Gut microbiota plays a key role in the control of body weight. In the present review the different ways in which it can modify the energy homeostasis of the host are exposed, based on its capacity to modify the metabolism of the individual and its contribution in the energy consumption regulation. With the current evidence, it is not clear what microbiota profile is associated with the presence of obesity, although in animal models it seems to be related to a higher proportion of bacteria of the Firmicutes phylum, to the detriment of those of the Bacteroidetes phylum. Other factors clearly involved would be the diversity in the gut microbiota or its possible functional changes. More studies in humans are needed to clarify how dysbiosis can influence weight control. On the other hand, probiotics directly affect the gut microbiota, modulating its composition and, possibly, its functionality. A large number of studies in humans have evaluated the impact of probiotics on obesity. Although this intervention may have a potentially beneficial effect, more effort is needed to clarify which strains of probiotics should be recommended, at what dose and for how long.
La microbiota intestinal tiene un papel determinante en el control del peso corporal. En la presente revisión se exponen las diferentes vías por las que puede modular la homeostasis energética del huésped, en base a su capacidad modificadora del metabolismo del individuo y su contribución en la regulación del aprovechamiento energético. Con las evidencias actuales, no está claro cuál es el perfil de microbiota que se atribuye a la presencia de obesidad, aunque en modelos animales parece relacionarse con una mayor proporción de bacterias del filo Firmicutes, en detrimento de las del filo Bacteroidetes. Otros factores claramente implicados serían la diversidad en la microbiota intestinal o sus posibles cambios funcionales. Son necesarios más estudios en humanos para poder esclarecer cómo la disbiosis puede influir en el control ponderal. Por otra parte, los probióticos afectan directamente la microbiota intestinal, modulando su composición y, posiblemente, su funcionalidad. Un gran número de estudios en humanos han evaluado el impacto de los probióticos en la obesidad. A pesar de que esta intervención puede tener un potencial efecto beneficioso, es preciso esclarecer qué cepas de probióticos deben recomendarse, en qué dosis y durante cuánto tiempo.
The prevalence of obesity is increasing worldwide, especially in industrialised countries, and is a major global health issue, linked to the onset of multiple comorbidities such as hypertension, type 2 diabetes mellitus, non-alcoholic fatty liver disease and cardiovascular disease. According to data from the Nutrition and Cardiovascular Risk Study in Spain, the most recent population study of cardiovascular risk factors in the country, 16.5% of the population is overweight (body mass index [BMI] 25–30kg/m2), 21.7% is mildly or moderately obese (BMI 30–40kg/m2), and 1.2% are severely or morbidly obese (BMI >40kg/m2).1
From a simplistic point of view, the pathophysiology of obesity can be explained by a positive energy balance, with more energy consumed or eaten than expended, which, when continued for a long period of time, leads to build-up of fat in the adipocytes and, consequently, weight gain. However, the pathophysiology of this disease is much more complex than this, with additional factors playing a role, such as basal metabolic rate, genetic and environmental factors. These factors have an even greater impact on individuals’ weight gain.2 Of the different environmental factors, eating habits and physical activity play a significant role, although there are other environment-related aspects involved in the onset of obesity.
These include the composition of the individual's microbiota and dysbiosis or imbalance processes that may cause changes in the microbiota composition and/or function. Over the last decade, a very close link has been established between composition and changes in gut microbiota and obesity, both in experimental and human models.3,4 Probiotic microorganisms have also become more popular due to the increasing number of studies demonstrating that certain strains have health-promoting properties.5 The role of microbiota in the pathophysiology of obesity and the impact of probiotic treatment is reviewed below.
Gut microbiotaGut microbiota can be defined as the community of microorganisms that live in the intestines. Prior to birth, the gut is sterile and it becomes fully colonised during the first year of life. Mode of delivery and breastfeeding play an important role in the stabilisation of the microbiota.6 The microbiota then changes with age, dietary habits and environmental factors, including antibiotic treatment.7 In line with the above, recent investigations show that 80-90% of the bacterial phylotypes in the human gut are members of two phyla, Bacteroidetes (gram-negative, e.g. Bacteroides and Prevotella) and Firmicutes (gram-positive, e.g. Clostridium, Enterococcus, Lactobacillus, Ruminococcus), followed by Actinobacteria (gram-negative, e.g. Bifidobacterium) and Proteobacteria (gram-negative, e.g. Helicobacter, Escherichia).8–10
Gut microbiota makes an important contribution to human metabolism11 since it modulates host nutrition and energy consumption through the production of vitamins (K, folic acid and B12), absorption of electrolytes and minerals, fermentation of indigestible components of the host's diet and production of short-chain fatty acids (SCFA)10; it also influences homeostasis of the intestinal epithelium, development of the immune system, protection against pathogens and drug metabolism.11,12
Microbiota and obesityOf all the functions outlined above, there has been increasing concern over the last 10 years about the role of the gut microbiota in energy homeostasis and, more specifically, its behaviour in metabolic diseases such as obesity. There are multiple studies in both animal models and humans linking alterations in gut microbiota with the presence of obesity.
