cardiovascular disease is one of the main complications of people with type 2 diabetes (T2D). The ESC/ESA 2019 lipid guide has led to a change in dyslipidemia control. We analyse the evolution of the lipid profile, the fulfillment of the LDL-C targets, how patients are classified and the impact of this guide on lipid control in T2D patients.
Materials and methodsA prospective cohort study from 2017 to 2020 from a cohort of 297 T2D out of a total of 1229 (95% confidence level). We classified patients according to their cardiovascular risk (CVR) and whether they met or their low-density cholesterol (LDL-C) goal.
ResultsAge: 62.58 ± 10.68; 52.79% men. Mean LDL-C levels 116.2 at baseline and 100.2 mg/dl at four years (p < .001). They met their individualized LDL-C target after publication of the guide: 57 (21.67%). There were more controls who were under 65 (57.9% vs 36.9% p < .01; RR 0.83), men (66.7% vs 49.5% p < .05; RR 0, 86) and smokers (17.5% vs 7.8% p < .05). 74.23% had a high CVR and a target LDL-C <70 mg/dl.
ConclusionsSince the publication of the ESC/ESA 2019 lipid guide, a decrease in LDL-c levels has been observed. Only one in five patients fulfill their individualized LDL-C target. Male patients, under 65 years of age and smokers presented an advantage in meeting their goal. Most T2D patients have a high CVR, and the predominant LDL-C target is less than 70 mg/dl.
La enfermedad cardiovascular supone una de las principales complicaciones de las personas con diabetes tipo 2 (DM2). La guia ESC/ESA 2019 de lípidos ha supuesto un cambio en control de dislipemia. Analizamos la evolución del perfil lipídico, el cumplimiento de los objetivos de c-LDL, como clasifica a los pacientes y el impacto de esta guía en el control lipídico en pacientes con DM2.
Materiales y métodosEstudio de cohortes prospectivo de 2017 a 2020 de una cohorte de 297 DM2 de un total de 1229(nivel confianza 95%).Clasificamos a los pacientes en función de su riesgo cardiovascular (RCV) y si cumplía o su objetivo de colesterol de baja densidad (c-LDL).
ResultadosEdad: 62,58 ± 10,68; 52,79% hombres. Niveles de c-LDL medio 116,2 al inicio y 100,2 mg/dL a los cuatro años años (p < 0,001). Cumplían su objetivo individualizado de c-LDL después de la publicación de la guía: 57 (21,67%). Hubo más controlados que eran menores de 65 (57,9% vs 36,9% p < 0,01; RR 0,83), varones (66,7% vs 49,5%;p < 0,05; RR 0,86) y fumadores (17,5%vs7,8%;p < 0,05). El 74,23% tenía un RCV alto y un objetivo c-LDL <70 mg/dl.
ConclusionesDesde la publicación de la guía de lípidos ESC/ESA 2019 se observa un descenso en los niveles de c-LDL. Aun así, solo uno de cada cinco pacientes cumple su objetivo individualizado de c-LDL. Presentaron una ventaja de cumplir su objetivo los pacientes con sexo varón, menores de 65 años y fumadores. La mayoría de los pacientes DM2 tienen un RCV alto y el objetivo de c-LDL predominante es inferior a 70 mg/dl.
Cardiovascular disease is one of the main complications in patients with type 2 diabetes mellitus (DM2), leading to high morbidity and mortality. Up to 80% of these patients die from cardiovascular disease (75% from coronary heart disease and 25% from cerebrovascular disease) and a similar percentage are hospitalised for cardiovascular disease.1 Optimal control of cardiovascular risk factors (CVR) can reduce the risk of developing these DM-related complication.2
The new recommendations of the latest European Society of Cardiology/European Society of Anaesthesiology (ESC/ESA) guidelines on lipid management highlight the importance of stratifying patients with DM2 according to their CVR. This stratification allows us to set a cholesterol control target linked to low-density lipoprotein (LDL-C) binding protein for choosing the appropriate lipid-lowering therapy.3 This latest update brings with it the novelty of distinguishing high-risk patients from very high-risk patients, whose LDL-C target should be below 55 mg/dl.
Except in the low CVR group,4 the benefit of meeting these targets is clear, as they have been developed not only based on randomised studies, but also through a compilation of existing knowledge. The results of a meta-analysis concluded that a 38 mg/dl reduction in LDL-C induced by statin therapy reduces all-home mortality by 9% and the incidence of major cardiovascular events by 21%.5
However, we know that meeting these lipid targets in practice is not easy. In the IMPROVE-IT clinical trial in patients after acute coronary syndrome, only 37% met the LDL-C target one month after randomisation.6 According to a study carried out in patients with dyslipidaemia in Italy in primary care, the percentage of those reaching the target was between 16% and 45%.7 These control figures are lower in DM2 according to a study carried out in Argentina, where the impact of the new recommendations was analysed and the result showed that only 13.3% of patients met their individualised target.8
Our objective was to analyse the evolution of the lipid profile of our patients with DM2, as well as to determine the degree of compliance with the LDL-C targets set by the new ESC/ESA 2019 lipid guidelines. In addition, we will study which characteristics other than LDL-C (arterial hypertension, degree of glycaemic control, body mass index, smoking, etc.) are associated with better compliance with these targets under conditions of routine clinical practice. Finally, we will analyse how adherence evolves during follow-up and which CVR profile is predominant in our population of patients with DM2.
