Positioning document and summary of recommendations recently published by the Working Group on Atherogenic Dyslipemia of the Spanish Society of Arteriosclerosis and by the European Society of Arteriosclerosis.
Documento de posicionamiento y resumen de las recomendaciones recientemente publicadas por el Grupo de Trabajo de Dislipemia Aterogénica de la Sociedad Española de Arteriosclerosis y por la Sociedad Europea de Arteriosclerosis.
Atherogenic dyslipidaemia is characterised by an increase in total plasma triglycerides (TG) and a decrease in high density lipoprotein cholesterol (HDL-C). Alongside these two lipid disorders which define atherogenic dyslipidaemia we find an increase in TG-rich and apolipoprotein B (apoB) carrier lipoproteins. This is usually a moderate increase and, on occasion, low-density lipoprotein cholesterol (LDL-C) values are close to normal, with predominantly small and dense LDL particles.1
Atherogenic dyslipidaemia is hugely important as it is associated with different conditions that are currently very prevalent among the general population, and which represent a high cardiovascular risk, such as overweight (37%), obesity (17%), diabetes (14%) and hyperglycaemia, and metabolic syndrome (30%).2,3 Moreover, atherogenic dyslipidaemia is in itself an indicator of high cardiovascular risk among subjects with diabetes. In this sense, atherogenic dyslipidaemia is associated with a higher risk of myocardial ischaemia or silent coronary artery disease in patients with type 2 diabetes and LDL-C levels <130mg/dl.4
In fact, in the Spanish population, the prevalence of atherogenic dyslipidaemia is elevated, with a presence in 34% of diabetic patients, 21% of high-risk patients with controlled LDL and 21–34% of patients with a history of any form of vascular disease (coronary, cerebral or peripheral artery).5
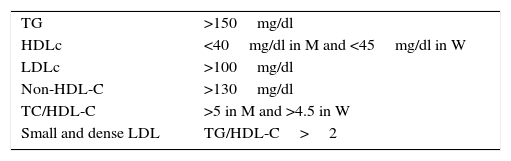
DiagnosisAtherogenic dyslipidaemia is characterised by hypertriglyceridaemia, which is a consequence of an increase in TG-rich lipoproteins, including their remnant particles, and due to the moderate elevation of LDLs, i.e. the group of atherogenic lipoproteins that contain apoB. All of the abovementioned lipoproteins can be quantified using non-HDL cholesterol (non-HDL-C) or apoB concentrations. Conversely, there is a decrease in HDL-C.
Along with these disorders, the presence of an increase in small and dense LDL particles, calculated indirectly using the TG/HDL-C ratio, and the increase in atherogenic indices, particularly total/HDL cholesterol, constitute the set of findings in this type of dyslipidaemia,6 a summary of which is presented in Table 1.
Atherogenic dyslipidaemia.
| TG | >150mg/dl |
| HDLc | <40mg/dl in M and <45mg/dl in W |
| LDLc | >100mg/dl |
| Non-HDL-C | >130mg/dl |
| TC/HDL-C | >5 in M and >4.5 in W |
| Small and dense LDL | TG/HDL-C>2 |
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; M: men; LDL: low-density lipoproteins; W: women; TG: triglycerides.
Non-HDL-C essentially represents the sum of lipoprotein cholesterol containing apoB, i.e. atherogenic lipoproteins that can be deposited along the arterial wall. As such, in patients with atherogenic cholesterol, it has been recommended that the most suitable therapeutic target is non-HDL-C or apoB, as both parameters have a better correlation with cardiovascular risk than LDL-C.7
Non-HDL-C has proven to be a significant cardiovascular risk factor that can replace apoB in routine practice, as it is more economic, reliable and easier to calculate, since it only requires subtracting the HDL-C from the total cholesterol – analytical parameters that are available at all healthcare facilities’ clinical laboratories.