Studies in animal modelsRole of the microbiota in the regulation of metabolismThe microbiota by itself can only cause weight gain. Therefore, the microbiota derived from genetically obese mice or mice rendered obese by diet can cause fat accumulation, and this is not mediated by increased food consumption. The first evidence for the role of the gut microbiota in obesity comes from studies conducted in germ-free (GF) mice, the gut of which is sterile, compared to conventional mice.13 At baseline, conventional mice had 40% more body fat than GF mice, regardless of food intake. Also, colonisation of GF mice with gut microbiota from conventional mice produced a significant increase in body weight and a 60% increase in body fat, a significant increase in triglyceride synthesis in the liver, leptin secretion and insulin resistance, regardless of food intake and total energy expenditure. Likewise, the transfer of gut microbiota from conventional mice to GF mice caused a significant increase in body weight and body fat compared to transplantation of microbiota from lean mice.14
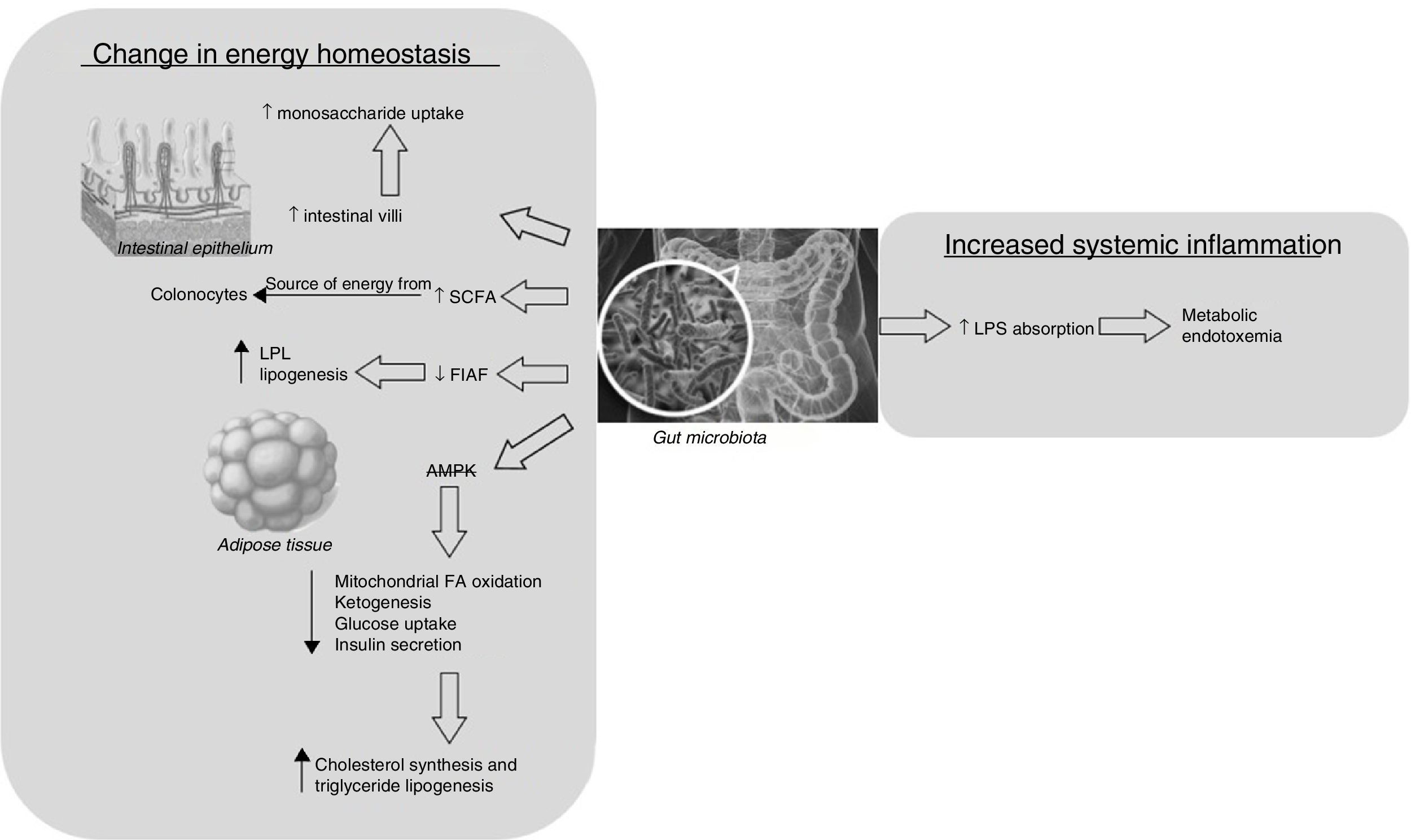
There are two main mechanisms that may explain why microbiota composition promotes obesity: altered host energy homeostasis and increased systemic inflammation (Fig. 1). The first mechanism would affect the hosts’ predisposition to extract more calories from their food and, consequently, to develop obesity and increased adiposity.13 This can occur by various pathways: (a) enhanced uptake of monosaccharides that would normally not be digestible, secondary to development of gut epithelium by the microbiota, increasing the density of intestinal villi capillaries15; (b) increased production of SCFA, which are used as a source of energy by the colonocytes16; (c) increased deposition of triglycerides in adipocytes. This mechanism may be caused by reduced intestinal expression of fasting-induced adipocyte factor by the microbiota. This hormone inhibits lipoprotein lipase, which is responsible for cellular uptake of fatty acids from lipoproteins and accumulation of triglycerides in adipocytes13; and (d) suppression by the gut microbiota of the release of AMP-activated protein kinase, which leads to reductions in mitochondrial FA oxidation, ketogenesis, glucose uptake and insulin secretion and potentiation of lipogenesis and cholesterol and triglyceride synthesis.17–19
