Familial Hypercholesterolemia (FH) is an autosomal dominant disease with an estimated prevalence between 1/200–250. It is under-treated and underdiagnosed. Massive data screening can increase the detection of patients with FH.
MethodsStudy population: Residents in the health coverage area (N: 195.000 inhabitants) and with at least one determination of cholesterol linked to low-density lipoproteins (LDLC) carried out between January 1, 2010 and December 30, 2019. The highest LDL-C values were selected. Exclusion criteria: nephrotic syndrome, hypothyroidism, Hypothyroid treatment or triglycerides > 400 mg/dL. Seven algorithms suggestive of Familial Hypercholesterolemia Phenotype (HF-P) were analyzed, selecting the most efficient algorithm that could easily be translated into clinical practice.
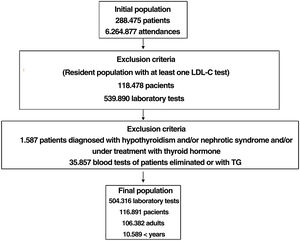
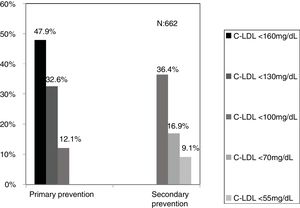
ResultsBased on 6.264.877 assistances and 288.475 patients, after applying the inclusionexclusion criteria, 504.316 tests were included, corresponding to 106.382 adults and 10.509 < 18 years. The selected algorithm presented a prevalence of 0.62%. 840 patients with HF-P were detected, 55.8% being women and 178 < 18 years old, 9.3% had a history of cardiovascular disease (CVD) and 16.4% had died. 65% of the patients in primary prevention had LDL-C values > 130 mg/dL and 83% in secondary prevention values > 70 mg/dL. A ratio of 7.64 (1–18) patients with HF-P per analytical requesting physician was obtained.
ConclusionsMassive data screening and patient profiling are effective tools and easily applicable in clinical practice for the detection of patients with FH.
La Hipercolesterolemia Familiar (HF) es una enfermedad autósómica dominante con una prevalencia estimada entre 1/200–250. Se encuentra infratratada e infradiagnosticada. El rastreo masivo de datos puede incrementar la detección de pacientes con HF.
MétodosPoblación a estudio: Residentes en la zona sanitaria de cobertura (N: 195.000 habitantes) y con al menos una determinación de colesterol ligado a lipoproteínas de baja densidad (C-LDL) realizada entre el 1 de Enero de 2010 y el 30 de Diciembre de 2019. Se seleccionaron los valores más altos de C-LDL. Criterios de exclusión: síndrome nefrótico, hipotiroidismo, tratamiento hipotiroideo o triglicéridos > 400 mg/dL. Se analizaron 7 algoritmos sugestivos de fenotipo de Hipercolesterolemia Familiar (FHF). Se seleccionó el algoritmo más eficaz y de fácil traslación a la práctica clínica.
ResultadosPartiendo de 6.264.877 asistencias y 288.475 pacientes tras aplicar los criterios de inclusión-exclusión se incluyeron 504.316 analíticas correspondiendo a 106.382 adultos y 10.509 < 18 años.El algoritmo seleccionado presentó una prevalencia de 0.62%.Se detectaron 840 pacientes con fenotipo de Hipercolestereolemia Familiar (FHF) siendo el 55.8% mujeres y 178 < 18 años, El 9.3% tenían antecedentes de enfermedad cardio-vascular (ECV) y 16.4% habían fallecido.El 65% de los pacientes en prevención primaria presentaron valores de C-LDL > 130 mg/dL y el 83% en prevención secundaria valores > 70 mg/dL.Se obtuvo una ratio de 7.64 (1–18) pacientes con HF-P por médico solicitante de analítica.
ConclusionesEl rastreo masivo de datos y el perfilado de pacientes son herramientas eficaces y fácilmente aplicables en práctica clínica para la detección de pacientes con HF.
Familial hypercholesterolaemia (FH) is the most common autosomal dominant disease associated with cardiovascular disease (CVD).1 Its estimated prevalence is 1/313 (1/250−1/397),1 which is approximately 34 million patients worldwide. Its prevalence in Spain is around 1/282 for genetically defined FH2 and 1/192 for the clinical FH phenotype3 (FH-P), which means that there are between 166,000 and 244,000 patients with FH in Spain, of whom only 12%–15% are diagnosed. In patients with FH not on lipid-lowering treatment (LLT), 50% of males will develop heart disease (HD) before the age of 50 and 30% of females before the age of 60.4 It is estimated that 1/31 patients with HD and 1/15 patients with premature HD are affected by FH.5 Patients with FH not only develop more premature CVD compared to the general population, but also more diffuse heart disease, with a higher prevalence of ST-elevation infarction, greater polyvascular involvement6 and twice the recurrence of ACS in the first year following the event, and have a worse prognosis.7 A study of the Dyslipidaemia registry of the Spanish Atherosclerosis Society shows that early initiation of LLT and for a duration of at least five years considerably reduces the risk of CVD in patients with FH.8 It is estimated that for every 1000 treated patients aged 35–85 years with FH, 101 cardiovascular events would be avoided, which if extrapolated to the European population would mean avoiding some 210,000 cardiovascular events.9 The SAFEHEART registry has shown that early initiation of LLT and a duration of at least five years considerably reduces the risk of CVD in patients with FH. The SAFEHEART registry has shown that only 3.4% of patients achieve the target of LDL-C < 100 mg/dL.10 In our setting, only 3% of patients with FH-P who do not achieve therapeutic targets, despite being on optimal LLT, are treated with proprotein convertase subtilisin/kexin type 9 (iPCSK9) inhibitors.11
The World Heart Federation has recently highlighted HF as a priority public health problem, requiring global action.12 The current ability to perform mass data screening in electronic health records (EHRs) could be an excellent opportunity to increase the detection of patients with HF.
