The assessment and prevention of cardiovascular risk (CVR) that persists in patients with dyslipidaemia despite treatment and achievement of goals specific to the plasma concentration of cholesterol linked to low density (c-LDL) is a clinical challenge today, and suggests that conventional lipid biomarkers are insufficient for an accurate assessment of CVR.
Apart from their lipid content, there are other lipid particle characteristics. The results of this study show that there are a number of lipoprotein compounds that determine atherogenic potential and its influence on the CVR. However, such additional characteristics cannot be analysed by the techniques commonly used in clinical laboratories. Nuclear Magnetic Resonance (NMR), is a technique that allows a detailed analysis to be made of the amount, composition, and size of lipoproteins, as well as providing more information about the detailed status of lipid metabolism and CVR in dyslipidaemia patients.
In this article a group of lipidologists from the Spanish Society of Arteriosclerosis review the existing evidence on the atherogenic mechanisms of particles and describe the technical basis and interpretation of the profiles lipoproteins obtained by MRI, with special reference to the test available in Spain (Liposcale®). Likewise, the main patient profiles are defined as such that an analysis would provide information of greater clinical interest. These include: a) Suspected mismatch between lipid concentrations and particles, a common situation in diabetes, obesity, metabolic syndrome, (b) Early atherothrombotic cardiovascular disease (ECVA) or recurrent without CVR factors to justify it; c) Lipid disorders, rare or complex, such as extreme concentrations of c-HDL, and d) Clinical situations where classical analytical techniques cannot be applied, such as very low c-LDL values.
La evaluación y prevención del riesgo cardiovascular (RCV) que persiste en los pacientes con dislipidemia a pesar del tratamiento y de haber alcanzado los objetivos específicos de la concentración plasmática de colesterol unido a lipoproteínas de baja densidad (c-LDL) es un reto clínico en la actualidad, y sugiere que los biomarcadores lipídicos convencionales resultan insuficientes para una evaluación precisa del RCV. Más allá de su contenido lipídico, existen otras características propias de las partículas lipoproteicas que determinan su potencial aterogénico y su influencia en el RCV. Sin embargo, dichas características adicionales no pueden ser analizadas por las técnicas utilizadas habitualmente en los laboratorios clínicos. La espectroscopia por resonancia magnética nuclear (RMN), es una técnica que permite un análisis detallado de la cantidad, composición y tamaño de las lipoproteínas y proporciona información más detallada del estado del metabolismo lipídico y del RCV en los pacientes dislipémicos. En este artículo un grupo de lipidólogos de la Sociedad Española de Arteriosclerosis revisa la evidencia existente sobre los mecanismos aterogénicos de las partículas lipoproteicas y describen el fundamento técnico y la interpretación de los perfiles lipoproteicos obtenidos mediante RMN, haciendo especial referencia al test disponible en España (Liposcale®). Así mismo se definen los principales perfiles de pacientes en los que dicho análisis aportaría una información de mayor interés clínico, los cuales son: a) Sospecha de discordancia entre las concentraciones de lípidos y el número de partículas, situación frecuente en la diabetes, la obesidad, el síndrome metabólico y la hipertrigliceridemia; b) Enfermedad cardiovascular aterotrombótica (ECVA) precoz o recurrente sin factores de RCV que la justifiquen; c) Trastornos lipídicos infrecuentes o complejos como las concentraciones extremas de c-HDL, d) Situaciones clínicas en las que las técnicas analíticas clásicas no pueden aplicarse, como los valores de c-LDL muy bajos.