The second mechanism, which is related to a systemic inflammation process, was described by Cani et al.20, who observed, after administering a fat-enriched diet to a group of mice, an increase in the gram-negative-to-gram-positive ratio in the gut microbiota and, therefore, increased intestinal absorption of bacterial fragments, such as lipopolysaccharides, causing the so-called “metabolic endotoxemia”, which is classically associated with chronic inflammation and other metabolic diseases related to obesity.
Type of microbiota and its influence on obesityOnce it had been established that gut microbiota has an impact on the host metabolism, research focused on clarifying which type of microbiota was associated more directly with weight gain. First, the presence of certain strains of microorganisms has been associated with the presence of obesity. In animal models, it has been concluded, more or less unanimously, that an increase in the ratio of gram-positive bacteria (Firmicutes) to gram-negative bacteria (Bacteroidetes, actinobacteria and proteobacteria) is related to the presence of obesity.4,8,21
Other authors seem to suggest that the gut microbiota can be modified through diet. Turnbaugh et al.22 colonised GF mice with human faeces and fed them a Western-style diet versus a low-fat, plant polysaccharide-rich diet for 2 weeks and then transplanted their microbiota into GF mice. The GF mice that received the microbiota of those mice fed the Western-style diet gained more weight than those which received transplants from those fed a low-fat diet. In another study, an increase in Firmicutes and a lower ratio of Proteobacteria and Actinobacteria (i.e. Bifidobacterium spp.) was observed in mice fed a high-fat diet.23 An increased ratio of Firmicutes to Bacteroidetes was also observed both in mice with obese and lean phenotypes when fed a high-fat diet.24
Studies in humansA few years after the initial animal model studies, the first papers focusing on the determination of gut microbiota in humans and its link to obesity began to emerge.