Material and methodsDescriptive study using mass EHR analysis comparing different algorithms for detecting patients with FH-P.
Objective of the study: To evaluate the diagnostic efficacy of different algorithms for the detection of FH-P through screening of EHRs13–19 (Table 1). To describe the clinical characteristics of patients detected by an algorithm that is effective, easy to translate to clinical practice and approximates the prevalence of FH-P described in the recent systematic review by Hu et al. .31 (95% confidence interval [CI] .20–.44) in adults20 and .46% (95% CI .41–.52) in children under 18 years of age.3
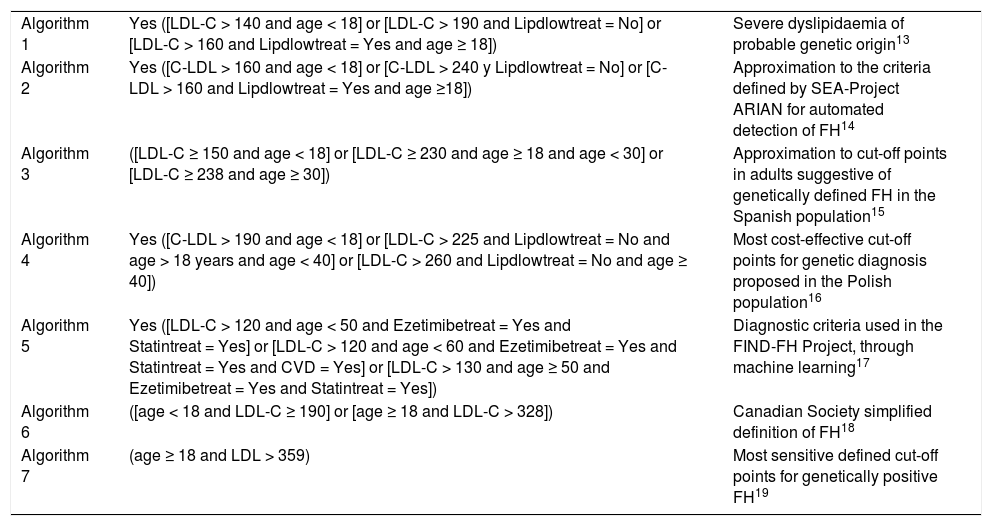
Defined search algorithms for detecting patients with FH phenotype.
| Algorithm 1 | Yes ([LDL-C > 140 and age < 18] or [LDL-C > 190 and Lipdlowtreat = No] or [LDL-C > 160 and Lipdlowtreat = Yes and age ≥ 18]) | Severe dyslipidaemia of probable genetic origin13 |
| Algorithm 2 | Yes ([C-LDL > 160 and age < 18] or [C-LDL > 240 y Lipdlowtreat = No] or [C-LDL > 160 and Lipdlowtreat = Yes and age ≥18]) | Approximation to the criteria defined by SEA-Project ARIAN for automated detection of FH14 |
| Algorithm 3 | ([LDL-C ≥ 150 and age < 18] or [LDL-C ≥ 230 and age ≥ 18 and age < 30] or [LDL-C ≥ 238 and age ≥ 30]) | Approximation to cut-off points in adults suggestive of genetically defined FH in the Spanish population15 |
| Algorithm 4 | Yes ([C-LDL > 190 and age < 18] or [LDL-C > 225 and Lipdlowtreat = No and age > 18 years and age < 40] or [LDL-C > 260 and Lipdlowtreat = No and age ≥ 40]) | Most cost-effective cut-off points for genetic diagnosis proposed in the Polish population16 |
| Algorithm 5 | Yes ([LDL-C > 120 and age < 50 and Ezetimibetreat = Yes and Statintreat = Yes] or [LDL-C > 120 and age < 60 and Ezetimibetreat = Yes and Statintreat = Yes and CVD = Yes] or [LDL-C > 130 and age ≥ 50 and Ezetimibetreat = Yes and Statintreat = Yes]) | Diagnostic criteria used in the FIND-FH Project, through machine learning17 |
| Algorithm 6 | ([age < 18 and LDL-C ≥ 190] or [age ≥ 18 and LDL-C > 328]) | Canadian Society simplified definition of FH18 |
| Algorithm 7 | (age ≥ 18 and LDL > 359) | Most sensitive defined cut-off points for genetically positive FH19 |
CVD: cardiovascular disease; Ezetimibetreat: Ezetimibe treatment; FH: familiar hypercholestereolaemia; LDL-C: low-density lipoprotein cholesterol; Lipdlowtreat: lipid-lowering treatment; Statintreat: Statin treatment.
Inclusion criteria: Residents in the catchment area, with a census population of 195,000 inhabitants, for whom at least one laboratory test of low-density lipoprotein cholesterol (LDL-C) was available, performed in a hospital, outpatient, or social healthcare setting) between 1 January 2010 and 30 December 2019. When more than one blood test was available, the on with the highest LDL-C value was selected. Patients under LLT were analysed qualitatively (under LLT or without LLT), according to the inclusion criteria of the selected algorithm. Exclusion criteria: diagnosis of nephrotic syndrome, hypothyroidism, treatment with thyroid hormone or triglycerides (TG) > 400 mg/dL.
The clinical data assessed were sex, age, total cholesterol (TC), HDL-cholesterol (HDL-C), TG, LDL-C, LLT prescription, health problems associated with the patient recorded in primary care and hospital discharge diagnoses, admissions for CVD and cardiovascular causes of death. Internal validation of data quality was performed by analysis of 25 randomly selected health records.
Statistical analysisThe quantitative data are presented with mean and standard deviation (SD). Categorical variables are expressed as percentages. Differences between groups will be analysed using the Student's t-test for continuous variables with estimated reduction of degrees of freedom, in case of non-homogeneity of variances, and the χ2 test for categorical variables. The 95% confidence intervals of proportions and means are also presented. A P-value <.05 is considered statistically significant. SAS Enterprise Guide v7.15 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis.
