Extracellular matrix (ECM) deposit is the result of a physiological response to an insult. When this becomes chronic, changes occur in the composition, structure and accumulation of components in the ECM giving rise to fibrosis, which plays a direct role in adverse remodelling. On a cardiovascular level, fibrosis alters the structure of the myocardial tissue or of the vascular wall, conferring rigidity, loss of elasticity and, ultimately, compromising the function of the tissue or organ affected. Fibrosis occurs when the synthesis of fibrillar collagen (types I and III), a fundamental component of the ECM, predominates over degradation. In hypertensive patients, rather than an increase in the collagen content, an alteration in the assembly or cross-linking of its fibres has been described, contributing to myocardial rigidity and ultimately resulting in heart failure.1 Thus, fibrosis is a determining factor in vascular senescence.2
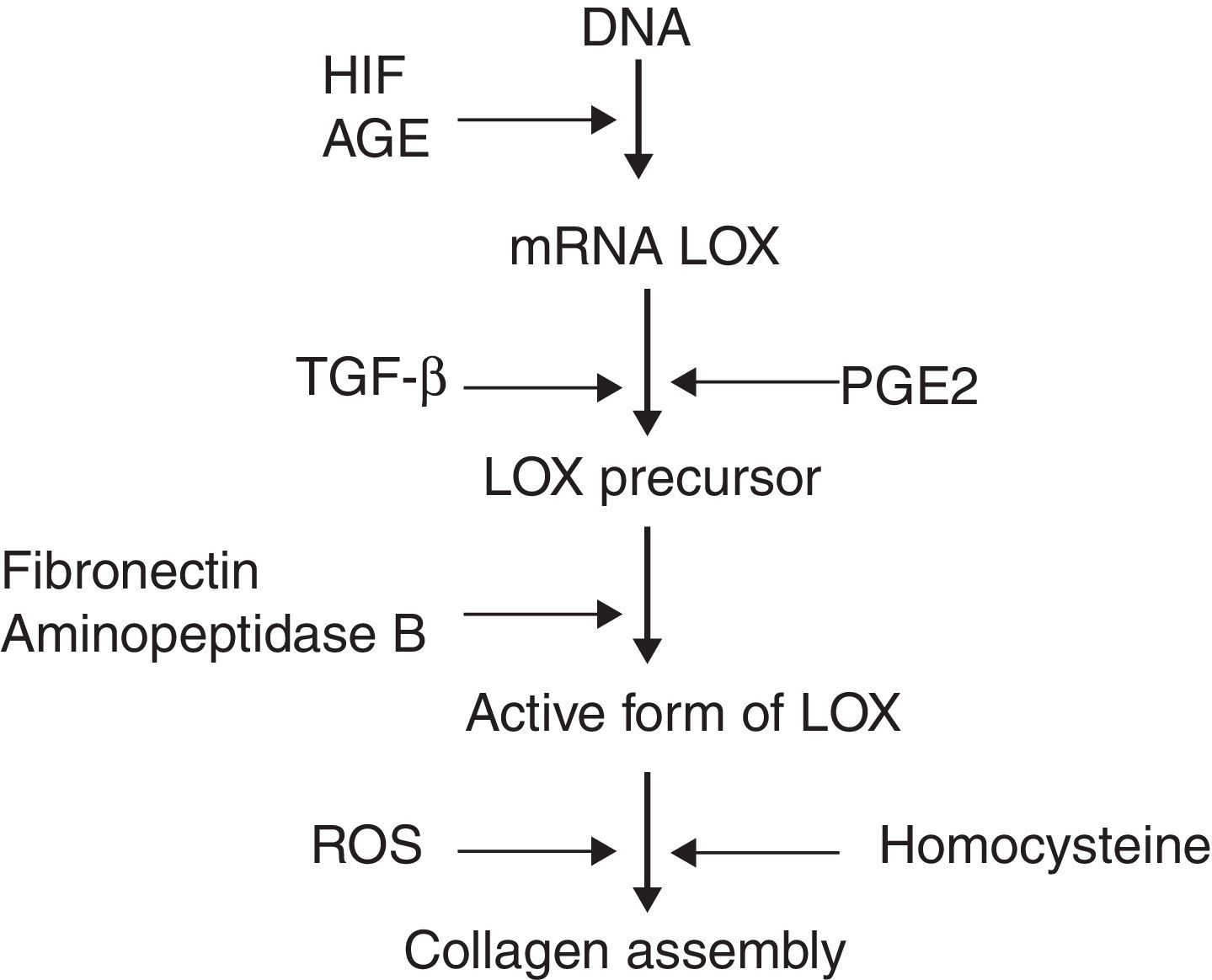
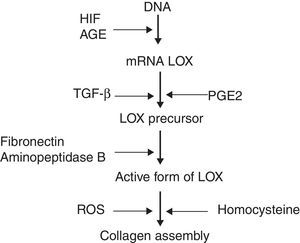
Recent studies have identified molecules which play a role in the processing of collagen. Prominent among these is the enzyme lysyl oxidase (LOX), a copper-dependent amine oxidase which participates in the covalent assembly of the collagen and elastin fibres in the ECM. Additionally, LOX controls the expression of genes involved in cell migration and differentiation, and also modulates the biological activity of growth factors.3 LOX can be regulated on three levels (Fig. 1): synthesis of the precursor by fibroblasts and myofibroblasts, transformation of the precursor into the mature enzyme, and direct stimulation of enzyme activity. It has been observed that the increase of reactive oxygen species (ROS) related to oxidative stress stimulates LOX activity in fibroblast cultures.
There is clinical and experimental evidence suggesting that the deregulation of LOX could be an important factor in the development of cardiovascular diseases.3 The collagen assembly impacts the quality of LOX deregulation and affects the function of the damaged tissue or organ. One important cause of heart failure, which has a five-year mortality rate of 50%, is adverse tissue remodelling accompanied by interstitial fibrosis. There are currently no effective therapies capable of halting these processes. On a myocardial level, the degree of cross-linking determines the rigidity, thickness and resistance of the degradation, all of which have a bearing on the functional properties of the left ventricle.3 In patients with hypersensitive cardiopathy and chronic heart failure, an increase in LOX expression in the fibrotic myocardium has been observed, suggesting that the overexpression of LOX may significantly compromise cardiac function.4 There has also been shown to be a correlation between this enzyme and circulating biomarkers for heart failure. Finally, LOX has been related with vascular risk factors, with components of metabolic syndrome and with pulmonary arterial hypertension.5,6