Type of microbiota and its influence on obesityAlthough it was concluded, more or less unanimously, in animal models that an increase in the ratio of gram-positive bacteria (Firmicutes) to gram-negative bacteria (Bacteroidetes, Actinobacteria and Proteobacteria) is related to the presence of obesity, studies in humans have not been as conclusive. In fact, there is a series of papers defending this idea. In 2006, one year after their first experimental observation in mice,8 Ley et al.9 confirmed that obese subjects, compared with lean subjects, had a higher proportion of Firmicutes and a relatively low proportion of Bacteroidetes. This study also showed that the ratio of Firmicutes to Bacteroidetes was similar to the profile of a thin person after losing weight by following a low-fat or low-carbohydrate diet for 18 months. Likewise, Santacruz et al.25 observed reduced numbers of Bacteroides and increased numbers of Staphylococcus, Enterobacteriaceae and Escherichia coli in obese compared to normal-weight pregnant women. Other papers supporting this line of research observed a significantly higher level of Lactobacillus species (genus belonging to the phylum Firmicutes) in obese patients.26 Specifically, a higher level of Lactobacillus reuteri (L. reuteri) and lower levels of Lactobacillus casei/paracasei, Lactobacillus plantarum (L. plantarum) and Bifidobacterium animalis are associated with obesity.27 Other studies do not make such a conclusive link between the ratio of such bacteria and obesity. On analysing a cohort of twin pairs, Turnbaugh et al. detected higher levels of Bacteroidetes and Actinobacteria in lean subjects compared with obese subjects, with no significant differences in the proportion of Firmicutes. It is also important to note that this study detected that obese subjects had less diverse microbiota.2
Contrary to the initial hypothesis, multiple studies oppose the idea that Firmicutes are the most abundant group of bacteria in the gut of overweight individuals. Ducan et al.28 reported no differences in phyla between obese and non-obese subjects. Furthermore, no significant changes were observed on examining the proportion of Bacteroidetes in the faeces of obese patients following a weight maintenance diet or a weight loss programme. Likewise, another study29 found somewhat more Bacteroidetes in obese subjects than in normal-weight individuals and showed that one genus of Bacteroides (i.e. Prevotella) was particularly high in obese subjects. However, it was also observed that, following weight loss secondary to a gastric bypass, these individuals had a higher proportion of Gammaproteobacteria (phylum Proteobacteria) and proportionally fewer Firmicutes. Similarly, Schwiertz et al.30 and Collado et al.31 demonstrated that the ratio of Firmicutes to Bacteroidetes shifted in favour of Bacteroidetes in overweight or obese patients. The reason why these studies do not always agree is the fact that they use less standardised methodologies, less homogeneous populations and more divergent lifestyles and diets in comparison with animal models.
As a whole, all these data support the fact that the link between obesity and microbiota is not due to the proportion of the major groups of bacteria, but to small changes or more specific variations in each species.32,33 As a result, there are studies that support the idea that low levels of Bifidobacterium (belonging to the phylum Actinobacteria)25,30,31,34 and high levels of Staphylococcus aureus (S. aureus) (belonging to the phylum Firmicutes)31,34 are related to obesity. One example of this is the paper by Kalliomäki et al.34, which observed higher levels of Bifidobacterium spp. in children of normal weight at the age of 7 compared to those beginning to show signs of being overweight. The relevance of this study is due to the fact that its results support the idea that changes in microbiota composition may precede the status of being overweight.34 The authors also observed that the levels of S. aureus were lower in children of normal weight than in those becoming overweight years later. The authors speculated that S. aureus may act as a trigger of low-grade inflammation. Likewise, Collado et al.31 observed higher levels of Bacteroides spp. and S. aureus in stool samples from overweight women than in normal-weight women. They also found a positive correlation between total Bacteroides spp. levels and BMI, both before and during pregnancy. It should be noted that higher levels of bifidobacteria were observed not only in normal-weight women compared to overweight women, but also in women who gained less weight during pregnancy.
Is weight control only affected by the type of microbiota?An extensive assessment of the relationship between BMI and the taxonomic composition of the gut microbiome in the ‘Human Microbiome Project’ dataset has recently been conducted. The results were compared to those obtained in another large metagenomic study of the gut microbiota, the MetaHIT study, as well as to two smaller studies that specifically sampled lean and obese individuals. There was no association between BMI and the taxonomic composition or diversity of the microbiome in the ‘Human Microbiome Project’ cohort.35 Furthermore, inter-study variability was found to far exceed differences in composition between lean and obese individuals within each study and the authors concluded that this suggests that there is no simple taxonomic signature of obesity in the gut microbiota. An identical conclusion was reached in a meta-analysis of indicator taxa in the microbiome and general features of the microbiota associated with obesity.36
Another important point to highlight is that certain studies, instead of correlating the type of microbiota observed with the risk of developing obesity, defend the idea that it is the limited diversity of the gut microbiota that may predispose individuals to weight gain.37–39 This fact may be closely linked to evidence that a lack of diversity in the gut microbiota of the Western population40–42 may influence the increase in overweight and obesity rates in the same environment.