Ethical considerationsThe data analysis was performed under current legislation (Regulation [EU] 2016/679 of the European Parliament and of the European Council of 27 April 2016 on Data Protection [GDPR]).21 A pseudonymised database was created with a working methodology involving two different teams, one overseeing the data screening and the other in charge of drawing up the list of patients per doctor. A member of the team with expertise in bioinformatics who was not involved in preparing the project, or in the detailed analysis, extracted and prepared the data matrix. The study was rated low risk in the data protection impact assessment. The study protocol was approved by the reference clinical research ethics committee, including approval of the informed consent waiver for the FH-P patient screening phase.
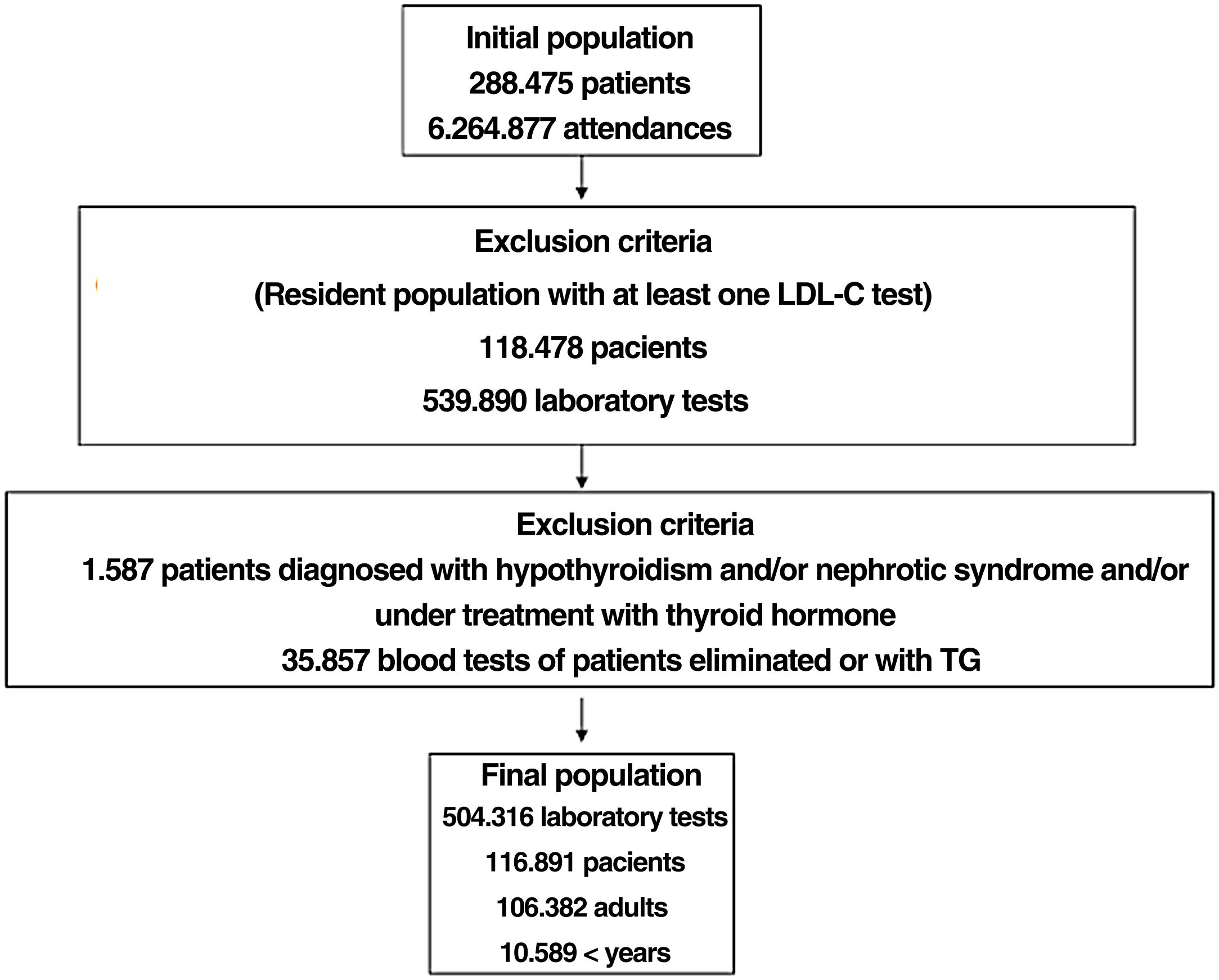
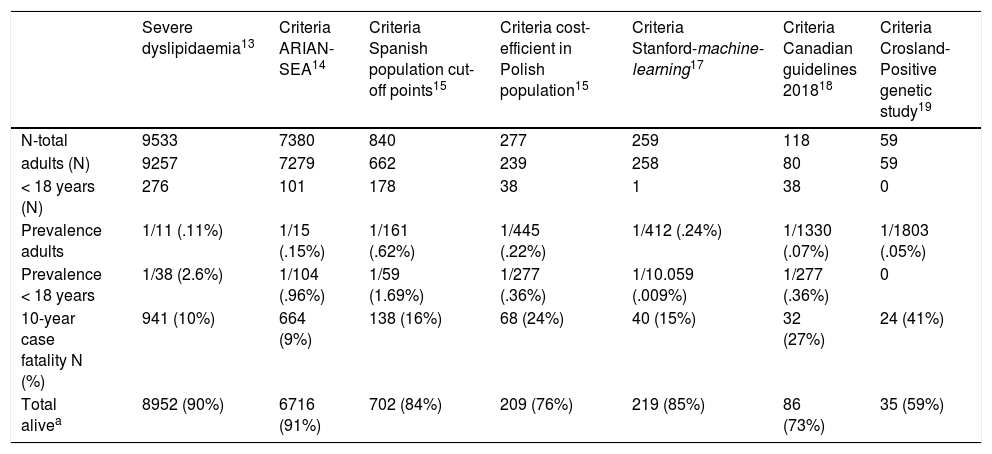
ResultsA total of 6,264,877 clinical attendances corresponding to 288,475 patients were assessed, including residents and temporary or transient population. After applying the inclusion-exclusion criteria, the final sample included in the study was 504,316 biochemical analyses corresponding to 116,891 patients, 106,382 adults and 10,509 < 18 years (Fig. 1). The prevalence of FH-P and 10-year case-fatality, according to the different algorithms used, is shown in Table 2.