In the article by Varona et al. in this edition of Clin Invest Arterioscler,7 the role of LOX overexpression on the ECM structure and on vascular oxidative stress is analysed in a murine model. To this end, the authors used transgenic mice which overexpress LOX, conducting a quantitative analysis of the internal elastic lamina, immunohistochemical studies, determination of NADPH oxidase and H2O2 and cultures with smooth muscle cells. The results show that transgenic mice present with alterations in the vascular collagen assembly and in the elastin structure, as well as with increased oxidative stress, detected through an increase in H2O2. Accordingly, the vascular overexpression of LOX induced the expression of elastogenic proteins in smooth muscle cells. It was also observed in vitro that catalase, an anti-oxidant agent, mitigated elastin fibre organisation. Taken together, the results of this interesting experimental study evince that LOX plays a fundamental role in vascular remodelling, mediated in part by oxidative stress and by an effect on the smooth muscle cells. A subsequent preclinical approach would be interesting with a view to analysing whether the inhibition of LOX over both physiopathological processes, which have a significant impact on vascular homeostasis, could be applicable to cardiovascular diseases characterised by an increase in this enzyme. In this regard, a recent murine model study found that the inhibition of LOX with antibodies, or by means of genetic manipulation, reduced stress-induced cardiac fibrosis, prevented dilatation and improved the heart's systolic and diastolic function.8 On a clinical level, the chronic administration of torasemide was effective in mitigating LOX expression, collagen assembly and rigidity in the left ventricle in patients with heart failure.4
In recent years, in addition to LOX, new candidates which help in the deregulation of collagen and fibrosis have emerged.9 Salient among these are Cardiotrophin-1, Galectin-3, NADPH oxidases NOX2 and 4, neutrophil gelatinase-associated lipocalin (NGAL) and microRNAs, since their excess or deficiency may result in alterations in the fibrillar collagen. It is hoped that in the coming years it will be possible to develop agents that, by selectively modulating the action of these molecules, will have enormous anti-fibrotic potential which can be transferred to the clinical setting in the short term.