Material and methodsWe conducted a prospective, longitudinal, fixed cohort study, based on a previous descriptive study, whose objective was to assess the degree of glycaemic control in patients with DM2 in our health area. This comprises 2 clinics serving an urban population of 18,481 people over 18 years of age. The cross-sectional descriptive data are the subject of a publication by the authors.9 The study was approved by the Local Ethics Committee, the ethical requirements expressed in the Helsinki declaration and its subsequent amendments were met, and the Spanish data protection law was therefore complied with.
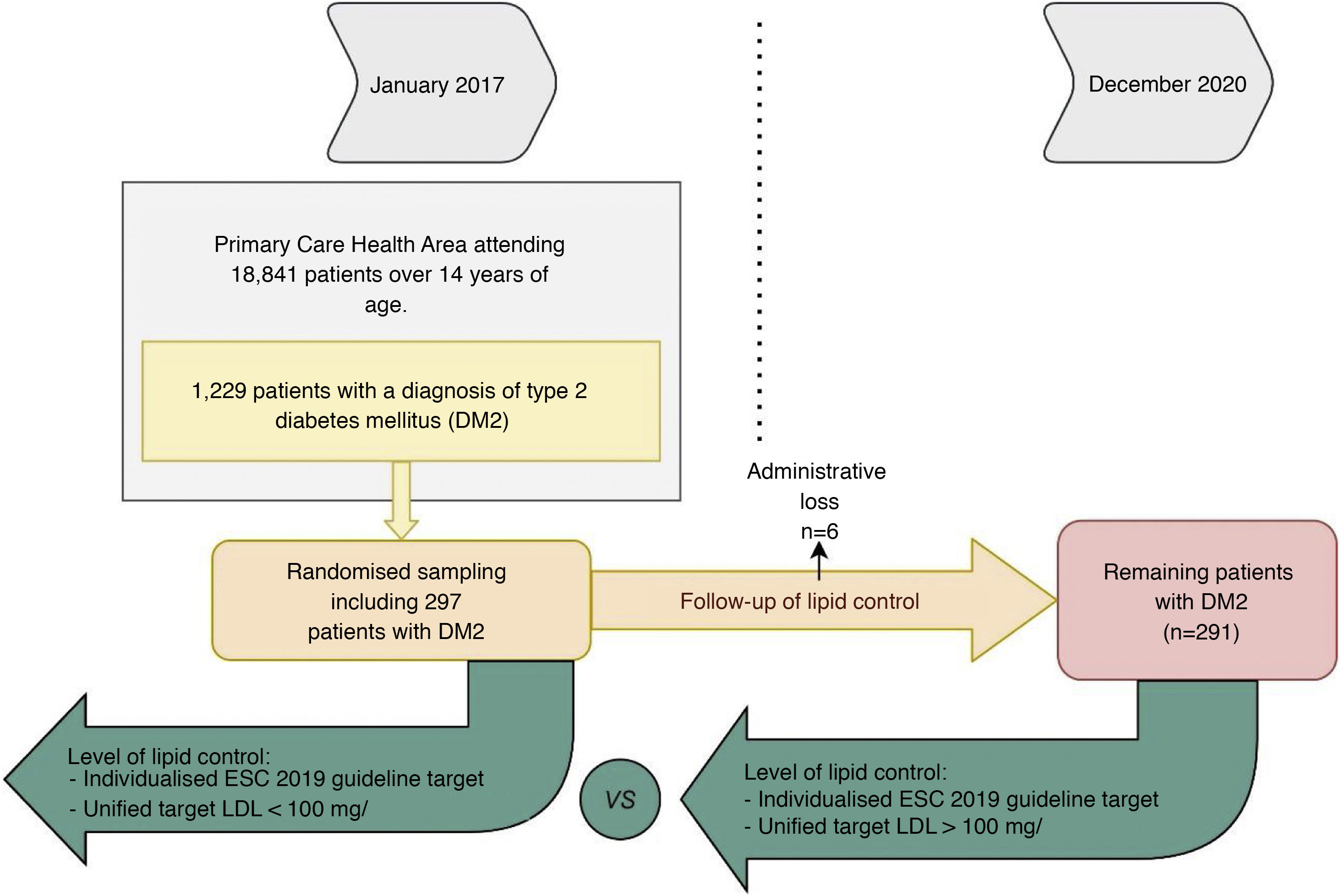
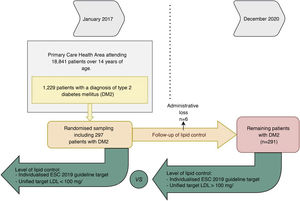
We considered data from patients with DM2 registered in January 2017, being reassessed in December 2020: 48 months later. Of the 1229 patients with DM2 in our health area, a simple random sampling of 297 patients was performed to obtain a representative sample (95% confidence level; margin of error < 5%). Administrative losses during the follow-up period were excluded (n = 6). Of the remaining 291 patients we analysed their degree of lipid control at baseline and at the end of follow-up (Fig. 1). To analyse the variables related to lipid control we selected those patients who had a LDL-C determination during the 4 years of follow-up (n = 263). To observe the evolution of lipid levels we selected those patients who had LDL-C and total cholesterol determinations at baseline and at the end of the study (n = 170).