Prevalence of HF-P and 10-year case-fatality based on different search algorithms.
| Severe dyslipidaemia13 | Criteria ARIAN-SEA14 | Criteria Spanish population cut-off points15 | Criteria cost-efficient in Polish population15 | Criteria Stanford-machine-learning17 | Criteria Canadian guidelines 201818 | Criteria Crosland-Positive genetic study19 | |
|---|---|---|---|---|---|---|---|
| N-total | 9533 | 7380 | 840 | 277 | 259 | 118 | 59 |
| adults (N) | 9257 | 7279 | 662 | 239 | 258 | 80 | 59 |
| < 18 years (N) | 276 | 101 | 178 | 38 | 1 | 38 | 0 |
| Prevalence adults | 1/11 (.11%) | 1/15 (.15%) | 1/161 (.62%) | 1/445 (.22%) | 1/412 (.24%) | 1/1330 (.07%) | 1/1803 (.05%) |
| Prevalence < 18 years | 1/38 (2.6%) | 1/104 (.96%) | 1/59 (1.69%) | 1/277 (.36%) | 1/10.059 (.009%) | 1/277 (.36%) | 0 |
| 10-year case fatality N (%) | 941 (10%) | 664 (9%) | 138 (16%) | 68 (24%) | 40 (15%) | 32 (27%) | 24 (41%) |
| Total alivea | 8952 (90%) | 6716 (91%) | 702 (84%) | 209 (76%) | 219 (85%) | 86 (73%) | 35 (59%) |
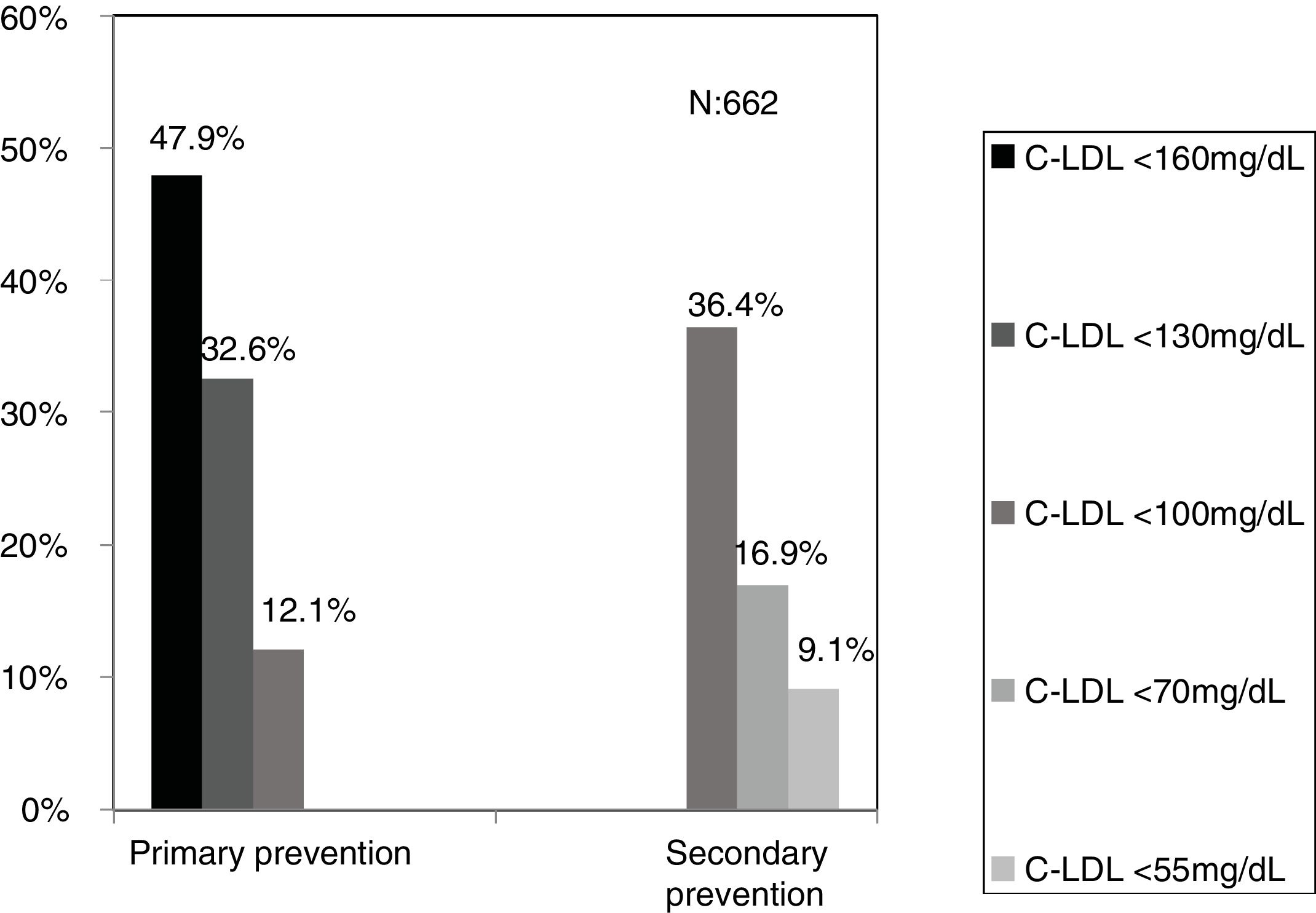
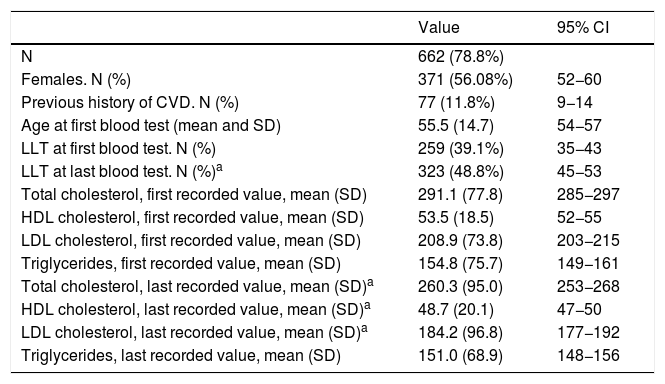
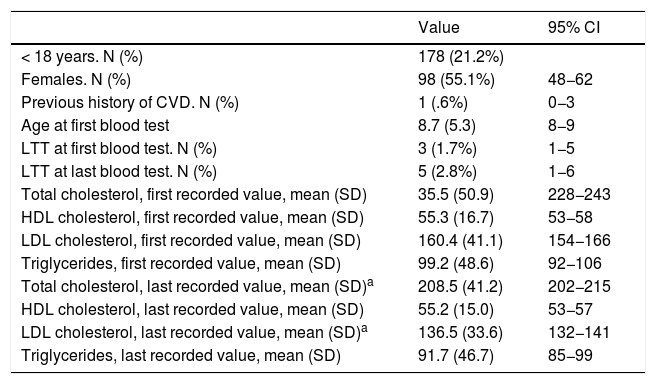
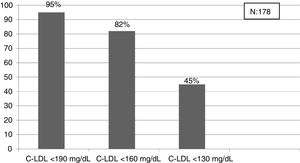
The selected algorithm was based on the cut-off points of LDL-C suggestive of genetic FH in the Spanish population (LDL ≥ 150 and age <18 or LDL ≥ 230 and age ≥ 18 < 30) or LDL ≥ 238 and age ≥30).15 This algorithm detected a .61% prevalence of FH-P. According to this criterion, 840 patients with FH-P were detected, of whom 178 were younger than 18 years. Fifty-five point eight percent were female and 78 (9.3%) had a history of CVD (Table 3). Of the population, 16.4% (n = 138) died during the 10-year follow-up. Of the total patients with FH-P, 259 (39%) adults were on LLT and 38 patients < 18 years with LDL-C > 190 mg/dL, of whom only seven were on LLT and only one was receiving statins plus ezetimibe. The number of blood tests performed per patient was 7.4 (1–43). The mean LDL-C in the blood test closest to 31 December 2019 was 184.2 (96.8) and 136.5 (33.6) mg/dL for adults and those <18 years, respectively. It was observed that the mean LDL-C decreased significantly between the first and the last recorded blood tests (Tables 3 and 4). Of the patients in primary prevention, 65% had LDL-C values > 130 mg/dL and 83% of those in secondary prevention had values > 70 mg/dL (Fig. 2). Of those aged under 18 years of age, 55% had values < 130 mg/dL (Fig. 3). Only nine patients had a coded diagnosis of FH on clinical discharge (ICD-10:E78.01), available since January 2018 in Catalonia. The mean number of patients with FH-P per physician requesting blood tests was 7.64 (1–18). There was 100% agreement in the internal validation process.
