Haptoglobin is a protein involved in the protection against oxidative damage caused by iron in haemoglobin. This protein is polymorphic, with 3 isomorphs prevalent in the population. The carriers of the Hp2-2 isoform have a lower antioxidant capacity and, in the population with diabetes, an increased risk of subclinical vascular disease and cardiovascular complications. The objective of this study was to evaluate whether this isomorphy is associated with an increased risk of carotid arteriosclerosis in subjects with and without diabetes, and free of cardiovascular disease.
Patients and methodsA study was conducted in a population between 45 and 74 years of age, randomly selected from the northwest area of Madrid. The participants were characterised in terms of their glycaemic status by oral glucose overload and the determination of the concentration of Hb1Ac. The haptoglobin phenotypes in all of them were determined by means of an immunoenzymatic assay, and the presence of carotid arteriosclerosis by ultrasound.
ResultsOf the 1256 participants included in the present analysis (mean age 61.6 ± 6 years, 41.8% males), the distribution of the isoforms of haptoglobin was as follows: Hp1-1: 13.3%, Hp1-2: 48.5%, and Hp2-2: 38.2%. In comparison with subjects Hp1-1 and Hp1-2, those with the Hp2-2 phenotype had a higher prevalence of dyslipidaemia (53.3% vs 43%; p < .0001) and arterial hypertension (39.2% vs. 32.2%, p = .012), and they more frequently received treatment with statins (31.5% vs 21.6%, p < .0001), and with antihypertensive agents (38.4% vs 30.8%, p = .006). The carriers of the Hp2-2 isoform had a higher prevalence of carotid plaques (OR: 1.35, 95% CI: 1.07–1.69, p = .011), with no differences in that prevalence as regards the glycaemic status. There were no differences in the intima-media thickness between the different phenotypes. The relationship of the Hp2-2 phenotype with the presence of plaques in the carotid was independent of age, gender, presence of risk factors (dyslipidaemia, hypertension and diabetes), the concentration of LDL-cholesterol, C-reactive protein and uric acid, blood pressure, and treatment with statins, and hypertensive drugs (OR: 1.31, 95% CI 1.01–1.70, p = .044).
ConclusionSubjects with the Hp2-2 phenotype of haptoglobin have a higher prevalence of carotid arteriosclerosis, which is independent of the presence of other cardiovascular risk factors and their glycaemic status.
La haptoglobina es una proteína implicada en la protección frente al daño oxidativo producido por el hierro de la hemoglobina. Esta proteína es polimórfica, con 3 isomorfas prevalentes en la población. Los portadores de la isoforma Hp2-2 tienen una menor capacidad antioxidante y, en la población con diabetes, un mayor riesgo de enfermedad vascular subclínica y de complicaciones cardiovasculares. Nuestro objetivo fue evaluar si dicha isomorfa se asocia con un mayor riesgo de arteriosclerosis carotidea en sujetos con y sin diabetes, libres de enfermedad cardiovascular.
Pacientes y MétodosEstudio realizado en una población de entre 45 y 74 años de edad, seleccionada aleatoriamente del área noroeste de Madrid. Los participantes fueron caracterizados en cuanto a su estatus glucémico mediante una sobrecarga oral de glucosa y la determinación de la concentración de HBA1c. A todos ellos se les determinó el fenotipo de la haptoglobina mediante un ensayo inmunoenzimático y la presencia de arteriosclerosis carotídea mediante ecografía.