The scientific evidence regarding the association between high LDL-C levels and an increased risk of cardiovascular disease is strong and indisputable. However, even with adequate LDL-C control, a considerable percentage of subjects remain who maintain a high vascular risk attributable to other lipid disorders, such as hypertriglyceridaemia and decreased HDL-C. The greatest cardiovascular risk is found when disorders in these three fractions coexist: LDL-C>130mg/dl, HDL-C<40mg/dl and TG>150mg/dl.8
A meta-analysis by Andersson et al.,9 on 62,154 patients included in 8 studies, showed that non-HDL-C had a better correlation with cardiovascular risk than LDL-C; moreover, the subjects who reached their therapeutic target for LDL-C but not non-HDL-C had a risk increase of 32% compared to those who achieved both targets. Similarly, a recent study has revealed that atheromatous plaque progression was more closely related to non-HDL-C than LDL-C concentrations. Accordingly, the lowest levels of non-HDL-C and TG showed a significant association with plaque regression through the different categories of cardiovascular risk.10
We can therefore consider that, in patients with atherogenic dyslipidaemia, the main risk predictor—and thus the primary control target—is non-HDL-C.11 Furthermore, it has been established that the cardiovascular risk presented by subjects with atherogenic dyslipidaemia is twice or three times that of the general population, and in the majority of cases, they have a high cardiovascular risk.12,13
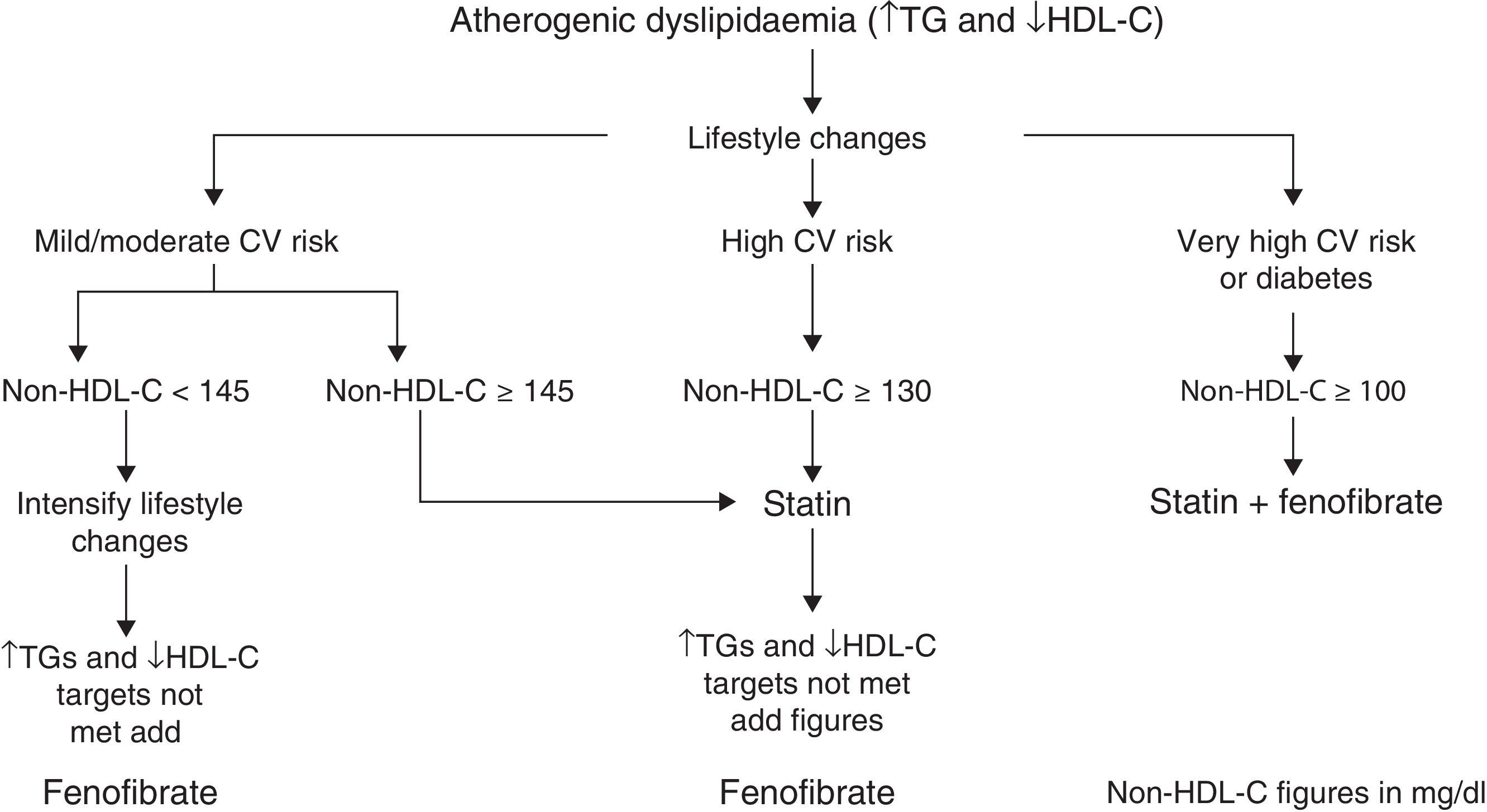
As such, we can consider non-HDL-C as the main therapeutic target in cases of atherogenic dyslipidaemia. The calculation of non-HDL-C based on the total cholesterol minus HDL-C is established as the target according to the cardiovascular risk; its values have been set as those for the target LDL-C plus 30mg/dl.