Cardiovascular disease (CVD) is the principle cause of mortality in Europe, where it is responsible for more than 4 million deaths a year. It is also a major cause of morbidity for the population and is an enormous economic and care burden for healthcare systems.1 Primary and secondary prevention based on evaluation of cardiovascular risk (CVR) is still a fundamental strategy to reduce the impact and consequences of CVD with an atherotrombotic origin (ACVD).2,3
Dyslipidaemia is a qualitative or quantitative alteration of plasma lipoproteins, including an increase in the concentration of cholesterol transported by low density lipoproteins (c-LDL). It is primordial when evaluating the risk of atherosclerotic disease, and it is a factor that can be modified and has played a fundamental role in prevention strategies over recent decades.2,3 Nevertheless, in spite of the progress that has been made, episodes of ACVD are seen in patients who are not classified as being high risk on current evaluation scales, and even in subjects whose plasma c-LDL concentration is within the target levels set by the guides. These episodes form what is known as residual risk, i.e., risk that persists in spite of achieving target c-LDL concentrations and other conventional CVR factors.4,5 This suggests that other lipid alterations and alterations of other types also influence CVR. Respecting lipid metabolism, it has therefore been shown that atherogenic dyslipidaemia (AD), defined as the imbalance between triglyceride-rich atherogenic lipoproteins that contain apo B and anti-atherogenic lipoproteins that contain apo A1, the increase in residual cholesterol and qualitative alterations in LDL particles are associated with residual risk.5–7 These alterations may increase the risk of CV events by up to 71%, even in patients treated pharmaceutically who have achieved target c-LDL concentrations in plasma.8
There are therefore other magnitudes associated with the lipid metabolism that is involved in atherogenesis and which go beyond the concentration of cholesterol in plasma as a total amount as well as of c-LDL and the cholesterol associated with the set of atherogenic lipoproteins (non-HDL cholesterol [c-non HDL]). Thus the characteristics of the lipoprotein particles themselves determine their atherogenic potential.9 Moreover, in the context of medical care, patients are often encountered in whom the conventional lipid biomarkers are insufficient, or who have limitations for determining them, so that their CVR may be under-estimated.10
Knowledge of the number, size and composition of lipoproteins completes the information about lipid metabolism and makes it possible to undertake a more complete evaluation of the clinical situation of patients. Study of lipoprotein particles using nuclear magnetic resonance (NMR) imaging makes this metabolic evaluation possible. This review covers the advanced analysis of lipoproteins using the Liposcale®, which was developed in Spain, which enables this technique to be used in clinical practice. It is based on the analysis of a serum or plasma sample using diffusion-ordered two-dimensional NMR that makes it possible to go a step further in the study of lipoproteins using NMR, as a single analysis gives the concentration and lipid composition of the lipoproteins on the one hand, and particle size on the other. Two-dimensional diffusion-ordered NMR is based on studying particle mobility within a fluid (serum or plasma) which is associated with the size of the particles, so that it may be considered to be a new generation technique within the context of this methodology. This technique makes it possible to directly measure the quantity, size and composition of lipoprotein fractions and subfractions.11
The purpose of this paper is to establish a series of recommendations for the use of this technique in clinical practice, based on a review of the scientific evidence now available on the use of Liposcale® advanced lipoprotein analysis.
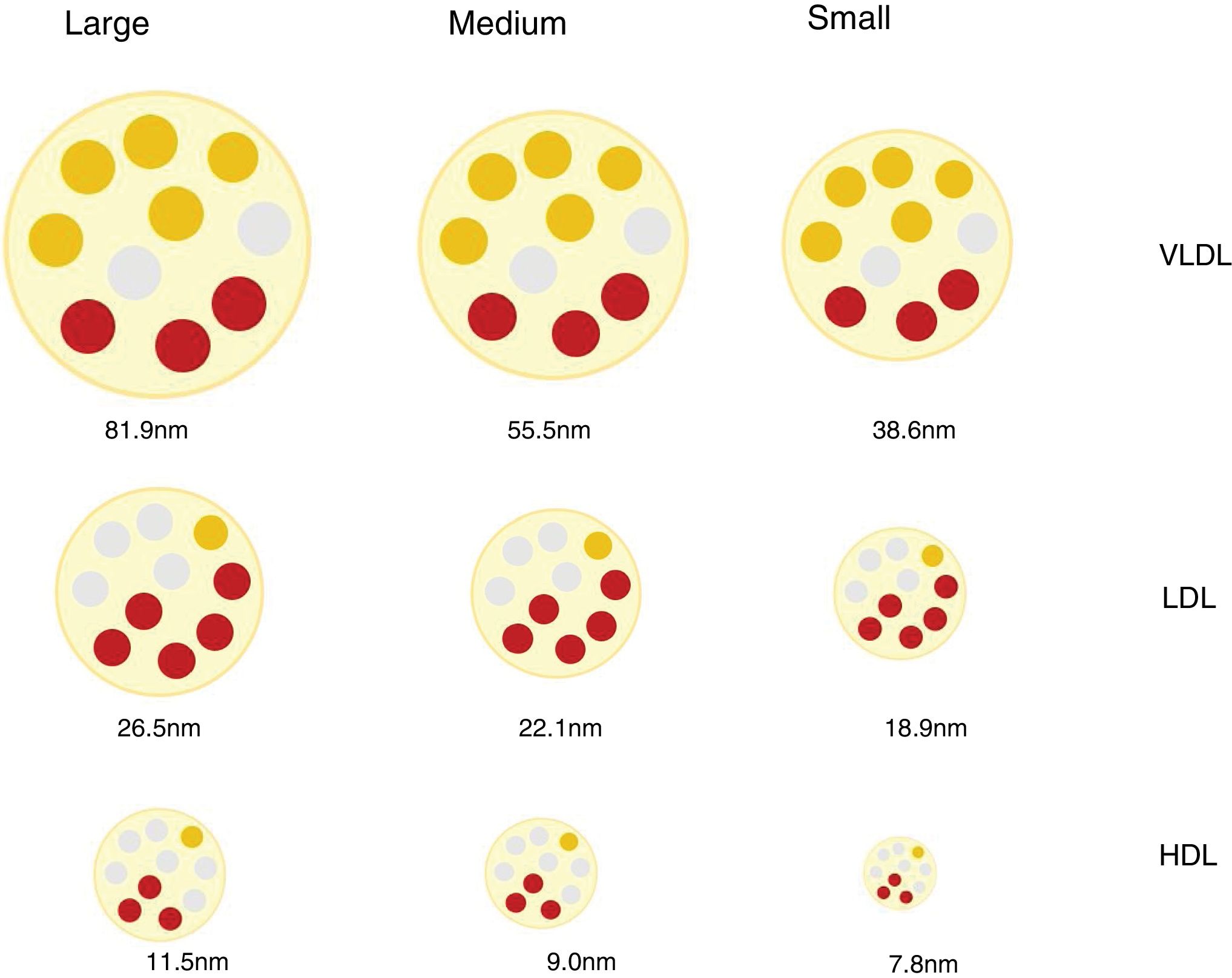
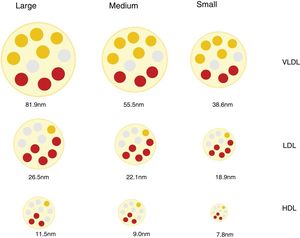