ResultadosDe los 1256 participantes incluidos en el presente análisis (edad media 61,6 ± 6 años, 41,8% varones), la distribución de las isoformas de la haptoglobina fue la siguiente: Hp 1-1: 13,3%, Hp1-2: 48,5% y Hp2-2: 38,2%. En comparación con los sujetos Hp1-1 y Hp1-2, aquellos con el fenotipo Hp2-2 tuvieron una mayor prevalencia de dislipemia (53,3% vs 43%, p < 0,0001) e hipertensión arterial (39,2% vs 32,2%, p = 0,012), y recibieron con más frecuencia tratamiento con estatinas (31,5% vs 21,6%, p < 0,0001) y con antihipertensivos (38,4% vs 30,8%, p = 0,006). Los portadores de la isoforma Hp2-2 tuvieron una mayor prevalencia de placas carotideas (OR 1,35; IC95% 1,07–1,69, p = 0,011), sin diferencias en dicha prevalencia en función del estatus glucémico. No existieron diferencias en el grosor íntima-medio entre los diferentes fenotipos. La relación del fenotipo Hp2-2 con la presencia de placas en carótida fue independiente de la edad, sexo, presencia de factores de riesgo (dislipemia, hipertensión y diabetes), de la concentración de colesterol-LDL, proteína C reactiva y ácido úrico, de la presión arterial, y del tratamiento con estatinas y antihipertensivos (OR 1,31; IC95% 1,01–1,70, p = 0,044).
ConclusiónLos sujetos con el fenotipo Hp2-2 de la haptoglobina tienen una mayor prevalencia de arteriosclerosis carotidea, que es independiente de la presencia de otros factores de riesgo cardiovascular y de su estatus glucémico.
Haptoglobin is a glycoprotein the main biological function of which lies in its non-covalent binding to free haemoglobin to form a complex that will be subsequently cleared by the reticuloendothelial system through CD163-mediated endocytosis.1 Haptoglobin, in this way, prevents the potential oxidative damage of iron contained in the haemoglobin molecule.1 In the absence of this clearing mechanism, haemoglobin favours the production of free radicals and, subsequently, the oxidation of LDL cholesterol, an early mechanism in the development of arteriosclerosis.2 Similarly, it promotes the oxidation of HDL cholesterol, which leads to the creation of dysfunctional HDL.3 Haptoglobin can also bind to apolipoprotein A1 at the same binding site of the LCAT enzyme,4 which reduces the activity of the enzyme, altering reverse cholesterol transport and preventing the normal maturation of HDL particles.
The haptoglobin gene is located on the long arm of chromosome 16 and is polymorphic, with two codominant alleles, Hp1 and Hp2, whose products give rise to three isoforms, Hp1-1, Hp1-2 and Hp2-2, with frequencies of 16, 48 and 36%, respectively.5
Hp2-2 subjects have a lower antioxidant capacity than Hp1 carriers. This is because they have a lower plasma concentration of haptoglobin, because their haptoglobin has a lower affinity for haemoglobin, since the plasma clearance of the Hp-haemoglobin complex is slowed.5 This fact is more evident in patients with diabetes, who are subject to greater oxidative stress. In addition, in this population, Hp2-2 homozygotes have reduced CD163 expression and have difficulties in transferring from the haem to the haptoglobin as a result of haemoglobin glycation.6
These alterations have a translation in the symptoms. Several studies have shown that Hp2-2 subjects have an increased risk of cardiovascular complications, especially the diabetic population.1 However, there are few studies that have evaluated whether such a genotype is associated with an increased risk of subclinical vascular disease, and have been preferably performed in the diabetic population.7–9
Our study evaluates the presence of subclinical carotid disease in relation to the haptoglobin phenotype in subjects free of cardiovascular disease, with and without diabetes, randomly selected from a Spanish population.
Patients and methodsThe Screening PRE-diabetes and type 2 DIAbetes (SPREDIA-2) study is a prospective population cohort study conducted in the Autonomous Community of Madrid. The characteristics of this study and the selected population have been previously published.10 In summary, 1592 subjects between 45 and 74 years of age randomly selected from the population of the northwest area of Madrid were included. Participants were called on an empty stomach for the performance of lab work, an oral overload with 75 g of glucose, anthropometric measurements and the performance of a carotid ultrasound. Of the total participants, 1256 had no cardiovascular disease at the time of inclusion, had a carotid ultrasound, and had a reading of the haptoglobin phenotype, being included in the present analysis.