Consequently, the target non-HDL-C will be (Table 2):
- –
<145mg/dl in subjects with moderate risk, an unusual situation in clinical practice among cases of atherogenic dyslipidaemia, as these subjects usually present lipid characteristics or an association with other risk factors (abdominal obesity, metabolic syndrome or diabetes) which increase the cardiovascular risk evaluation.
- –
<130mg/dl in subjects with a high risk.
- –
<100mg/dl in subjects with a very high risk.
Lipid targets in atherogenic dyslipidaemia.
| Primary: non-HDL-C | |
| Moderate CV risk (rare situations) | <145mg/dl |
| High CV risk: abdominal obesity, metabolic syndrome, CVRF association, diabetes | <130mg/dl |
| Very high CV risk: cardiovascular disease, type 2 diabetes with target organ damage or serious CVRF | <100mg/dl |
| Secondary: TG and HDL-C | |
| After achieving the primary target | TG<150mg/dl HDL-C>40mg/dl M, >45mg/dl W |
HDL-C: high-density lipoprotein cholesterol; non-HDL-C: cholesterol bound to atherogenic lipoproteins (total cholesterol minus high-density lipoprotein cholesterol); CV: cardiovascular; CVRF: cardiovascular risk factor; M: men; W: women; TG: triglycerides.
The secondary targets are TG and HDL-C concentrations.
Hypertriglyceridaemia is an independent cardiovascular risk factor and TG levels <150mg/dl14 are considered optimal. As such, although it has not been clearly established, the therapeutic target may be considered as <150mg/dl.
Plasma HDL-C concentrations <40mg/dl in men and <45mg/dl in women are also considered an independent cardiovascular risk factor and, as a result, figures exceeding those mentioned above would be desirable.14,15
Points of consensus in the clinical assessment of atherogenic dyslipidaemiaBased on the available data and the most significant clinical evidence put forth with respect to atherogenic dyslipidaemia-associated cardiovascular risk, the key points are as follows:
- –
Hypertriglyceridaemia is an independent risk factor that is exacerbated in the presence of elevated LDL-C or low HDL-C levels. It is one of the key aspects of lipid-related residual vascular risk.
- –
In order to assess overall cardiovascular risk, the determination of TG and HDL-C is fundamental.
- –
The most practical markers for assessing the risk attributable to atherogenic dyslipidaemia are non-HDL-C (value above the LDL-C concentration) and TG (as a marker of remnant TG-rich lipoproteins).
- –
Non-HDL-C is the most suitable therapeutic target for controlling cardiovascular risk in patients with atherogenic dyslipidaemia. It has a good correlation with apoB (the ideal marker, but widespread availability for routine employment is lacking), is easy to calculate, presents no additional cost and has great stability for calculating risk.
Atherogenic dyslipidaemia is a critical element that clearly contributes to the residual risk that remains following treatment with statins. This lipoprotein disorder is under-diagnosed, under-treated and under-controlled, which is particularly relevant in patients with a high cardiovascular risk and those with diabetes.16
It is necessary to consider the approach to atherogenic dyslipidaemia based on the scientific evidence available in order to improve its treatment and adherence to the same.
Lifestyle changesThe incorporation of a healthy diet, regular physical exercise and smoking cessation are the first measures for reducing cardiovascular risk in all patients.
The Mediterranean diet, with a reduced calorie intake in case of overweight or abdominal obesity, is accompanied by clear cardiovascular benefits and increased longevity. As well as having beneficial effects on lipid profiles, it has positive effects on hypertension and hyperglycaemia. Alcohol consumption should be moderated or avoided in cases of moderate to severe hypertriglyceridaemia.
Aerobic exercise is also paramount in atherogenic dyslipidaemia and in preventing and treating metabolic syndrome, hyperglycaemia, diabetes and cardiovascular disease.
Pharmacological treatmentDue to the fact that most patients with atherogenic dyslipidaemia have a high or very high risk of developing cardiovascular disease, it is generally necessary to combine lifestyle changes with different lipid-lowering drugs.