Finally, as in animal models, it can also be concluded that, in the case of humans, a low-calorie diet modifies the microbiota composition, generally increasing the proportion of Bacteroidetes and reducing the proportion of Firmicutes, and is also accompanied by weight loss.9,43,44 One study conducted in overweight adolescents even shows that a certain gut microbiota composition may potentiate the efficacy of dietary interventions in weight loss.45
Probiotics and obesityThe classic approach to obesity involves making lifestyle changes and restricting bariatric surgery to the most severe cases. The main limitation of the conventional treatment of diet and physical activity is its limited efficacy, both in the short and long term.46 Bariatric surgery, however, which is the most effective treatment for obesity, can achieve remission of comorbidities.47,48 Nevertheless, surgery is not exempt from potential complications and it is therefore necessary to find new therapeutic strategies for obesity control, in combination with adjuvant lifestyle changes, such as new drugs or the use of probiotics as treatment. This was the motive for early studies to analyse the efficacy of probiotics as a possible way of controlling obesity.
Probiotics were defined by the Food and Agriculture Organization and by the World Health Organization as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”.49 These microorganisms do not permanently colonise the gut and must remain alive along the entire length of the digestive tract. Therefore, to be considered good candidates, bacterial strains must have certain features that contribute to host colonisation: tolerance of low pH in the stomach, resistance to bile salts and adherence to the host epithelium.50
Probiotics interact with the host through pattern recognition receptors in intestinal cells, such as Toll-like receptors, and these can play multiple roles in the individual's body. The mechanisms of action of probiotics associated with obesity control may be modulation of endogenous microbiota functions which affects interaction with the host, competitive exclusion of pathogens, improved epithelial barrier function and other innate immune responses, modulation of fat absorption and excretion, reduced endotoxemia and inflammation, and modulation of numerous genes involved in hepatic lipogenesis or lipolysis in adipose tissue.51–54
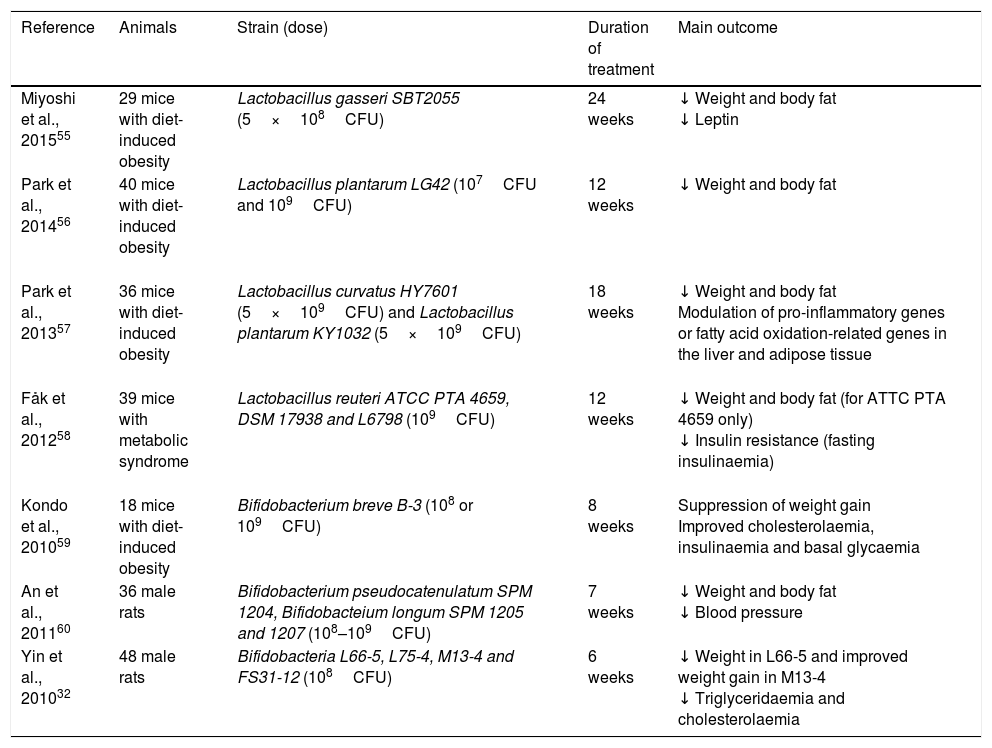
Studies in animal modelsThere are a large number of studies (Table 1) that have observed a decrease in body weight and body fat in obese mice after introducing different strains of Lactobacillus: Lactobacillus gasseri SBT2055 for 24 weeks55, L. plantarum LG42 for 12 weeks56, Lactobacillus curvatus HY7601 and L. plantarum KY1032 for 18 weeks57, L. reuteri ATCC PTA 465958; among others.11 Other metabolic changes, such as decreased leptin levels55, reduced resistance to insulin58 or modulation of pro-inflammatory genes or fatty acid oxidation-related genes in the liver and adipose tissue, have also been noted.57
Use of probiotics for weight control: animal model studies.