The study variables collected from the computerised history were: demographic data, HbA1c, body mass index, glomerular filtration rate (GFR), time of evolution of DM2 and pharmacological groups related to dyslipidaemia: statins, fibrates or ezetimibe. To calculate GFR we used the CKD-EPI formula, considering chronic kidney disease when GFR was < 60 ml/min/1.73 m2, and to establish the presence of microalbuminuria, 30−300 g/mg of albumin in urine in at least one determination. We considered obese those patients with a body mass index ≥ 30 kg/m2, and major adverse cardiovascular event to have a documented episode of myocardial infarction or stroke in the clinical history. We also documented whether the patient had been admitted to hospital for heart failure during follow-up. In terms of blood pressure, we considered uncontrolled those patients with blood pressure levels above 140 mmHg systolic or 90 mmHg diastolic in clinical conditions. Smoking was considered in those patients who presented active smoking in their list of problems or toxic habits in the digital health history. It should be noted that not all patients had all the variables studied recorded, highlighting that 28 patients (9.62%) had no LDL-C control during the 4 years of follow-up and 106 (36.42%) at the start of the study. During follow-up, weight was not recorded in 74 patients (25.42%), blood pressure in 54 (18.20%), albuminuria in 27 (9.27%) and creatinine in 18 (6.18%).
We divided patients into 2 groups based on whether their dyslipidaemia was controlled according to their last LDL-C level. To determine whether or not they were controlled, we established a unified target of LDL-C < 100 mg/dl on the one hand, and an individualised target based on each patient's CVR according to the latest ESC/ESA dyslipidaemia dislipidemia3 (Table 1). We established a unified target of LDL-C < 100 mg/dl because it was historically set as a single target and we considered it to be the "minimum target" to be met in patients with DM2.
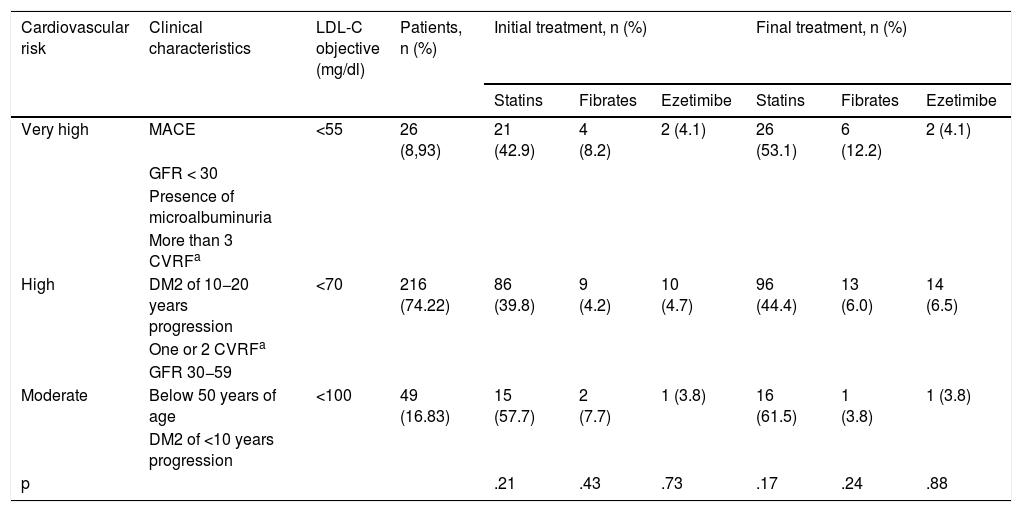
Classification of the cardiovascular risk of the patients of the study and their consequent LDL cholesterol objective.
| Cardiovascular risk | Clinical characteristics | LDL-C objective (mg/dl) | Patients, n (%) | Initial treatment, n (%) | Final treatment, n (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Statins | Fibrates | Ezetimibe | Statins | Fibrates | Ezetimibe | ||||
| Very high | MACE | <55 | 26 (8,93) | 21 (42.9) | 4 (8.2) | 2 (4.1) | 26 (53.1) | 6 (12.2) | 2 (4.1) |
| GFR < 30 | |||||||||
| Presence of microalbuminuria | |||||||||
| More than 3 CVRFa | |||||||||
| High | DM2 of 10−20 years progression | <70 | 216 (74.22) | 86 (39.8) | 9 (4.2) | 10 (4.7) | 96 (44.4) | 13 (6.0) | 14 (6.5) |
| One or 2 CVRFa | |||||||||
| GFR 30−59 | |||||||||
| Moderate | Below 50 years of age | <100 | 49 (16.83) | 15 (57.7) | 2 (7.7) | 1 (3.8) | 16 (61.5) | 1 (3.8) | 1 (3.8) |
| DM2 of <10 years progression | |||||||||
| p | .21 | .43 | .73 | .17 | .24 | .88 | |||
CVRF: cardiovascular risk factors; DM2: diabetes mellitus type 2; GFR: glomerular filtration rate; LDL-C: LDL cholesterol, low-density lipoprotein cholesterol; MACE: major adverse cardiovascular events.
To establish CVR we considered age, GFR, time of progression of DM2, whether a major adverse cardiovascular event had occurred, the presence of microalbuminuria as target organ damage and the number of CVR factors (obesity, smoking or uncontrolled HTN).