Lipoprotein particles, beyond cholesterol concentrationInteraction with the arterial wall and atherogenic mechanismsLipoproteins are spherical particles with a core that is chiefly composed of non-polar lipids (cholesterol esters and triacylglycerols. Their surface is composed of a single layer of polar lipids (phospholipids and free cholesterol) and proteins (apoproteins). These particles carry cholesterol and triglycerides to the tissues.12 The different proportions of lipids and proteins in the lipoproteins have different densities and allows them to be classified as chylomicrons, very low density lipoproteins (VLDL), intermediate density lipoproteins (IDL), low density lipoproteins (LDL) and high density lipoproteins (HDL). There is an inverse relationship between lipoprotein particle density and the size. Thus the former are larger in size and less dense, while the smaller and denser.12 The different classes of lipoprotein may in turn be divided into subclasses, as neither the size nor the density of lipoproteins are really discrete values (Fig. 1).
These differences in the number, size and composition of lipoproteins are relevant in the atherosclerotic process, and they influence atherogenicity.9,13 (e.g., small dense LDL particles in diabetes mellitus and metabolic syndrome14,15) and in associated CVR.16,17
Atherogenesis is a progressive and complex interaction between cells and circulating molecules, the vascular wall and modifications in blood flow characteristics that lead to the formation of lipid deposits on the arterial intima and the activation of an inflammatory response. Lipoproteins play a major role in this process.18
LDL particles and other lipoproteins associated with apo B (IDL, VLDL) with a diameter of less than 70 nm are able to pass freely through intact arterial wall. They are able to interact with wall proteoglycans and are susceptible to being retained in the extracellular matrix.9,19 Depending on the form of retention and the response, once the endothelial barrier has been crossed, a selective retention of LDL may take place at susceptible places. This is particularly so for small particles, due to their capacity to bind to the intima extracellular matrix proteoglycans. This is the primary event in the atherogenic process, and it triggers a series of modifications in the lipoproteins and local responses, including endothelial alterations with an increase of permeability to LDL particles, monocyte recruitment that facilitates the formation of foam cells, and inflammatory changes which lead in turn to more retention and the progression of the atheromatous plaque.9,20–22
The concentration and size of circulating particles and the permeability of the arterial wall are factors that condition the transendothelial influx of lipoproteins, so that a high concentration of particles and a small diameter favour passage towards the intima, increasing its atherogenic potential.9,20 The capacity to retain LDL particles is also conditioned by their affinity with proteoglycans, which is influenced by the protein and lipid composition of the lipoproteins.22
Triglyceride-rich IDL and VLDL lipoproteins are highly heterogeneous in terms of size, density and composition, and there is also an inverse relationship between their size and capacity to pass through the vascular endothelium.23 The residual VLDL particles generated following the enzymatic action of lipase lipoprotein (LLP) are smaller and cholesterol-enriched. Smaller diameter particles may penetrate the arterial wall and be captured directly by macrophages, giving rise to foam cells that are trapped in the intima, where they interact with other enzymes and mediators that contribute to the inflammatory response and plaque formation.9,24
HDL do not contain apo B and they play an essential role in cholesterol transport from peripheral tissues to the liver, within a heterogeneous and complex biological function which includes anti-inflammatory and cytoprotective effects. The composition of HDL is an important conditioning factor in the said functions, and it changes in situations associated with inflammation, obesity or hypertriglyceridaemia.25 The function of HDL has been proven to be altered in patients with diabetes mellitus, ACVD, chronic kidney disease (CKD) or systemic and autoimmune diseases such as sarcoidosis, so that the originally protective function of these lipoproteins is transformed into an atherogenic effect as the result of modifications produced by the inflammatory response.25,26 Respecting this, the inverse correlation in healthy individuals between c-HDL and CV mortality is reduced in patients with stable coronary disease, and it disappears in those with unstable disease.25