| Reference | Animals | Strain (dose) | Duration of treatment | Main outcome |
|---|---|---|---|---|
| Miyoshi et al., 201555 | 29 mice with diet-induced obesity | Lactobacillus gasseri SBT2055 (5×108CFU) | 24 weeks | ↓ Weight and body fat ↓ Leptin |
| Park et al., 201456 | 40 mice with diet-induced obesity | Lactobacillus plantarum LG42 (107CFU and 109CFU) | 12 weeks | ↓ Weight and body fat |
| Park et al., 201357 | 36 mice with diet-induced obesity | Lactobacillus curvatus HY7601 (5×109CFU) and Lactobacillus plantarum KY1032 (5×109CFU) | 18 weeks | ↓ Weight and body fat Modulation of pro-inflammatory genes or fatty acid oxidation-related genes in the liver and adipose tissue |
| Fåk et al., 201258 | 39 mice with metabolic syndrome | Lactobacillus reuteri ATCC PTA 4659, DSM 17938 and L6798 (109CFU) | 12 weeks | ↓ Weight and body fat (for ATTC PTA 4659 only) ↓ Insulin resistance (fasting insulinaemia) |
| Kondo et al., 201059 | 18 mice with diet-induced obesity | Bifidobacterium breve B-3 (108 or 109CFU) | 8 weeks | Suppression of weight gain Improved cholesterolaemia, insulinaemia and basal glycaemia |
| An et al., 201160 | 36 male rats | Bifidobacterium pseudocatenulatum SPM 1204, Bifidobacteium longum SPM 1205 and 1207 (108–109CFU) | 7 weeks | ↓ Weight and body fat ↓ Blood pressure |
| Yin et al., 201032 | 48 male rats | Bifidobacteria L66-5, L75-4, M13-4 and FS31-12 (108CFU) | 6 weeks | ↓ Weight in L66-5 and improved weight gain in M13-4 ↓ Triglyceridaemia and cholesterolaemia |
CFU: colony-forming unit; ↓: decrease.
Similar studies have been conducted in animal models on Bifidobacterium treatment in obesity, showing weight loss or decreased body fat: Bifidobacterium breve B-3 for 8 weeks59, Bifidobacterium pseudocatenulatum SPM 1204, Bifidobacterium longum SPM 1205 and 1207 for 7 weeks60 or Bifidobacteria L66-5 for 6 weeks.32 Decreased cholesterolaemia, glycaemia and insulinaemia59 or lower concentrations of leptin or lipase60 were also reported, among other beneficial effects.
Studies in humansFew studies have been conducted in humans to date to examine the effect of probiotics on body weight. In comparison with the mainly favourable results of animal model studies, there is little evidence from human studies for recommending the use of probiotics in treating obesity.