The diagnosis of prediabetes and diabetes was made according to the criteria of the ADA.11 Prediabetes was defined as not having diabetes but having a baseline glucose between 100 and 125 mg/dl (altered baseline glucose) or an A1C concentration of between 5.7 and 6.4%, or a blood glucose at 2 h from the OGTT between 140 to 199 mg/dl (glucose intolerance). Diabetes was defined as a previous diagnosis of diabetes, being treated with antidiabetic drugs or having a baseline blood glucose ≥126 mg/dl or a concentration of A1C ≥ 6.5% or a blood glucose level at 2 h after the OGTT ≥ 200 mg/dl. Subjects without altered baseline glucose, without glucose intolerance and with an A1C < 5.7% were considered normoglycaemic.
The haptoglobin phenotype (Hp1-1, Hp2-1 and Hp2-2) was determined qualitatively by an immunoenzymatic assay (VIT-A710-01 M Haptotype, Vitro S.A.),12 according to the protocol established by the trading house. The trial presented an intra-and intertrial accuracy of 1.8 and 10.6%, respectively.
A Doppler ultrasound was performed of both carotids with a 7.5 mHz probe (SonoSite MicroMaxx Ultrasound, SonoSite Inc., Bothell, Wash, USA). The presence of plaques throughout the extension of the common carotid artery, carotid bulb and internal and external carotid arteries was explored bilaterally. The existence of carotid plaques was defined as a focal thickening >1.5 mm or a thickening >50% of the value of the surrounding intima-media thickness (IMT).13 Images of the distal wall of the common carotid artery, 1 cm prior to the bifurcation, were obtained at three different viewing angles. The IMT was obtained by automated software (SonoSite, SonoCalc IMT Software, SonoSite Inc., Bothell, Wash, USA) as the average of the mean and maximum thickness measured in each of the six segments (three different angles on the right and left side).
Statistical analysisQuantitative variables are presented as mean and standard deviation. Qualitative variables, as percentages. Comparisons between the quantitative variables were made using the t-test, and those of the qualitative variables, using the chi-square test. A logistic regression analysis was performed using the "enter" method to assess the independent relationship of the haptoglobin phenotype with the presence of carotid plaques. Variables with p < 0.10 in the bivariate analysis and the clinically relevant ones were included in the model, trying to avoid overfitting. The magnitude of the associations was expressed as OR and 95% CI. The data were analysed using SPSS for Windows, version 9.0; IBM Corp, Armonk, New York, USA.
Ethical considerationsThe study was approved by the Clinical Research Ethics Committee of the Hospital Carlos III in Madrid, complying with the Declaration of Helsinki and international guidelines for epidemiological studies (Geneva, 1991). All participants signed an informed consent form.
ResultsA total of 1256 subjects were included in the analysis. The prevalence of Hp1-1, Hp1-2 and Hp2-2 phenotypes was 13.3% (n = 167), 48.5% (n = 609) and 38.2% (n = 480). Since the risk of cardiovascular complications has been associated with the Hp2-2 phenotype, subjects with Hp1-1 and Hp1-2 phenotypes were grouped for statistical calculations.
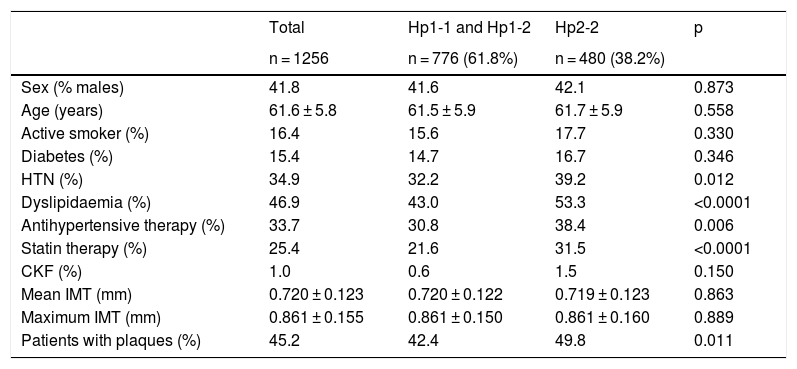
The characteristics of the population according to the haptoglobin phenotype can be seen in Tables 1 and 2. Subjects with the Hp2-2 phenotype had a higher prevalence of self-referred dyslipidaemia and hypertension. They also received antihypertensive and lipid-lowering therapy more frequently.