Characteristics of the adult population selected based on the LDL-C cut-off points defined in the Spanish population for genetically defined FH.
| Value | 95% CI | |
|---|---|---|
| N | 662 (78.8%) | |
| Females. N (%) | 371 (56.08%) | 52−60 |
| Previous history of CVD. N (%) | 77 (11.8%) | 9−14 |
| Age at first blood test (mean and SD) | 55.5 (14.7) | 54−57 |
| LLT at first blood test. N (%) | 259 (39.1%) | 35−43 |
| LLT at last blood test. N (%)a | 323 (48.8%) | 45−53 |
| Total cholesterol, first recorded value, mean (SD) | 291.1 (77.8) | 285−297 |
| HDL cholesterol, first recorded value, mean (SD) | 53.5 (18.5) | 52−55 |
| LDL cholesterol, first recorded value, mean (SD) | 208.9 (73.8) | 203−215 |
| Triglycerides, first recorded value, mean (SD) | 154.8 (75.7) | 149−161 |
| Total cholesterol, last recorded value, mean (SD)a | 260.3 (95.0) | 253−268 |
| HDL cholesterol, last recorded value, mean (SD)a | 48.7 (20.1) | 47−50 |
| LDL cholesterol, last recorded value, mean (SD)a | 184.2 (96.8) | 177−192 |
| Triglycerides, last recorded value, mean (SD) | 151.0 (68.9) | 148−156 |
95% CI: 95% confidence interval of the mean value or corresponding proportion; LTT: lipid-lowering treatment.
Characteristics of the Spanish population < 18 years with LDL-C > 150 mg/dL for genetically defined FH.
| Value | 95% CI | |
|---|---|---|
| < 18 years. N (%) | 178 (21.2%) | |
| Females. N (%) | 98 (55.1%) | 48−62 |
| Previous history of CVD. N (%) | 1 (.6%) | 0−3 |
| Age at first blood test | 8.7 (5.3) | 8−9 |
| LTT at first blood test. N (%) | 3 (1.7%) | 1−5 |
| LTT at last blood test. N (%) | 5 (2.8%) | 1−6 |
| Total cholesterol, first recorded value, mean (SD) | 35.5 (50.9) | 228−243 |
| HDL cholesterol, first recorded value, mean (SD) | 55.3 (16.7) | 53−58 |
| LDL cholesterol, first recorded value, mean (SD) | 160.4 (41.1) | 154−166 |
| Triglycerides, first recorded value, mean (SD) | 99.2 (48.6) | 92−106 |
| Total cholesterol, last recorded value, mean (SD)a | 208.5 (41.2) | 202−215 |
| HDL cholesterol, last recorded value, mean (SD) | 55.2 (15.0) | 53−57 |
| LDL cholesterol, last recorded value, mean (SD)a | 136.5 (33.6) | 132−141 |
| Triglycerides, last recorded value, mean (SD) | 91.7 (46.7) | 85−99 |
95% CI: 95% confidence interval of the mean value or corresponding proportion; LTT: lipid-lowering treatment.