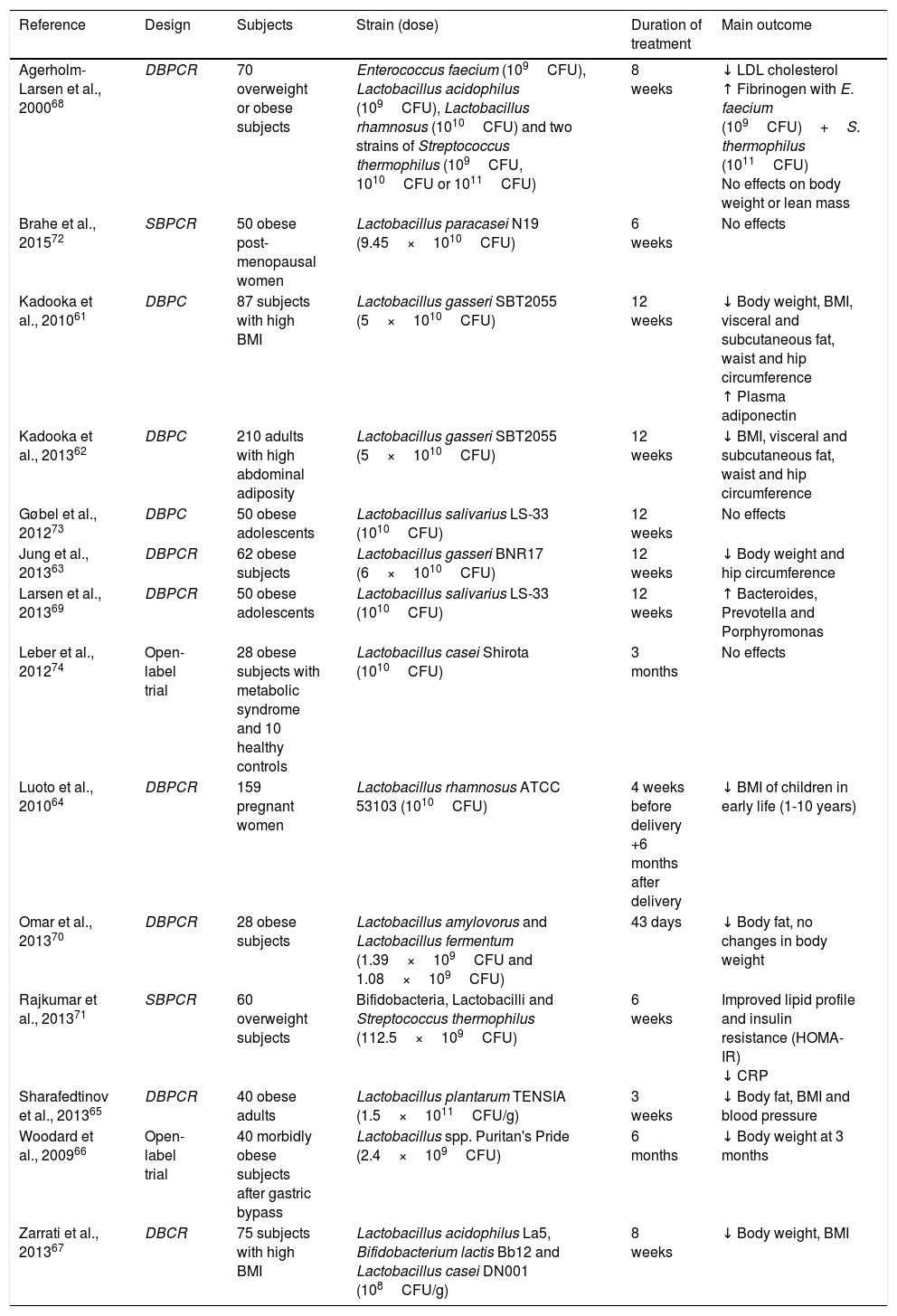
Based on the long tradition of using lactic acid bacteria with no harmful effects on human health, bacteria from the genera Lactobacillus and Bifidobacterium have an established history of safe use and have received GRAS (Generally Recognised as Safe) status from the Food and Drug Administration. As a result, these two groups of bacteria have been evaluated the most (Table 2). Not all studies show a positive relationship between probiotic use and obesity control. While some have linked the administration of different strains of bacteria (L. gasseri SBT2055, L. gasseri BNR17, Lactobacillus rhamnosus ATCC 53103, L. plantarum TENSIA, Lactobacillus spp. Puritan's Pride, Lactobacillus acidophilus La5, Bifidobacterium lactis Bb12 and L. casei DN001) to weight loss61–67, others have observed positive metabolic changes but with no change in weight parameters.68–71 Other papers, however, showed no significant changes with probiotic use as a treatment for obesity.72–74
Use of probiotics for weight control: human studies.