Characteristics of the population without known cardiovascular disease depending on the haptoglobin phenotype.
| Total | Hp1-1 and Hp1-2 | Hp2-2 | p | |
|---|---|---|---|---|
| n = 1256 | n = 776 (61.8%) | n = 480 (38.2%) | ||
| Sex (% males) | 41.8 | 41.6 | 42.1 | 0.873 |
| Age (years) | 61.6 ± 5.8 | 61.5 ± 5.9 | 61.7 ± 5.9 | 0.558 |
| Active smoker (%) | 16.4 | 15.6 | 17.7 | 0.330 |
| Diabetes (%) | 15.4 | 14.7 | 16.7 | 0.346 |
| HTN (%) | 34.9 | 32.2 | 39.2 | 0.012 |
| Dyslipidaemia (%) | 46.9 | 43.0 | 53.3 | <0.0001 |
| Antihypertensive therapy (%) | 33.7 | 30.8 | 38.4 | 0.006 |
| Statin therapy (%) | 25.4 | 21.6 | 31.5 | <0.0001 |
| CKF (%) | 1.0 | 0.6 | 1.5 | 0.150 |
| Mean IMT (mm) | 0.720 ± 0.123 | 0.720 ± 0.122 | 0.719 ± 0.123 | 0.863 |
| Maximum IMT (mm) | 0.861 ± 0.155 | 0.861 ± 0.150 | 0.861 ± 0.160 | 0.889 |
| Patients with plaques (%) | 45.2 | 42.4 | 49.8 | 0.011 |
Biochemical and anthropometric values in 1256 participants without known cardiovascular disease on the basis of the haptoglobin phenotype.
| Total | Hp1-1 and Hp1-2 | Hp2-2 | p | |
|---|---|---|---|---|
| n = 1256 | n = 776 (61.8%) | n = 480 (38.2%) | ||
| Glucose (mg/dl) | 105 ± 19 | 104 ± 17 | 106 ± 20 | 0.068 |
| Hb1Ac (%) | 5.8 ± 0.6 | 5.7 ± 0.5 | 5.8 ± 0.6 | 0.201 |