For data collection, a standardised protocol was developed, and volunteer physicians were trained. Each patient’s CVR, drug treatment and current lipid control were assessed.
Quantitative variables are shown with their mean value, standard deviation, and range (minimum-maximum), and qualitative variables with the number of patients and their frequency. To compare quantitative variables, we used the Student's t-test, previously checking its applicability with the Lilliefors normality test and Levene's test of equality of variances. In the analysis of independent qualitative variables, we used the Chi-square test and Fisher's exact test. For qualitative dependent variables we used McNemar’s test. For statistically significant variables we obtained their relative risk (RR) with a 95% confidence interval (CI). In the correlation analysis, we used Pearson's parametric correlation test and a dot plot to represent the correlation. We used the statistical package R (R Foundation for Statistical Computing, Vienna, Austria), specifically Rcmdr 4.0.3. For all hypothesis tests a risk () of .05 was set.
ResultsThe mean age of the patients studied was 62.58 ± 10.68 years (range: 37−91). 59.79% had an age ≥ 65 years, with a predominance of men (52.79%). The mean time of evolution of DM2 was 8.83 ± 5.89 years. Regarding clinical status at the start of follow-up, 43.31% were obese, 28.99% had uncontrolled HTN, 28.57% had chronic kidney disease and 9.28% were smokers. The mean HbA1c at the end of the study was 7.37 ± 1.64 and the mean body mass index was 30.49 ± 5.12. During the 4 years of follow-up 10.73% of patients had a major adverse cardiovascular event and 5.19% at least one admission for heart failure. Regarding CVR factors, 41.58% had one, 12.04% had two and 1.37% had three. We calculated the individual CVR of each patient and 8.93% were at moderate risk, 74.23% at high risk and 16.84% at very high risk. We observed a higher percentage of patients on statins in the moderate risk group (p = .17), fibrates in the very high-risk group (p = .24) and ezetimibe in the high-risk group (p = .73). In addition, there has been a generalised increase in the use of lipid-lowering drugs during follow-up (Table 1).
The mean LDL-C level was 117.8 ± 51.5 mg/dl at baseline and 101.9 ± 45.7 mg/dl at 4 years, and the total cholesterol level was 197.64 ± 42, 9 mg/dl at baseline and 176.5 ± 44.9 mg/dl at 4 years, thus resulting in a 15.98 mg/dl decrease in LDL-C (95% CI −21.94 to −10.0; p < .001) and a 20.79 mg/dl decrease in total cholesterol (95% CI −27.3 to −14.3; p < .001).