| Reference | Design | Subjects | Strain (dose) | Duration of treatment | Main outcome |
|---|---|---|---|---|---|
| Agerholm-Larsen et al., 200068 | DBPCR | 70 overweight or obese subjects | Enterococcus faecium (109CFU), Lactobacillus acidophilus (109CFU), Lactobacillus rhamnosus (1010CFU) and two strains of Streptococcus thermophilus (109CFU, 1010CFU or 1011CFU) | 8 weeks | ↓ LDL cholesterol ↑ Fibrinogen with E. faecium (109CFU)+S. thermophilus (1011CFU) No effects on body weight or lean mass |
| Brahe et al., 201572 | SBPCR | 50 obese post-menopausal women | Lactobacillus paracasei N19 (9.45×1010CFU) | 6 weeks | No effects |
| Kadooka et al., 201061 | DBPC | 87 subjects with high BMI | Lactobacillus gasseri SBT2055 (5×1010CFU) | 12 weeks | ↓ Body weight, BMI, visceral and subcutaneous fat, waist and hip circumference ↑ Plasma adiponectin |
| Kadooka et al., 201362 | DBPC | 210 adults with high abdominal adiposity | Lactobacillus gasseri SBT2055 (5×1010CFU) | 12 weeks | ↓ BMI, visceral and subcutaneous fat, waist and hip circumference |
| Gøbel et al., 201273 | DBPC | 50 obese adolescents | Lactobacillus salivarius LS-33 (1010CFU) | 12 weeks | No effects |
| Jung et al., 201363 | DBPCR | 62 obese subjects | Lactobacillus gasseri BNR17 (6×1010CFU) | 12 weeks | ↓ Body weight and hip circumference |
| Larsen et al., 201369 | DBPCR | 50 obese adolescents | Lactobacillus salivarius LS-33 (1010CFU) | 12 weeks | ↑ Bacteroides, Prevotella and Porphyromonas |
| Leber et al., 201274 | Open-label trial | 28 obese subjects with metabolic syndrome and 10 healthy controls | Lactobacillus casei Shirota (1010CFU) | 3 months | No effects |
| Luoto et al., 201064 | DBPCR | 159 pregnant women | Lactobacillus rhamnosus ATCC 53103 (1010CFU) | 4 weeks before delivery +6 months after delivery | ↓ BMI of children in early life (1-10 years) |
| Omar et al., 201370 | DBPCR | 28 obese subjects | Lactobacillus amylovorus and Lactobacillus fermentum (1.39×109CFU and 1.08×109CFU) | 43 days | ↓ Body fat, no changes in body weight |
| Rajkumar et al., 201371 | SBPCR | 60 overweight subjects | Bifidobacteria, Lactobacilli and Streptococcus thermophilus (112.5×109CFU) | 6 weeks | Improved lipid profile and insulin resistance (HOMA-IR) ↓ CRP |
| Sharafedtinov et al., 201365 | DBPCR | 40 obese adults | Lactobacillus plantarum TENSIA (1.5×1011CFU/g) | 3 weeks | ↓ Body fat, BMI and blood pressure |
| Woodard et al., 200966 | Open-label trial | 40 morbidly obese subjects after gastric bypass | Lactobacillus spp. Puritan's Pride (2.4×109CFU) | 6 months | ↓ Body weight at 3 months |
| Zarrati et al., 201367 | DBCR | 75 subjects with high BMI | Lactobacillus acidophilus La5, Bifidobacterium lactis Bb12 and Lactobacillus casei DN001 (108CFU/g) | 8 weeks | ↓ Body weight, BMI |
BMI: body mass index; CFU: colony-forming unit; CRP: C-reactive protein; DBPC: double-blind, placebo-controlled clinical trial; DBPCR: double-blind, placebo-controlled, randomised clinical trial; LDL: low density lipoprotein; SBPCR: single-blind, placebo-controlled, randomised clinical trial; ↓: decrease; ↑: increase.
The different findings may be due to inconsistent study methodologies, less homogeneous study populations, sample size, wide variability in study strains and short intervention time.
To conclude, the studies conducted have confirmed the influence of microbiota on the host metabolism, with a focus on its role in the regulation of energy homeostasis and its pathogenic role. However, more extensive epidemiological studies are required to be able to confirm whether the relationship between microbiota and obesity is due to diversity in bacterial flora, the presence of specific species in the gut, possible functional changes in gut microbiota or a combination of different factors.
With regard to the role of probiotics as a treatment for obesity, available evidence is controversial and therefore additional studies are required to assess the therapeutic use of probiotics for treating obesity.
Conflicts of interestThe authors declare that there is no conflict of interest regarding the publication of this article.
Please cite this article as: Fontané L, Benaiges D, Goday A, Llauradó G, Pedro-Botet J. Influencia de la microbiota y de los probióticos en la obesidad. Clín Investig Arterioscler. 2018;30:271–279.