | 370.7 | 367.1a | ||||||||||
AD: atherogenic dyslipidemia; OR: odds ratio; CI: 95% confidence interval; P: P-value, significant (<0.05); AIC: Akaike's information criterion.
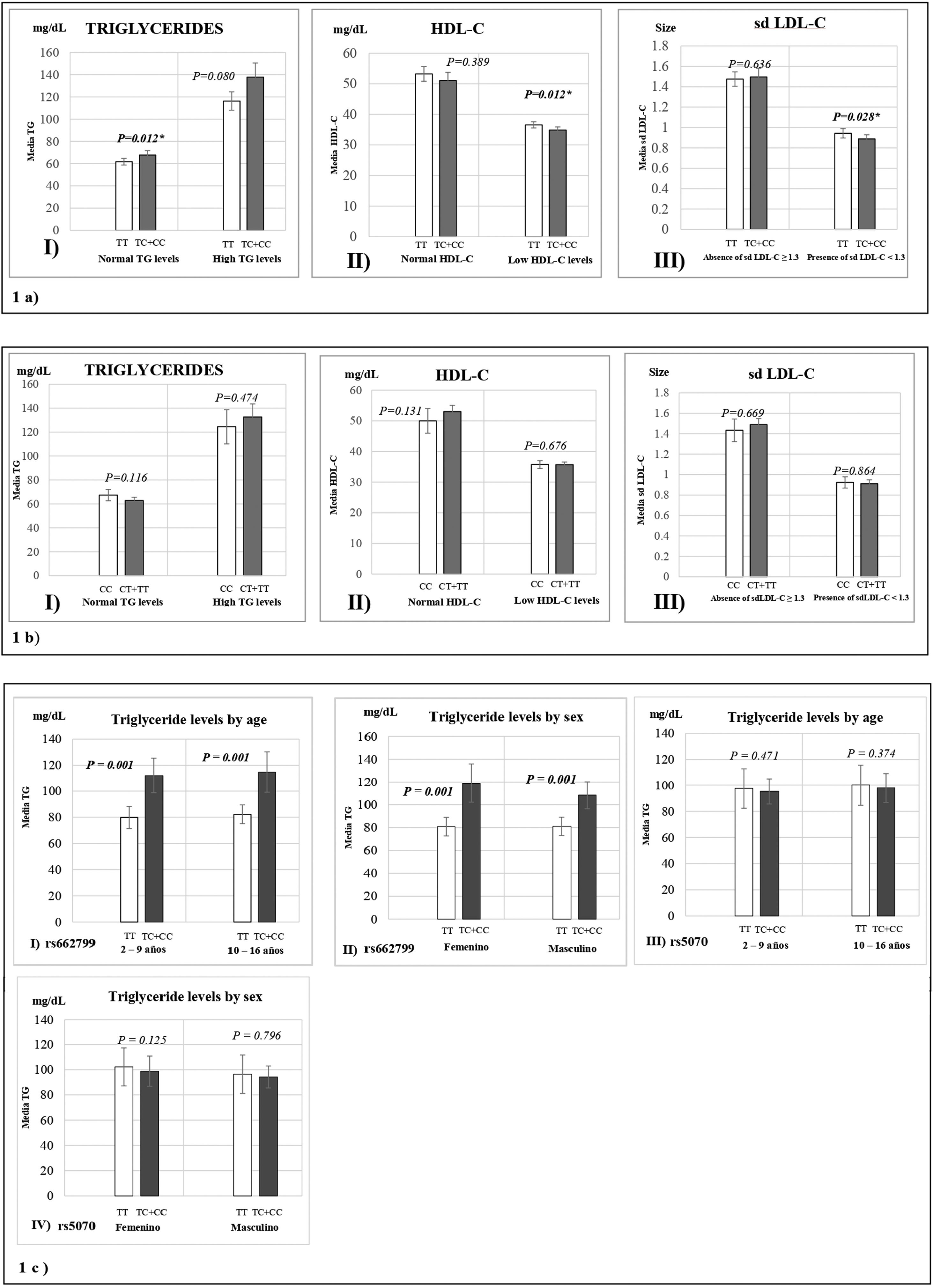
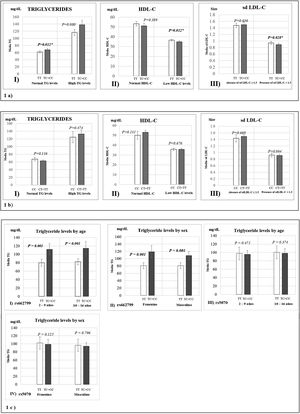
Fig. 1 represents the means of biochemical parameters and their association with the rs662799 and rs5070 polymorphisms. The data reveal that minors carrying the rs662799 (C) polymorphism had higher concentrations of TG levels in the control group (p=0.012). For the group of cases, low HDL-C levels, as well as small sd LDL-C particle sizes were found (p=0.028) (Fig. 1a), TG levels tend to increase in carriers, but no significant differences was found. The data also showed that rs662799 polymorphism carriers had higher concentrations of TG in both age groups (0–9 years and 10–16 years) and for both sexes (p=0.001) (Fig. 1c I and II).
Association graphs between the rs662799, rs5070 polymorphism and biochemical values. (a) Association graphs between the rs662799, and biochemical parameters. (b) Association graphs between the rs5070 polymorphism and biochemical parameters. (c) Association graphs between rs662799 and rs5070 polymorphisms and triglyceride levels by age group and sex. TG: triglycerides; HDL-C: high-density lipoprotein cholesterol; sd LDL-C: small and dense low-density lipoproteins cholesterol; mg/dL; milligram/deciliter; p: significant (p<0.05); U-Mann Whitney.
However, these differences were not observed in carriers of the rs5070 (T) polymorphism and the biochemical parameters (Fig. 1b).