This paper shows the efficacy and feasibility of mass screening and profiling of clinical records in detecting patients with FH-P, in both primary and secondary prevention.
Ideally, a screening algorithm for FH-P patients should be efficient, effective, reliable, reproducible, and cost-efficient. The lower the cut-off point chosen, the more sensitivity is gained, but specificity is lost and the number of patients to be screened and the number of resources needed to ensure the intervention can be implemented are considerably increased.22 Of the algorithms analysed, that based on cut-off points for LDL-C suggestive of genetically defined FH in the Spanish population is the most effective when considering mass screening.15 This algorithm has a sensitivity of 91% and a positive predictive value of 71% for genetically defined FH.15 Screening in health registries with this algorithm would detect around 50% of the population with FH.3 It has been estimated that screening 17%–47% of patients with FH followed by a direct and reverse cascade testing strategy could achieve detection rates of around 80%,23 much higher than the current 12%–16%.24 However, using only LDL-C levels for cascade screening may leave up to 20%–40% of relatives with normal but mutation-positive lipid values undiagnosed,2 therefore it is important to supplement this with a clinical assessment. Currently, the Dutch Lipid Clinic Network Score (DLCNS) is the most widely used scale in our setting to select patients with DLCNS > 524 as candidates for genetic testing, although this scale can be difficult to apply and has many areas for improvement.25 Confirmation of FH by genetic study will allow us to detect between two and eight relatives through direct cascade screening.1
A study of 162,864 subjects in an Italian population with a cut-off point of LDL-C > 190 mg/dL showed a 2.9% prevalence of FH-P in the population without LLT and 3.5% in the treated population,26 higher values than those detected in the present study. In our case, the number of patients with possible FH-P to be analysed would be multiplied by 11 if the criteria for severe hypercholesterolaemia were used (LDL-C > 190 mg/dL). Mass screening for FH using the selected algorithm may be feasible and affordable in clinical practice, as the number of patients to be re-assessed by each physician is small, a mean of around seven patients. Increasing the cut-off points increases the positive predictive value for the genetic study, but fewer patients with low-moderate phenotypic expression of FH, but at high CVR, are detected. In our case, by using the cut-off point of LDL-C > 359 mg/dL19 we would detect a small subgroup of patients with the highest likelihood of a positive genetic study, which may be of interest for detecting new genetic mutations. On the other hand, this is an extremely high-risk subgroup of patients, with a 10-year case fatality rate of over 40% in the present series. The criterion of Mickiewicz et al.16 in the Polish population could also be effective and cost-efficient. Studies are needed in the Spanish population to evaluate the cost-effectiveness of a combined strategy of screening and cascade genetic testing.
Another strategy used in screening patients with FH is disease pattern detection using machine-learning techniques such as the FIND-HF project, with a positive predictive value of 88% for FH.17 This model is technically more difficult in that it requires screening of unstructured data. In the present study, we screened patients with early CVD or on combined LLT, obtaining a prevalence at the lower end of the expected range, indicating underuse of high-intensity LLT. In our series, only 3% of patients in the selected population were taking combined LLT. The scant coding of FH diagnosis in discharge diagnoses is also noteworthy, at around 1% in the present study, although it is probably masked within the generic diagnosis of hypercholesterolaemia.
Recently, Civeira F.'s group highlighted as a useful strategy in screening patients with genetically defined FH the combination of a baseline LDL-C greater than 220 mg/dL and/or greater than 130 mg/dL on LLT,2 with a prevalence in our series of 12.5%. This high prevalence may reflect poorer lipid control in the population of the present study.