| Total cholesterol (mg/dl) | 209 ± 37 | 209 ± 35 | 208 ± 39 | 0.697 |
| LDL cholesterol (mg/dl) | 134 ± 33 | 134 ± 32 | 133 ± 34 | 0.543 |
| HDL cholesterol (mg/dl) | 55 ± 15 | 54 ± 15 | 55 ± 15 | 0.751 |
| Triglycerides (mg/dl) | 103 ± 66 | 102 ± 70 | 103 ± 58 | 0.826 |
| Uric acid (mg/dl) | 5.2 ± 1.3 | 5.2 ± 1.3 | 5.3 ± 1.2 | 0.099 |
| C-reactive protein | 5.2 ± 6.4 | 5.4 ± 6.8 | 4.8 ± 5.6 | 0.081 |
| Body mass index (kg/m2) | 28.3 ± 4.7 | 28.3 ± 4.7 | 28.4 ± 4.7 | 0.609 |
| Waist circumference (cm) | 95 ± 12 | 95 ± 12 | 95 ± 12 | 0.390 |
| Systolic blood pressure (mmHg) | 125 ± 17 | 124 ± 17 | 126 ± 17 | 0.111 |
| Diastolic blood pressure (mmHg) | 77 ± 10 | 77 ± 10 | 78 ± 10 | 0.188 |
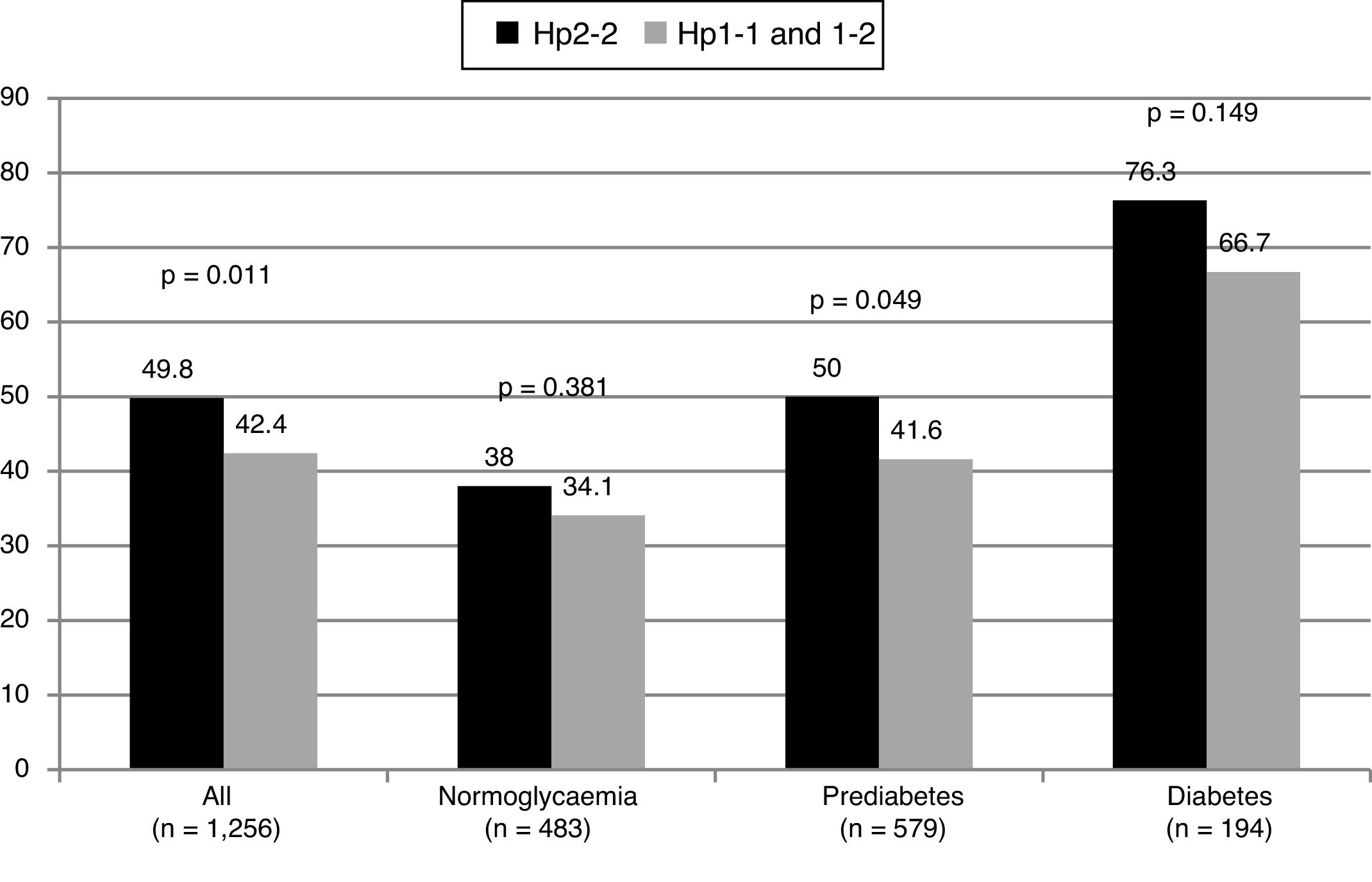
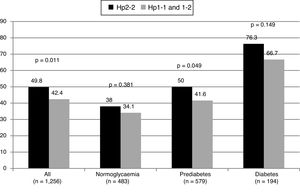
Subjects with the Hp2-2 phenotype had a higher prevalence of carotid plaques (OR: 1.35; 95% CI: 1.07–1.69; p = 0.011). No differences were observed in the common carotid IMT between the groups. The proportion of patients with plaques based on their glycaemic status can be seen in Fig. 1. The OR for the presence of plaques was similar in patients with and without diabetes (OR: 1.60; 95% CI: 0.84–3.06; p = 0.151 in diabetics, and OR: 1.30; 95% CI: 1.01–1.67; p = 0.044 in non-diabetics).