DiscussionIn Mexico, the most common dyslipidemia is hypertriglyceridemia.28 The lipid triad that makes up AD are present in the pediatric population,29 and these metabolic disorders continue to increase due to related factors such as obesity, poor diet, and sedentary lifestyle.30
The study showed an increase in BMI in both age groups of cases. An increase in values of blood pressure was found in minors with hypertriglyceridemia, these results were expected. Other research has shown that TG values are significantly elevated in infants with obesity,22 and increased blood pressure (BP).23
Likewise, there were significant differences in almost all lipid parameters except for LDL-C in both age groups, and in ApoB levels in minors between 10 and 16 years. Research work has shown that TG is strongly correlated with undesirable levels of serum lipid levels.5 On the other hand, when comparing the anthropometric variables of infants these presented significant differences as part of the phenomenon of natural growth of minors, however, there were no statistically significant differences in lipid levels among age groups.
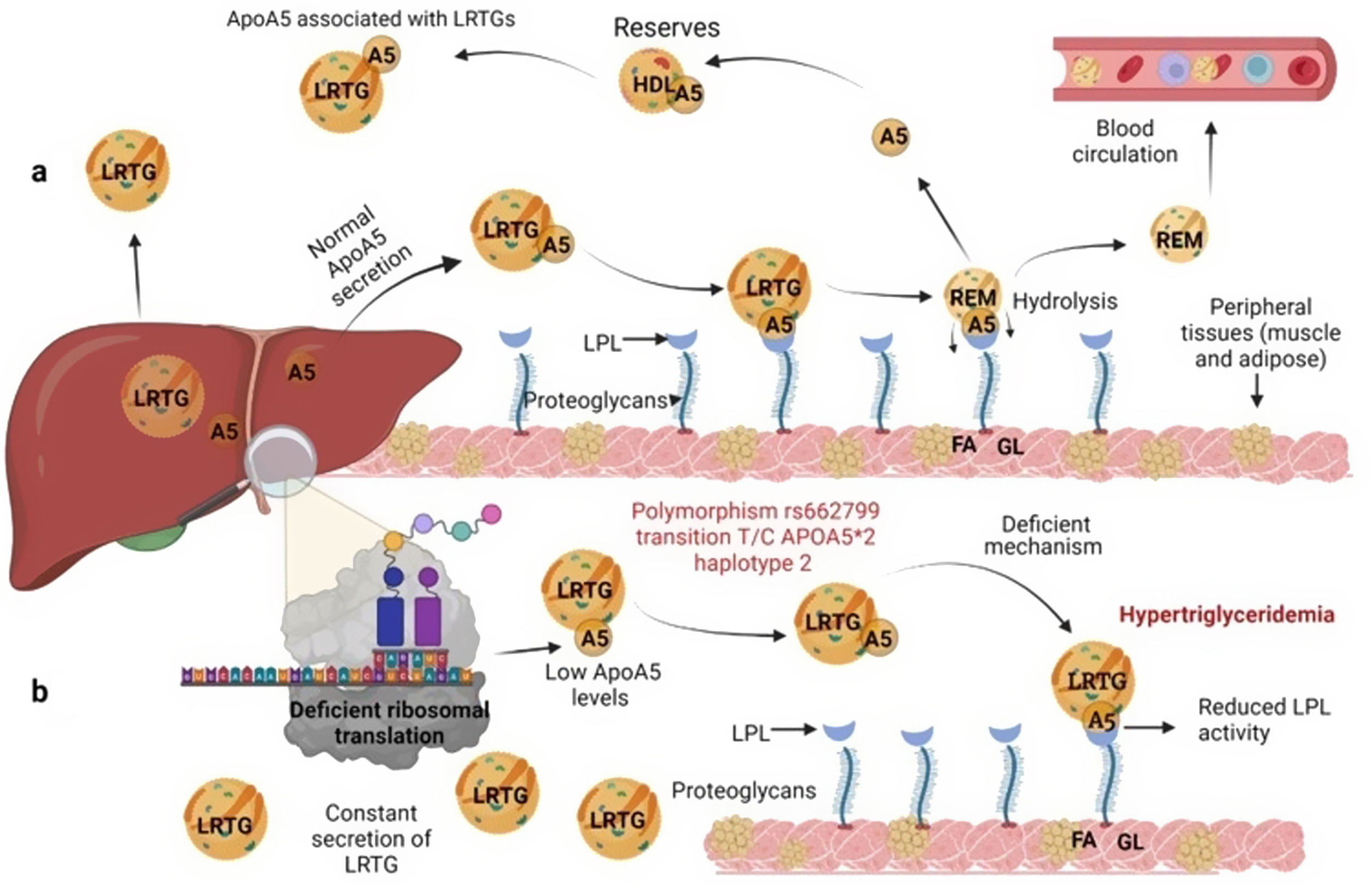
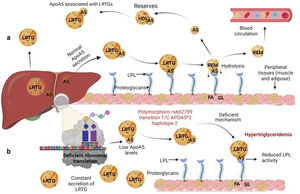
Several previous studies in the adult population associate the rs662799 polymorphism with dyslipidemia, and CVD.5,31 In this research, it was found that the overdominant model of rs662799 was associated with the risk of hypertriglyceridemia Additionally, this research showed that minors with rs662799(C) polymorphism had higher triglyceride concentrations in both age groups and for both sexes., Additionally, this research showed that minors with rs662799(C) polymorphism had higher triglyceride concentrations in both age groups