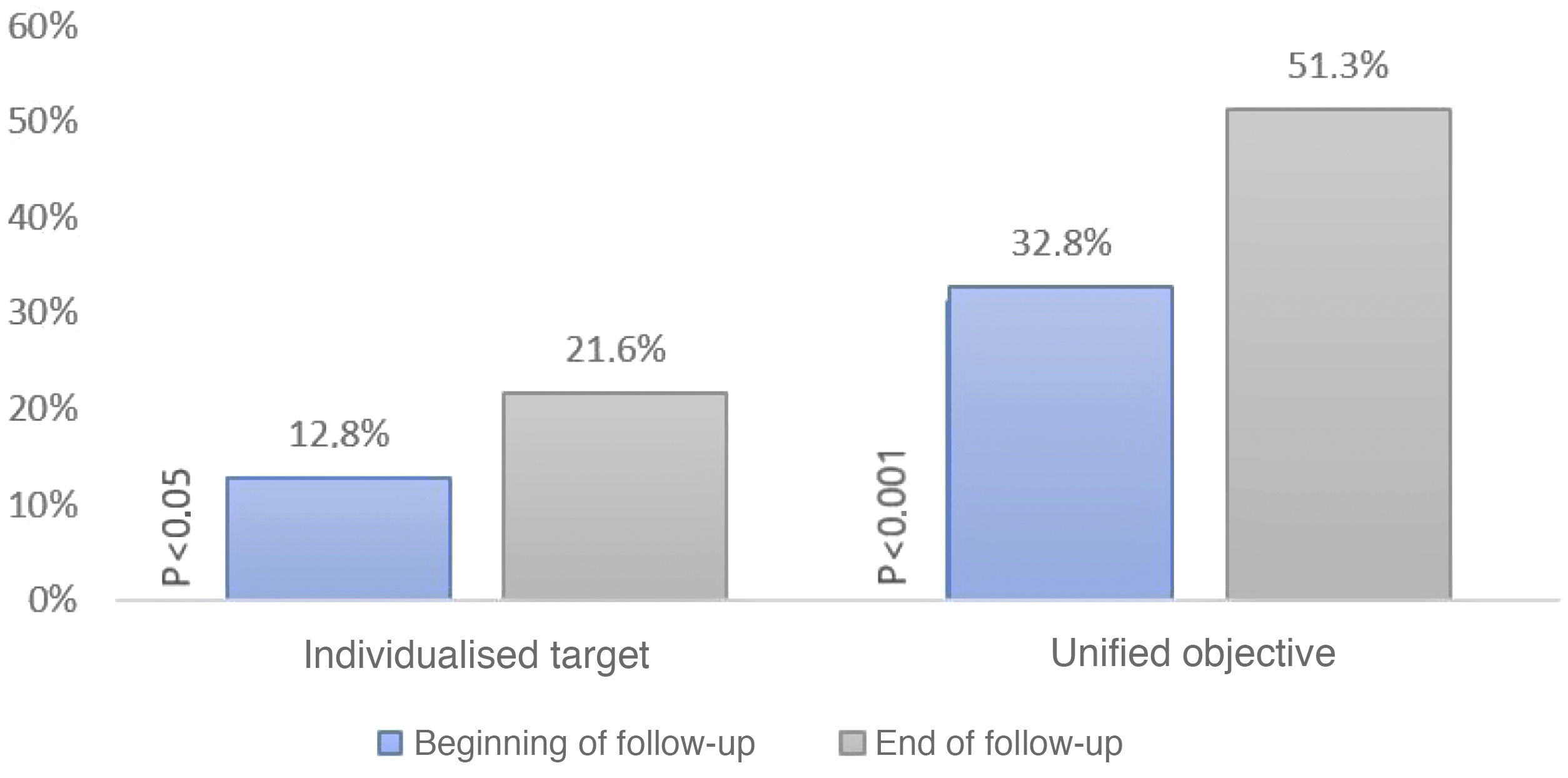
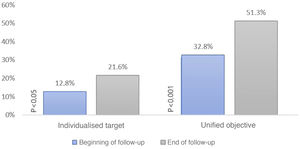
When setting individualised targets according to the ESC/ESA 2019 lipid guideline we obtained that 12.78% of patients were controlled at baseline and 21.57% at the end of follow-up (p < .05). Only 30% of patients who were controlled at baseline remained controlled at the end of follow-up. In contrast, if we set a unified LDL-C < 100 mg/dl target, 32.78% were controlled at baseline and 51.3% at the end of follow-up (p < .001); 56.6% of these patients who were controlled at baseline remained controlled at the end of follow-up (Fig. 2).
Percentage of patients meeting their LDL-C target at baseline and at the end of the study. The ordinate axis shows the percentage of patients meeting their target. The abscissa axis shows whether they met their individualised or unified target at baseline and at the end of the study. This graph shows an increase over time in the degree of control according to the two types of targets.
When establishing an individualised objective, we found a higher number of patients younger than 65 years in the controlled group than in the uncontrolled group (57.9 vs. 36.9%; p < .01; age: 65.2 vs. 67.9 years; p < .05), with an RR of .83 (95% CI .72–.95). Furthermore, we found a higher number of males in the controlled than in the uncontrolled group (66.7 vs. 49.5%; p < .05), with an RR of .86 (95% CI .75–.99), and of smokers (17.5 vs. 7.8%; p < .05). We obtained similar results when setting a unified target of LDL-C < 100 mg/dl. Regarding control of other risk factors, we observed a higher percentage of patients with uncontrolled HTN (31.8 vs. 28.3%; p = .64) and a lower percentage meeting their HbA1c target (30.5 vs. 38.8%; p = .78) in the group of patients who did not meet their individualised lipid target. We found no statistically significant differences for the rest of the variables studied, including the different pharmacological groups (p < .05) (Table 2).
Variables relating to lipid control.
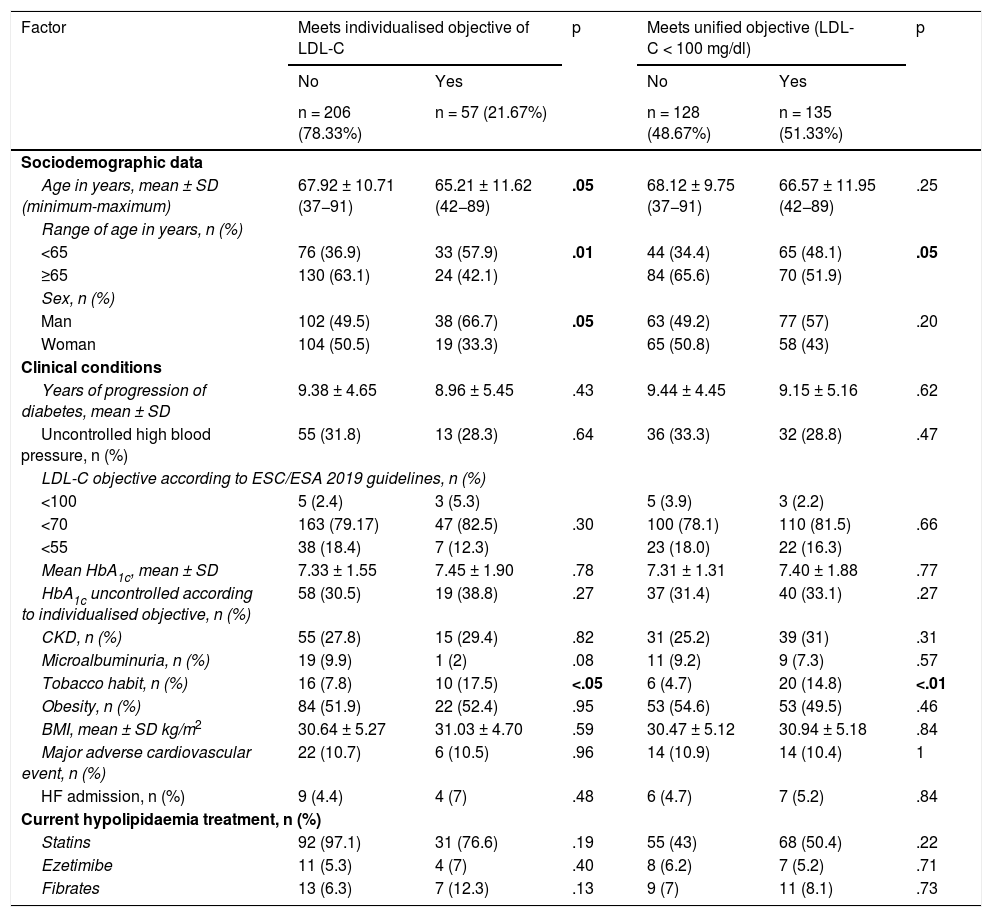
| Factor | Meets individualised objective of LDL-C | p | Meets unified objective (LDL-C < 100 mg/dl) | p | ||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| n = 206 (78.33%) | n = 57 (21.67%) | n = 128 (48.67%) | n = 135 (51.33%) | |||
| Sociodemographic data | ||||||