To establish whether the association between the haptoglobin phenotype and the presence of carotid plaques was independent of cardiovascular risk factors, we performed a logistic regression analysis adjusting for clinically relevant factors (age, sex, presence of diabetes) and factors associated with the haptoglobin phenotype (history of dyslipidaemia, history of hypertension, statin therapy, antihypertensive therapy, systolic blood pressure and uric acid concentration, CRP and LDL cholesterol), maintaining the relationship (OR: 1.31; 95% CI: 1.01–1.70, p = 0.044).
DiscussionOur results show that the subjects with the Hp2-2 haptoglobin phenotype have a higher prevalence of carotid arteriosclerosis than Hp1-1 and Hp1-2 subjects. These subjects also have a higher self-referred prevalence of dyslipidaemia and hypertension and, in parallel, greater use of statins and antihypertensives. However, the association between Hp2-2 phenotype and the presence of carotid plaques was independent of dyslipidaemia and hypertension, as demonstrated in the multivariate analysis.
Several studies have shown that the rate of cardiovascular complications is higher in Hp2-2 subjects. Although this fact has been mostly associated with populations with diabetes, it has also been observed in the general population.14,15 Despite this, not all studies are in agreement, since in some of them this relationship has not been found,16 and in others the results differ between patients with and without diabetes, suggesting that diabetic subjects carrying the Hp2-2 genotype could have less cardiovascular disease compared to a higher prevalence in H2-2 subjects without diabetes.17
The association between the haptoglobin phenotype and the presence of subclinical carotid disease has been evaluated in a very small number of studies and mostly in populations with diabetes, usually with a limited sample size. In a study conducted on 64-year-old women, the carriers of the Hp2-2 genotype had a higher prevalence of carotid plaques and a higher IMT in the carotid bulb, with no differences observed in the IMT of the common carotid artery.8 These associations, however, were only observed in women with long-standing diabetes. Haptoglobin phenotype did not influence carotid arteriosclerosis in participants without diabetes or in those with newly-diagnosed diabetes.8 Other studies have shown that subjects with diabetes and a Hp2-2 phenotype also have a greater IMT than subjects with an Hp1 allele.7,9 Our data extend the association between the Hp2-2 phenotype and subclinical carotid disease by demonstrating that it is present in both patients with and without diabetes and that it is independent of glycaemic status.
Our data also indicate an association between the presence of dyslipidaemia referred by the patient him/herself, statin therapy and the Hp2-2 phenotype. The relationship between Hp2-2 and cholesterol levels has barely been explored, although some large epidemiological studies have also observed that the Hp2 allele is associated with higher levels of total cholesterol and LDL cholesterol.18,19 Not all studies, however, have found these findings, perhaps because most of them are performed in people with diabetes, many of them already being treated with statins regardless of their cholesterol levels. Haptoglobin can interfere with lipoprotein metabolism by binding to apolipoprotein E20 and prevent its oxidation.21 The oxidation of apoE hinders lipid clearance.22 The higher affinity of Hp1 to bind to apoE and prevent oxidation would favour these subjects having a lower concentration of plasma cholesterol.
Similarly, our data indicate that subjects with a Hp2-2 phenotype have a higher self-referred prevalence of hypertension and receive antihypertensive therapy more frequently. Again, there are no data in this regard in the literature, although some studies have shown that these patients have more complications derived from hypertension and forms of hypertension more resistant to treatment.23,24
In any case, the higher prevalence of carotid plaques has been demonstrated independent of the presence of dyslipidaemia or hypertension and of the treatments for these risk factors. It has been postulated that the arteriosclerosis of these patients derives from the increased oxidation of LDL particles and alterations in the metabolism of HDL. In fact, several intervention studies have shown that antioxidant therapy with vitamin E in patients with diabetes is associated with a lower risk of cardiovascular complications.25 However, studies with a larger sample size that demonstrate that the Hp2-2 phenotype is associated with an increased risk of future cardiovascular complications, and that its determination improves the prediction of the risk obtained from classical risk factors are needed.