and for both sexes. It is necessary to take into account that the sum of other components such as diet, environment, and other genetic polymorphisms that were not analyzed in this study, also can promote the increase in TG concentrations.19 However, this research largely coincides with another study published in a Mexican child population, which revealed that the rs662799 polymorphism modulates TG levels in children and adolescents.5 Furthermore, European and Chinese young populations who have the SNP rs662799 have been shown increased TG plasma concentrations.32 Some studies have demonstrated that variations in nucleotide bases, result in modifications of the amino acid sequence of the ApoA5 molecule, causing a dysfunctional protein 33or decreasing their activity.34 ApoA5 is produced a majority in the liver,12 and can be secreted together with lipoproteins rich in triglycerides (LRTG), chylomicrons (QM) and very low density lipoprotein cholesterol (VLDL-C) 35(Fig. 3a), ApoA5 leads the LRTG to lipoprotein lipase (LPL) and initiates TG hydrolysis,36 generating remnant (Rem) particles.36 Also, ApoA5 is coupled to HDL-C to be reserved and it can be translocated and reused by other LRTG.12 It is suggested that carriers of the rs662799 polymorphism, 3A-3G, 751G-T, and 1891T-C (haplotype A5*2),37 present deficiency in ribosomal translation38 (Fig. 3b), generating reduced ApoA5 levels,34 decreasing LPL-mediated TG uptake,38 while the liver continues to steadily secrete LRTG, and promoting the cases of hypertriglyceridemia.33 We illustrate the mechanism of action of ApoA5 on TG modulation and the presence of the rs662799 SNP in Fig. 2.