| Age in years, mean ± SD (minimum-maximum) | 67.92 ± 10.71 (37−91) | 65.21 ± 11.62 (42−89) | .05 | 68.12 ± 9.75 (37−91) | 66.57 ± 11.95 (42−89) | .25 |
| Range of age in years, n (%) | ||||||
| <65 | 76 (36.9) | 33 (57.9) | .01 | 44 (34.4) | 65 (48.1) | .05 |
| ≥65 | 130 (63.1) | 24 (42.1) | 84 (65.6) | 70 (51.9) | ||
| Sex, n (%) | ||||||
| Man | 102 (49.5) | 38 (66.7) | .05 | 63 (49.2) | 77 (57) | .20 |
| Woman | 104 (50.5) | 19 (33.3) | 65 (50.8) | 58 (43) | ||
| Clinical conditions | ||||||
| Years of progression of diabetes, mean ± SD | 9.38 ± 4.65 | 8.96 ± 5.45 | .43 | 9.44 ± 4.45 | 9.15 ± 5.16 | .62 |
| Uncontrolled high blood pressure, n (%) | 55 (31.8) | 13 (28.3) | .64 | 36 (33.3) | 32 (28.8) | .47 |
| LDL-C objective according to ESC/ESA 2019 guidelines, n (%) | ||||||
| <100 | 5 (2.4) | 3 (5.3) | 5 (3.9) | 3 (2.2) | ||
| <70 | 163 (79.17) | 47 (82.5) | .30 | 100 (78.1) | 110 (81.5) | .66 |
| <55 | 38 (18.4) | 7 (12.3) | 23 (18.0) | 22 (16.3) | ||
| Mean HbA1c, mean ± SD | 7.33 ± 1.55 | 7.45 ± 1.90 | .78 | 7.31 ± 1.31 | 7.40 ± 1.88 | .77 |
| HbA1c uncontrolled according to individualised objective, n (%) | 58 (30.5) | 19 (38.8) | .27 | 37 (31.4) | 40 (33.1) | .27 |
| CKD, n (%) | 55 (27.8) | 15 (29.4) | .82 | 31 (25.2) | 39 (31) | .31 |
| Microalbuminuria, n (%) | 19 (9.9) | 1 (2) | .08 | 11 (9.2) | 9 (7.3) | .57 |
| Tobacco habit, n (%) | 16 (7.8) | 10 (17.5) | <.05 | 6 (4.7) | 20 (14.8) | <.01 |
| Obesity, n (%) | 84 (51.9) | 22 (52.4) | .95 | 53 (54.6) | 53 (49.5) | .46 |
| BMI, mean ± SD kg/m2 | 30.64 ± 5.27 | 31.03 ± 4.70 | .59 | 30.47 ± 5.12 | 30.94 ± 5.18 | .84 |
| Major adverse cardiovascular event, n (%) | 22 (10.7) | 6 (10.5) | .96 | 14 (10.9) | 14 (10.4) | 1 |
| HF admission, n (%) | 9 (4.4) | 4 (7) | .48 | 6 (4.7) | 7 (5.2) | .84 |
| Current hypolipidaemia treatment, n (%) | ||||||
| Statins | 92 (97.1) | 31 (76.6) | .19 | 55 (43) | 68 (50.4) | .22 |
| Ezetimibe | 11 (5.3) | 4 (7) | .40 | 8 (6.2) | 7 (5.2) | .71 |
| Fibrates | 13 (6.3) | 7 (12.3) | .13 | 9 (7) | 11 (8.1) | .73 |
This table shows the variables that may be related to lipid control. Variables are analysed separately according to whether they meet the individualised or unified lipid target. Age and years since diabetes onset are considered at baseline.
BMI: body mass index; CKD: chronic kidney disease; ESC/ESA: European Society of Cardiology/European Society of Anaesthesiology; HbA1c: glycosylated haemoglobin; HF: heart failure; LDL cholesterol, low-density lipoprotein cholesterol; SD: standard deviation.
In bold, statistically significant p values.