StatinsTreatment with statins will be initiated, choosing the type and dose that is necessary to achieve the therapeutic target, keeping in mind that increases in LDL-C and non-HDL-C are usually moderate. The benefits of statin treatment are well known and sufficiently demonstrated. A reduction of 1mmol/l (approximately 39mg/dl) is associated with a 21% reduction in the incidence of serious cardiovascular events and a 23% reduction in coronary events.17 One relevant fact is that 10-year treatment with statins in subjects with a low cardiovascular risk is related to a 23% reduction in non-fatal myocardial infarction events, in the case of low-dose statins, and a 53% reduction with more powerful statins, including a significant reduction in cardiovascular events.18,19
FibratesWhen the non-HDL-C and LDL-C targets have been achieved through treatment with statins, an unacceptable risk of cardiovascular events still remains due to the presence of the main components of atherogenic dyslipidaemia (hypertriglyceridaemia and decreased HDL-C). The administration of fibrates in these conditions corrects these lipid disorders and has additional cardiovascular benefits. In case of a contraindication or fibrate intolerance in patients with elevated TG levels, omega-3 fatty acids may prove beneficial.20,21 Fibrates have proven beneficial in primary and secondary prevention studies, as well as in the diabetic population, fundamentally in subgroups with atherogenic dyslipidaemia or any of its components, with a 28–30% reduction in cardiovascular events.
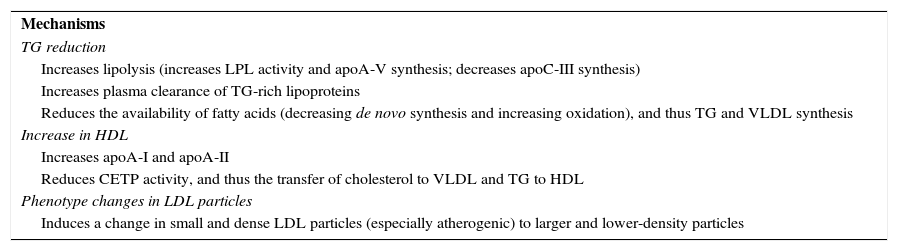
Fibrate-induced lipid changes are explained by modifications in the expressivity of various genes involved in lipid metabolism through peroxisome proliferator-activated receptors α (PPARα), with reductions in TG and LDL-C of 20–50% and 5–20%, respectively, a decrease in small and dense LDL particles, and a HDL-C increase of 5–20%. These effects are dependent on baseline concentrations11 and are shown in Table 3.22
Lipid effects of fibrates.
| Mechanisms |
| TG reduction |
| Increases lipolysis (increases LPL activity and apoA-V synthesis; decreases apoC-III synthesis) |
| Increases plasma clearance of TG-rich lipoproteins |
| Reduces the availability of fatty acids (decreasing de novo synthesis and increasing oxidation), and thus TG and VLDL synthesis |
| Increase in HDL |
| Increases apoA-I and apoA-II |
| Reduces CETP activity, and thus the transfer of cholesterol to VLDL and TG to HDL |
| Phenotype changes in LDL particles |
| Induces a change in small and dense LDL particles (especially atherogenic) to larger and lower-density particles |
apoA-I: apolipoprotein A-I; apoA-II: apolipoprotein A-II; apoA-V: apolipoprotein A-V; apoC-III: apolipoprotein C-III; CETP: cholesteryl ester transfer protein; HDL: high-density lipoproteins; LDL: low-density lipoproteins; LPL: lipoprotein lipase; TG: triglycerides; VLDL: very low-density lipoproteins.
The safest fibrate to combine with a statin is the fenofibrate; its addition achieves a greater cholesterol-lowering effect and control of non-HDL-C, TG and HDL-C.
Contrary to what happens with gemfibrozil, the combination of fenofibrate and a statin has demonstrated an excellent safety profile in all clinical studies including a large number of patients and a prolonged treatment period. Moreover, the effects of the fenofibrate-statin combination on the elevation of muscle and/or liver enzymes, or on temporary creatinine increases, do not differ from those observed in the monotherapy regimen and the reversibility of the effects is thus demonstrable.