Mechanism of action of triglyceride hydrolysis and the interaction with rs662799 polymorphism. LRTG; lipoproteins rich in triglycerides; ApoA5 and A5: apolipoprotein A5; LPL: lipoprotein lipase; REM: remnants; C-HDL: high density lipoprotein cholesterol; FA: fatty acids; GL: glycerol. Modified from Merkel and Heeren.36 (a) ApoA5 is secreted exclusively in the liver and initially, ApoA5 can be secreted together with LRTG. TG are hydrolyzed by the action of LPL. ApoA5 directs LRTG to LPL for hydrolysis, generating Rem particles. ApoA5 is coupled to C-HDL for its reserve so that it can be translocated and reused by other LRTGs. (b) The opposite situation in patients with the rs662799 polymorphism in APOA5, the C variant impairs the efficiency of ribosomal translation, this deficiency generates reduced ApoA5 levels, causing a reduction in LPL-mediated TG uptake activity, while the liver continues to secrete LRTG constantly, manifesting hypertriglyceridemia.
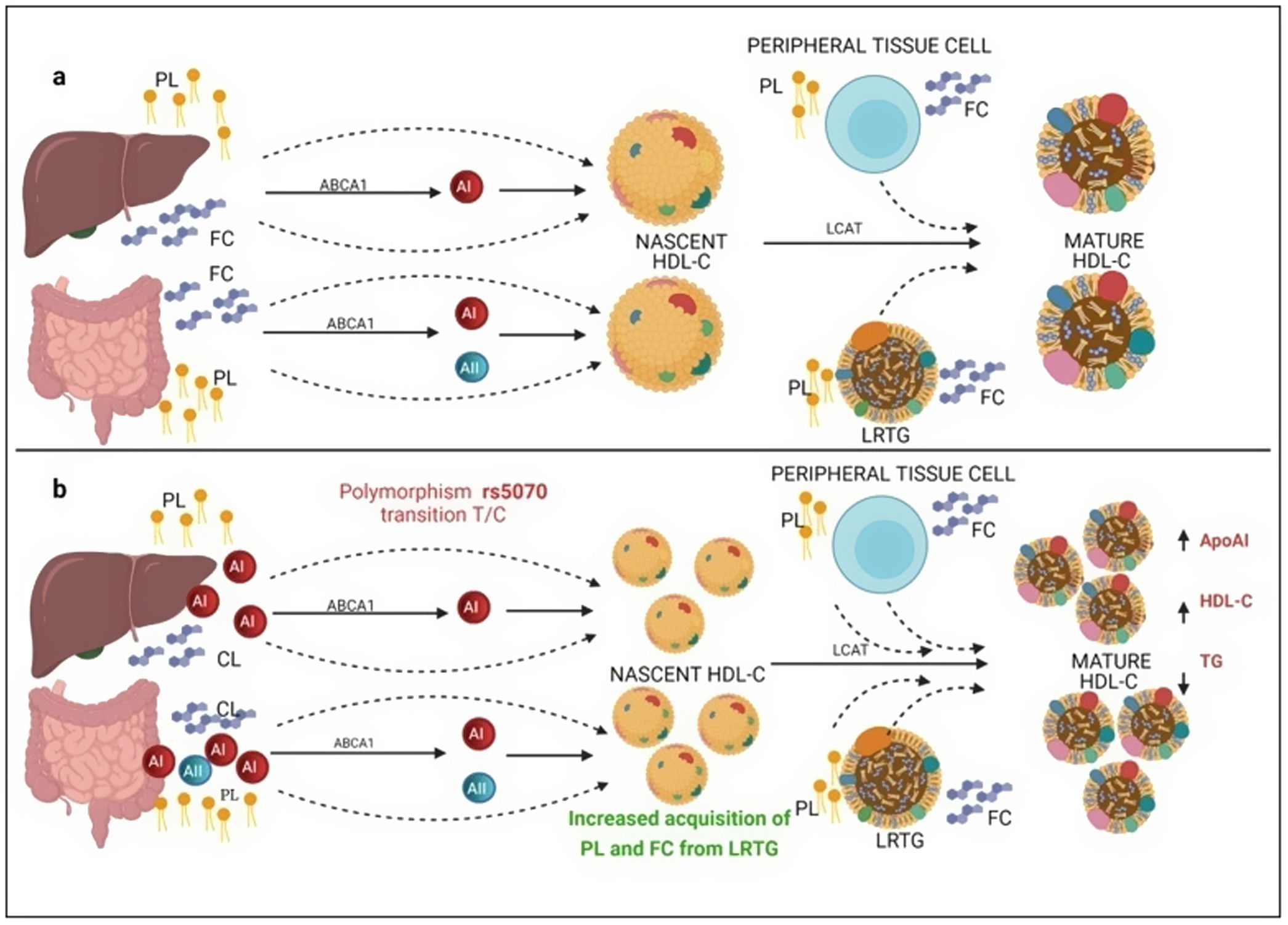
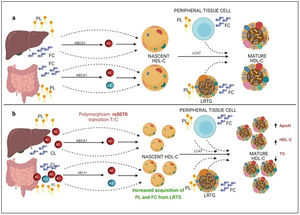
On the other hand, previous research has reported that common APOA1 genetic variants influence dyslipidemia.39 Studies in Korean population revealed that rs5070 polymorphism was associated with elevated TG levels,17 however, the association of rs5070 with TG has not been yet assessed in the Mexican population. Results found in other populations differ considerably with this investigation, the data presented