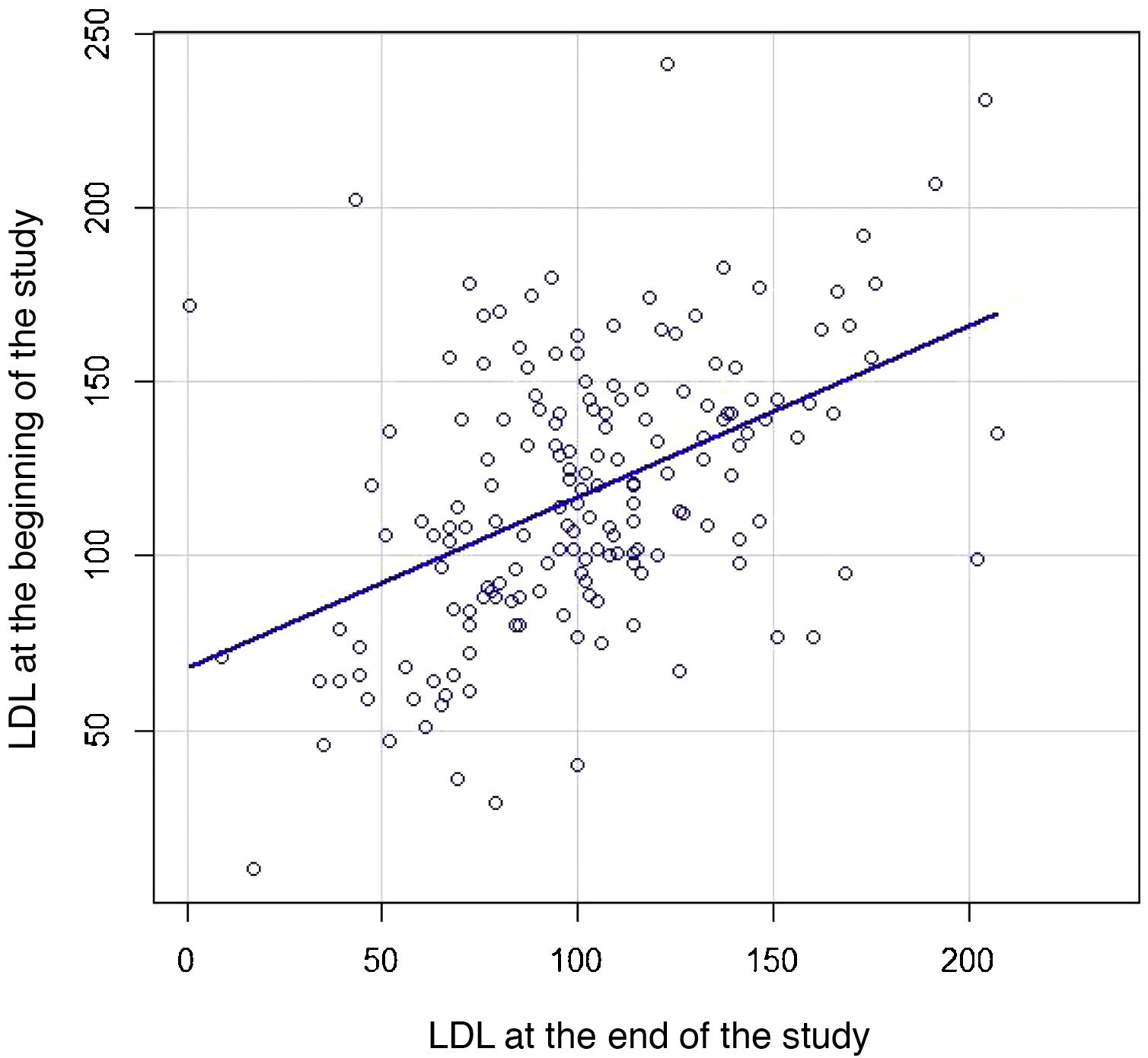
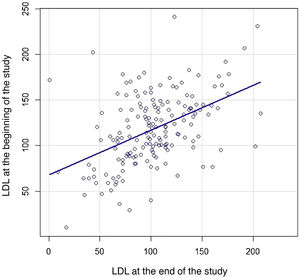
We then constructed a scatter plot (Fig. 3) to compare the individual LDL-C of each patient both at baseline and at the end of follow-up to study the strength of association between the two: LDL-C correlation 0.47 (95% CI .34–.58; p < .001).
DiscussionThree out of 4 patients had a high CVR, so this group accounts for the vast majority; secondly, patients with a very high CVR account for 17%, and finally, the group with a moderate CVR accounts for less than 9% (Table 2). This implies that the vast majority of patients with DM2 in our study had a target of less than 70 mg/dl, and the lowest percentage had a target of less than 100 mg/dl. We consider this data to be of particular relevance since historically the target of 100 mg/dl has been assigned to patients with DM2 and we observed that in actual practice it is a target reserved for a minority of patients.