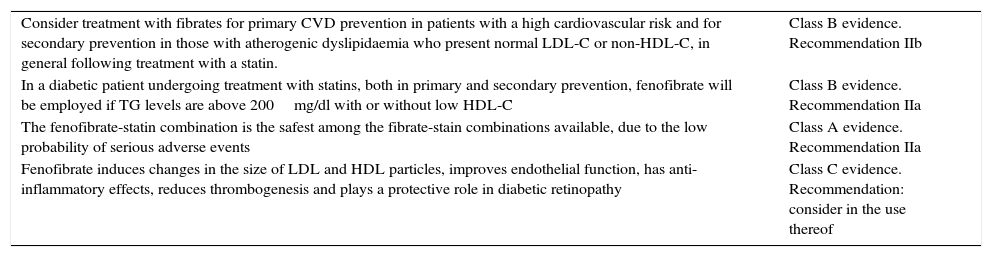
The main clinical evidence of fibrate treatment is shown in Table 4.15,23
Evidence of treatment with fibrates.
| Consider treatment with fibrates for primary CVD prevention in patients with a high cardiovascular risk and for secondary prevention in those with atherogenic dyslipidaemia who present normal LDL-C or non-HDL-C, in general following treatment with a statin. | Class B evidence. Recommendation IIb |
| In a diabetic patient undergoing treatment with statins, both in primary and secondary prevention, fenofibrate will be employed if TG levels are above 200mg/dl with or without low HDL-C | Class B evidence. Recommendation IIa |
| The fenofibrate-statin combination is the safest among the fibrate-stain combinations available, due to the low probability of serious adverse events | Class A evidence. Recommendation IIa |
| Fenofibrate induces changes in the size of LDL and HDL particles, improves endothelial function, has anti-inflammatory effects, reduces thrombogenesis and plays a protective role in diabetic retinopathy | Class C evidence. Recommendation: consider in the use thereof |
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; non-HDL-C: cholesterol bound to atherogenic lipoproteins (total cholesterol minus high-density lipoprotein cholesterol); CVD: cardiovascular disease; TG: triglycerides.
The results of clinical studies with fenofibrate have suggested, in addition to changes in the lipid profile, other vascular protection actions, such as increased nitric oxide expression and decreased oxidative stress. Fibrates exert an anti-inflammatory action on attenuating the production of inflammatory cytokines. Fenofibrate complements this anti-inflammatory action, significantly reducing levels of C-reactive protein, the CD40 ligand, monocyte chemoattractant protein-1 and the macrophage stimulating factor. It also reduces plasma fibrinogen concentrations by up to 20%, as well as thrombin-antithrombin complexes and plasminogen activator inhibitor-1. Another important extralipid effect is the slowdown of diabetic retinopathy progression, independent of glucose and lipid control. Furthermore, the abovementioned trials suggest that fenofibrate plays a protective role in diabetic nephropathy and neuropathy.23
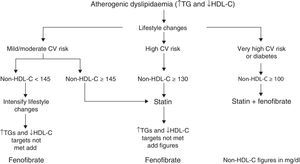
Based on these facts, the algorithm in Fig. 1 is established for treating atherogenic dyslipidaemia and controlling the accompanying cardiovascular risk.
Points of consensus in the treatment of atherogenic dyslipidaemia- –
Lifestyle changes, a low-fat diet with adequate calories for weight management, physical exercise and smoking cessation are very effective in all patients and the first step in dealing with atherogenic dyslipidaemia.
- –
Following lifestyle changes, the first safe and effective treatment employed in cardiovascular prevention is statins.
- –
Patients with hypertriglyceridaemia and low HDL-C, i.e. those with atherogenic dyslipidaemia, benefit from combined therapy with a statin and fenofibrate.
- –
The clinical practice guidelines and European Medicines Agency advocate fenofibrate plus a statin for the treatment of mixed hyperlipidaemia when TG and HDL-C levels are not adequately controlled.
- –
Evidence of the clinical benefit and safety of the statin-fenofibrate combination is robust. Combining both drugs in a single tablet simplifies the dosage and may facilitate compliance in the long term.
The authors declare that no experiments were conducted on human beings or animals for this research.
Confidentiality of dataThe authors declare that no patient data is contained in this article.
Right to privacy and informed consentThe authors declare that no patient data is contained in this article.
Conflicts of interestThere is no conflict of interest.
Please cite this article as: Ascaso JF, Millán J, Hernández-Mijares A, Blasco M, Brea A, Díaz Á, et al. Documento de consenso sobre el manejo de la dislipemia aterogénica de la Sociedad Española de Arteriosclerosis. Clin Invest Arterioscler. 2017;29:86–91.