do not disclose association with hypertriglyceridemia, oppositely the ORs data show a significant protection association in the additive risk model genotype T/T (OR=0.68, 95% CI: 0.47–0.99, p=0.03). It is important to mention that few studies have been done evaluating this polymorphism, and they do not provide a clear answer about the mechanisms related to TG levels. However, it is known that ApoA1 bound to HDL-C works as a surface acceptor of QM and VLDL-C during their catabolism,40 the protective effect may be involved with the biosynthesis and metabolism of HDL-C. ApoA1 is predominantly secreted by the liver and intestine as lipid-poor ApoA140 (Fig. 3) and acquires free cholesterol (FC) and phospholipids (PL) from enterocytes and hepatocytes, creating nascent HDL-C,41 as HDL-C travels through the circulation, it also picks up FC and PL generating mature HDL-C41 (Fig. 3a). We propose that carriers of the rs5070 polymorphism (Fig. 3b) manifest hepatic and intestinal overexpression of ApoA1, high levels of ApoA1, significantly increase nascent HDL-C levels and the overproduction of nascent HDL-C requires more FC and PL from peripheral tissues and LRTG, decreasing TG levels and producing more mature HDL-C. Therefore, this polymorphism acts as protection by being significantly associated with elevated ApoA1 and HDL-C (data not shown) and decreased TG levels. However, studies to test this possible hypothesis have yet to be carried out.