Our study has limitations from its cross-sectional design, which prevents drawing inferences about causation. However, it has a large sample size, participants have been randomly selected from the population and the haptoglobin phenotype was determined without knowing the situation of carotid arteriosclerosis.
In summary, the population with a haptoglobin Hp2-2 phenotype has a higher prevalence of dyslipidaemia and hypertension, and they are more frequently treated with statins and antihypertensives. These subjects also have a higher prevalence of carotid plaques, independent of the aforementioned risk factors.
FundingThe study has been partially funded by an unconditional grant from Novo Nordisk and the Fundación para el Fomento y Desarrollo de la Investigación Clínica [Foundation for the Promotion and Development of Clinical Research] (FYDIC).
Conflicts of interestThe authors state that this manuscript does not imply any conflict of interest with any of the authors.
The following authors have collaborated in the collection and analysis of data: Carmen de Burgos-Lunar, Paloma Gómez-Campelo, Belén Fernández Puntero, Luis Montesano Sánchez, David Vicent López, Pedro J. Fernández García, Jesús Castro Toro and Pedro Patrón Barandío.
We appreciate the collaboration of all the doctors of the ESPREDIA group: Concepción Aguilera Linde (C.S. Ciudad de los Periodistas), Álvaro R. Aguirre de Carcer Escolano (C.S. La Ventilla), Patricio Alonso Sacristán (C.S. Ciudad de los Periodistas), M. Jesús Álvarez Otero (C.S. Dr. Castroviejo), Paloma Arribas Pérez (C.S. Santa Hortensia), María Luisa Asensio Ruiz (C.S. Fuentelarreina), Pablo Astorga Díaz (C.S. Barrio Pilar), Begoña Berriatua Ena (C.S. Dr. Castroviejo), Ana Isabel Bezos Varela (C.S. José Marvá), María José Calatrava Triguero (C.S. Ciudad Jardín), Carlos Casanova García (C.S. Barrio Pilar), Ángeles Conde Llorente (C.S. Barrio Pilar), Concepción Díaz Laso (C.S. Fuentelarreina), Emilia Elviro García (C.S. Ciudad de los Periodistas), Orlando Enríquez Dueñas (C.S. Fuentelarreina), María Isabel Ferrer Zapata (C.S. El Greco), Froilán Antuña (C.S. Ciudad de los Periodistas), María Isabel García Lazaro (C.S. Ciudad de los Periodistas), M. Teresa Gómez Rodríguez (C.S. Barrio Pilar), África Gómez Lucena (C.S. La Ventilla), Francisco Herrero Hernández (C.S. La Ventilla), Rosa Julián Viñals (C.S. Dr. Castroviejo), Gerardo López Ruiz Ogarrio (C.S. Barrio Pilar), M. Carmen Lumbreras Manzano (C.S. José Marvá), Sonsoles Paloma Luquero López (C.S. Ciudad de los Periodistas), Ana Martínez Cabrera Peláez (C.S. Barrio Pilar), Montserrat Nieto Candenas (C.S. La Ventilla), María Alejandra Rabanal Carrera (C.S. Barrio Pilar), Ángel Castellanos Rodríguez (C.S. Ciudad de los Periodistas), Ana López Castellanos (C.S. La Ventilla), Milagros Velázquez García (C.S. Barrio Pilar) and Margarita Ruiz Pacheco (C.S. Dr. Castroviejo).
Please cite this article as: Mostaza JM, de Dios O, Lahoz C, Arribas M, Pérez Arroyo A, Salinero-Fort MA, et al.; en representación de los investigadores del estudio ESPREDIA. Fenotipo de la haptoglobina y presencia de enfermedad vascular subclínica: estudio poblacional. Clín Investig Arterioscler. 2020. https://doi.org/10.1016/j.arteri.2019.03.007