During the 4 years of follow-up, there was an increase in the percentage of patients meeting both individualised and unified LDL-C targets. Despite this increase in the percentage of controlled patients, the degree of control at the end of the study was much lower than desirable, with 51.3% meeting the unified target and only 21.57% meeting the individualised targets. For the latter, there were no significant differences between the different subgroups stratified by CVR. Although these results are not entirely comparable, we consider them to be inferior to those of a similar study conducted in China in patients with DM2. In this study, the target LDL-C < 70 mg/dl was set for patients with a history of cardiovascular disease and LDL-C < 100 for the rest, where the degree of control was 49,1%.10 In another study, conducted in Italy in patients with dyslipidaemia in primary care, only 16% met a target of less than 70 mg/dl and 45% met a target of less than 100 mg/dl.7 However, our results show higher control figures compared to another study conducted in Argentina in patients with DM2, in which only 13.3% of patients met their individualised target.8
Although there is no solid scientific evidence, it is known that patients with DM2 are hyporesponsive to statins because there is greater intestinal absorption of cholesterol.11 This may help to explain the degree of control of the subjects in our study, and those of the other study conducted in patients with DM28, which are lower than those published in other studies of patients with dyslipidaemia.7,11
As this was a study under conditions of routine clinical practice, this low compliance with the targets could be due to the therapeutic inertia of the professionals,12 low therapeutic adherence, which according to some studies is less than 60%,13 patient intolerance to lipid-lowering treatments, or that the targets are not appropriate in patients diagnosed with advanced diseases with a short life expectancy.14
Mean total cholesterol and LDL-C levels decreased during the follow-up period by 20.8 and 16.0 mg/dl, respectively. We believe that the new and more stringent LDL-C targets of the new ESC/ESA 2019 lipid guidelines are a factor that may have contributed significantly to the increase in the prescription of lipid-lowering drugs (Table 1) and, therefore, to the decrease in lipid levels. It could be thought that this decrease is also due to the ageing of the sample during follow-up, since from the age of 57, LDL-C levels begin to decrease physiologically.15 Although paradoxically patients younger than 65 years were more controlled in our study, with a 17% probability of being controlled compared to those older than 65 years, this could be due to a lower therapeutic inertia in younger patients.12
Something similar occurs in the patients who smoked in our study. We observed a higher percentage of smokers among the controlled patients, despite the fact that smokers are known to have higher cholesterol levels than the rest of the población.16 Smoking may lead to a higher perception of CVR, which could translate into an intensification of treatment promoted by the physician and/or greater compliance by the patient.
Males also had a lipid control advantage with a 14% higher probability of meeting their target. These results are in line with a 9-country multicentre study,17 which also found a gender disadvantage in women with dyslipidaemia, which according to the authors of the same study is attributed to a lower prevalence in women of high-risk coronary heart disease.
LDL-C values at baseline correlated moderately positively with those at the end of the study, so that each individual tends to have similar LDL-C values over time. Even so, only one in three patients meeting the individualised target remained controlled at the end of follow-up and half of those meeting the unified target. This indicates that the "fire and forget”18 strategy proposed for lipid control is not the most advisable for our population, as we observed that a large percentage of patients considered to be controlled are no longer controlled over time. Therefore, we consider it a better strategy to periodically monitor compliance with LDL-C targets.
When assessing the risk factors of a patient with DM2, it is not only LDL-C levels that must be addressed; it must be done from a multidisciplinary approach, seeking to meet the targets for blood pressure and glycaemic profile, smoking cessation, etc. It is known that the inability to achieve a target was associated with difficulty in achieving control of other risk factors, which further aggravates the riek.19 In our study we observed that in the group of patients who did not have off-target lipid levels there was a greater tendency not to control their blood pressure; however, we did not observe this trend in terms of achieving individualised HbA1c targets.
One of the main limitations of our study is the small sample size, which probably prevented us from obtaining other statistically significant results. One problem of observational studies is the lack of recording in the health history, especially of target organ damage (retinopathy and diabetic neuropathy), so that the percentage of patients with very high CVR is probably higher; this, together with other failures to record the variables observed, has been an important conditioning factor to be taken into account. Another limitation of our study lies in the fact that we did not consider 2 high urine albumin and GFR values over 3 months to classify patients as having microalbuminuria or chronic kidney disease, respectively, which may have led to an overestimation of these 2 entities.
A distinguishing feature of our study was the consideration of lipid control according to the individualised LDL-C target, unlike most of the studies reviewed in DM2, which indicate a single target of less than 100 mg/dl and 70 mg/dl, but not less than 55 mg/dl. The fact that this is a study conducted under conditions of routine clinical practice also adds much to the current literature. On the other hand, we have tried to avoid other types of biases that might have arisen if professionals had felt observed.
We consider it essential to study the reasons why treatment targets are not met, such as non-compliance and therapeutic inertia. This would allow us to design strategies to increase the percentage of patients who meet their treatment targets, thus reducing the possibility of cardiovascular events. It is also advisable to study the determinants that influence the factors that lead to non-compliance with these targets, such as female sex and patients over 65 years of age, in order to mitigate them as far as possible.
ConclusionsTo sum up, since the publication of the ESC/ESA 2019 lipid guideline we have observed a significant decrease in LDL-C levels in our cohort of patients with DM2. However, this decline has not been sufficient, with only one in 5 patients meeting their individualised LDL-C target, a result that is somewhat higher than in another study with similar characteristics. Male patients, those under 65 years of age and smokers had an advantage in meeting their target compared to the rest. Only one out of three patients who met their target at the start of the study were still controlled at the end of the study. The vast majority of our diabetic patients have a high CVR, so the predominant LDL-C target is below 70 mg/dl.
FinancingThis manuscript had no external funding.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Martín Enguix D, Hidalgo Rodríguez A, Sánchez Cambronero M, Aguirre Rodríguez JC. Aplicación de los objetivos individualizados definidos por la guía europea 2019 de lípidos en pacientes con diabetes tipo 2. Clin Investig Arterioscler. 2022;34:19–26.