Mechanism of C-HDL biosynthesis and the interaction with rs5070 polymorphism. ABCA1: ATP binding cassette subfamily A member 1; A1: apolipoprotein AI; AII; apolipoprotein AII; HDL-C: high density lipoprotein cholesterol; LCAT: lecithin cholesterol acyl transferase; PL: phospholipids; FC; free cholesterol; LRTG: lipoprotein rich in triglycerides. (a) ApoA1 is predominantly secreted by the liver and intestine as lipid-poor ApoA1, ApoA1 acquires FC and PL, creating nascent HDL-C, as HDL-C travels through the circulation, it also picks up more CL and PL from peripheral tissues, and LRTGs, generating mature HDL-C. (b) Carrying of rs5070 polymorphism manifests hepatic and intestinal overexpression of ApoA1 than increases the levels of nascent HDL-C, overproduction of nascent HDL-C needs to acquire more FC and PL, causing low TG levels and forming more mature HDL-C.
In this research, we also evaluated the magnitude of the association of SNP rs662799 with the presence of AD. It was found that allele C was associated with a higher risk of AD, whereas the dominant model showed a strong association with these lipid disorders. Until now, this is the first work that estimates the association of SNP rs662799 with AD in Mexicans. In addition, there are no studies carried out in the child population. However, the results reported in this work, are similar to other studies carried out in adults, in a multiethnic population, which report that SNP rs662799 predisposes to higher TG levels, more atherogenic LDL-C particles, and decreased HDL-C concentrations.3,42 It is assumed that the association of the APOA5 gene with AD is mainly due to the trigger on TG metabolism.43
Moreover, rs5070 polymorphism has been frequently studied with HDL-C concentrations,17 and to our knowledge, this is the first study to report an interaction between the rs5070 polymorphism and AD. Where the Turkish and Brazilian populations were associated with morbidities related to TG, HDL-C, VLDL-C, and LDL-C.18,44 Analyzing the results obtained in rs5070 polymorphism did not show a significant association with AD. The differences observed in these populations can be attributed to the fact that the genetic variations present in the APOA1 gene are under transcriptional regulation in the liver and can be explained by the effect of various factors that may intervene on transcription factors in hepatocellular regulation.45
One of the main limitations in this study was the lack of adjustment for these genetic polymorphisms related to lifestyle and dietary habits since diet plays an important role in developing dyslipidemia. In addition, we did not assess the parents’ history of dyslipidemia.
ConclusionThis study showed that SNP rs662799 was associated with cases of hypertriglyceridemia and AD in pediatric patients in southeastern Mexico. It was also determined that rs5070 NPS was not associated with cases of hypertriglyceridemia and did not contribute to the risk to present AD in this population.
Data access statementData supporting the findings of this study are available from the corresponding author [cirecta@ecosur.mx], Dr. César Antonio Irecta Nájera. Some information is omitted to protect the privacy of minors.
Author contribution[César Antonio Irecta Nájera], and [Armando Camilo Hernández Contreras] conceived and directed the project; [Héctor Ochoa-Díaz-López] designed and planned the study. [César Antonio Irecta Nájera], [Armando Camilo Hernández Contreras], and [Valeria Ovando Gómez] supervised data collection. [Valeria Ovando Gómez] performed the statistical analysis. [Soraya Amalí Zavaleta Muñiz], and [Valeria Ovando Gómez] contributed to the analysis and interpretation of the results. The first draft of the manuscript was written by [Valeria Ovando Gómez], and [César Antonio Irecta Nájera]. [Héctor Ochoa-Díaz-López], and [Soraya Amalí Zavaleta Muñiz] revised the manuscript with the important contribution of all coauthors. All authors approved the final version.
FundingThis work was supported by the National Council of Science and Technology (CONACYT), project number: 2016-01-2697.
Conflict of interestsThe authors declare no conflict of interest.
We thank the professionals of the medical unit of the ISSSTE Comitán de Domínguez, Chiapas, and Villahermosa, Tabasco.