The FAMCAT study, which focused on primary care and included 750,000 patients, showed the ability to detect FH through mass screening of EHR with a positive predictive value of 88% and a negative predictive value of 99%.27 The problem in implementing it is the lack of essential clinical data in electronic records. A study focusing on hospital discharges of coronary patients with early presentation found that only 60% reported a family history of premature CAD.28 It is estimated that 20% of patients < 50 years of age with myocardial infarction and a family history of premature CAD would present with FH, this percentage would rise to 60% if associated with LDL-C > 160 g/dL.29
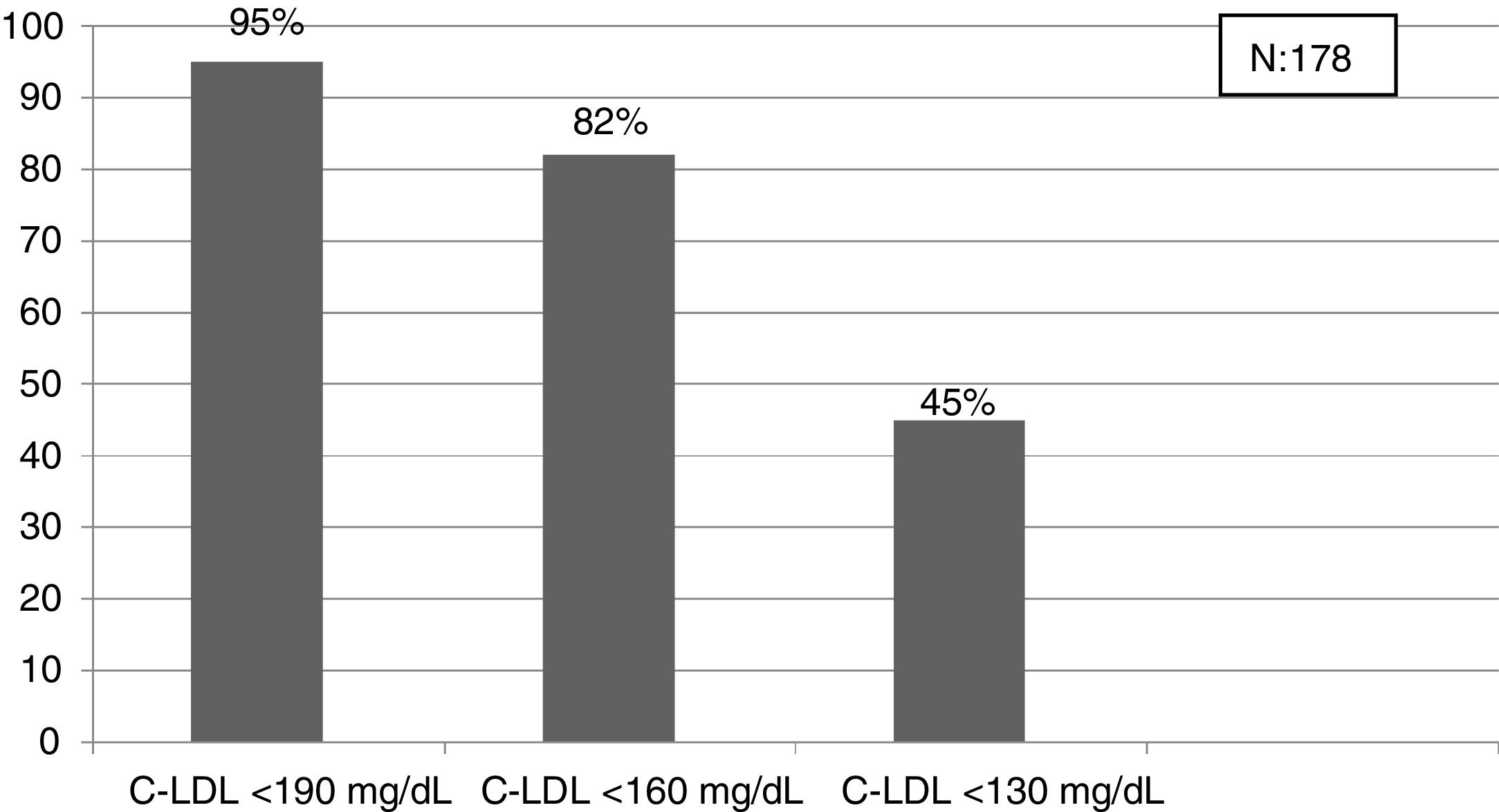
Detecting FH at an early age should be a priority to initiate early therapeutic measures.24 In our study, the criterion that obtained the prevalence closest to that expected in the population < 18 years was the cut-off point LDL-C > 190 mg/dL (definite FH, if secondary causes are excluded). In this population it would be better to lower the cut-off point to 160 mg/dL (probable FH with compatible family history) and even to 130 mg/dL (probable if one parent has genetically proven FH). This entire population will be candidates for education and engagement in healthy habits at least, and depending on the LDL-C values for LLT. Reverse cascade genetic screening of first-degree relatives of children with suspected FH can detect 84% of mutation-positive children and 63% of parents.30 A recent study has shown that the combination of direct and reverse cascade screening of one-year-old children, using immunisation campaigns, could detect 50% of the FH population in 17 years and 75% in 30 years.31 Mass data screening, together with direct and reverse cascade genetic screening could considerably reduce these periods of time.
A look through the big data window shows clinical practice where there is ample room for improvement and is an urgent call to action. In the present study, the selected FH-P population has high or very high CVR, with a CVD prevalence of 9% and a 10-year case fatality of 16%. Although our study shows a significant reduction in LDL-C over the time analysed, which is probably due to a better therapeutic approach to patients, there are few patients with FH-P in therapeutic targets, both in primary and secondary prevention, consistent with that observed in other studies in the FH population,10,32 and the lack of LLT in the paediatric and young population is particularly striking. Patient profiling techniques, following concentric circles based on CVR, mean we can prioritise in detecting patients starting with those at extreme risk,33 and allow cost-efficient use of the most innovative treatments.34,35
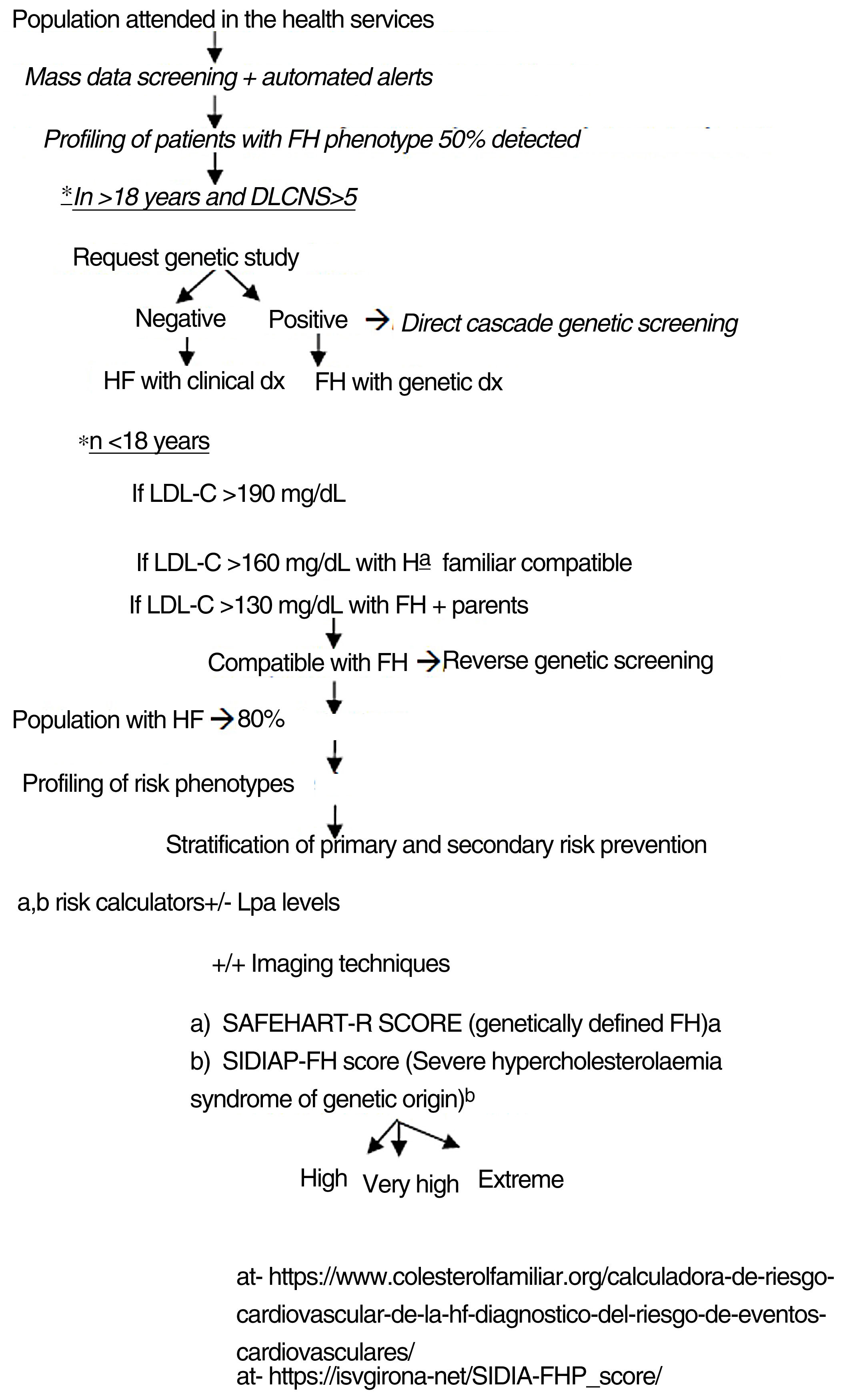
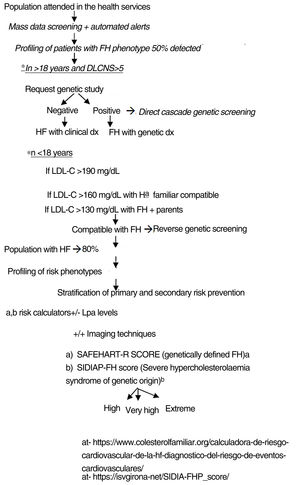
The authors propose a clinical programme to improve the quality of care for patients with FH-P, combining mass data screening, automated detection, patient profiling, clinical assessment, direct-reverse cascade genetic screening and CVR assessment (Fig. 4). The LOPDGDD 3/2018 authorises the re-identification of data as this is a situation of relevance to public health.21 Transparency, beneficence and proportionality are the principles governing the re-identification of patients. The teams overseeing profiling and re-identification should be different. The clinical stage should be led by the doctor responsible for each request for analysis, after informed consent.
A strength of the study to be highlighted is that the screening took place after merging databases from the different levels of care, and therefore we were able to analyse a significant number of patients and gain a cross-sectional view of healthcare. In terms of its limitations, this is a retrospective study with a time cut-off and focused on a specific geographical area, although its approximation to the expected prevalences indicates that it is useful in FH-P screening. Screening based on LDL-C values may overestimate the number of patients with genetically defined FH, particularly at the expense of patients with polygenic or combined hypercholesterolaemia, although they would be included together with FH in severe hypercholesterolemia syndrome of genetic origin,36 at high CVR and requiring intensive LLT. By not considering whether LDL-C values are in patients under LLT, the number with FH may be underestimated. However, selecting the highest LDL-C value selects at least 40% without LLT, is easy to do, allows the use of algorithms that assess LDL-C in patients under LLT and avoids imputation bias by generalising therapeutic response and adherence. Another limitation is the lack of access to genetic data and lipoprotein (a) levels.2 Criteria suggestive of FH-P needs to be defined in the Spanish population differentiated by age and sex. Unstructured data were not captured, although this does not invalidate the results as an initial screening proposal.
Mass data screening and patient profiling constitute an effective and easy-to-apply tool in clinical practice to detect FH. These techniques are a second opportunity to improve the diagnosis and treatment of patients with FH, in primary as well as secondary prevention.
- -
The prevalence of FH in Spain is around 1/282 and 1/192 for the FH phenotype.
- -
It is the most common cause of premature CVD. It is associated with more diffuse heart disease and a worse prognosis.
- -
It is underdiagnosed, with diagnosis rates of around 12%–15% and undertreated.
- -
It is a public health problem requiring urgent intervention.
- -
Mass data screening is an effective technique for the initial screening of patients with FH-P.
- -
Patient profiling enables us to identify patients with FH and at very high-extreme CVR.
- -
An effective and feasible screening algorithm in our setting could be one that uses LDL-C cut-off points suggestive of genetically defined FH in the Spanish population.
- -
These tools are easy to incorporate and can be used in daily clinical practice.
Own funds of Corporació de Salut del Maresme i al Selva.
Please cite this article as: Zamora A, Paluzie G, García-Vilches J, Gisbert OA, Méndez Martínez AI, Plana N, et al. El rastreo masivo de datos es una segunda oportunidad para mejorar el manejo de los pacientes fenotipo de hipercolesterolemia familiar. Clin Investig Arterioscler. 2021;33:138–147.