Discordance between the concentration in plasma of c-LDL and the concentration of LDL particlesLDL lipoproteins vary in size and this depends on the lipid content of their core, which in turn conditions the density of the particle. By using a range of analytical techniques different LDL particles have been identified and classified in an arbitrary and practical way as small, medium or large, with a density that is inversely proportional to their size.27
Different mechanisms intervene in the amount of cholesterol in LDL and in the genesis of small dense LDL lipoproteins (sdLDL) that contain less esterified and non-esterified cholesterol than large LDL particles. These mechanisms include the action of the cholesterol esters transport protein (CETP) and the lipase lipoprotein (LLP). In situations of metabolic alteration, such as the excess production of large VLDL particles, the resulting LDL are relatively cholesterol-depleted and triglyceride-enriched (TG). Subsequently, the latter reduce due to the action of hepatic lipase (HL), which reduces the size of LDL, giving rise to an increase in sdLDL particles.27,28 This same mechanism affects HDL,29 and this partially explains the relationship found between hypertriglyceridaemia, sdLDL and c-HDL deficit.27 It should be pointed out that these mechanisms which favour the production of sdLDL take place above all in metabolic situations associated with insulin resistance, such as those in AD, abdominal obesity, metabolic syndrome, kidney disease and chronic inflammatory processes.30
Due to the variability in cholesterol content of LDL particles, the concentration of c-LDL does not faithfully reflect the amount of particles that exist (p-LDL), especially under the said conditions. Thus a high amount of p-LDL may be associated with normal c-LDL concentrations, which would means that there is an excess of small particles low in cholesterol content, but with a high atherogenic potential. On the contrary, a high concentration of c-LDL may coexist with a normal amount of particles, which would mean that they are predominantly cholesterol-rich and larger in size, with less relative atherogenic potential.30 These situations are the basis for the concept of c-LDL:p-LDL discordance, the definition of which varies depending on the cut-off values selected, the population median for each parameter, and the absolute values established for each one as the threshold of normality.30 Several studies have shown that p-LDL have a closer association with the risk of progression of atherosclerosis and ACVD episodes than c-LDL and, in fact, in patients with discordance, the number of p-LDL is the best CVR indicator.16,31,32 Inversely, a low quantity of p-LDL has been shown to be a more sensitive indicator of lower CVR than c-LDL or c-non HDL.33
The most important consequence of the discordance between the c-LDL concentration and the amount of p-LDL is that it may lead to under- or over-estimations of a patient’s CVR.16,30,31 Given that the whole therapeutic strategy for cases of dyslipidaemia has the aim of preventing ACVD and that each patient is treated on the basis of their CVR level, an inaccurate or insufficient evaluation of their degree of CVR due to this discordance is highly important in practice. In fact, it is known that in monitoring statin treatment the c-LDL target is often insufficient for the purpose of controlling CVR, and it has been proven that therapeutic monitoring based on the number of LDL particles permits a better assessment of residual risk.10
The prognostic importance of the number, size and composition of lipoproteins in cardiovascular diseasesAs was pointed out above, according to the factors that influence and condition atherogenesis, the atherogenic potential of cholesterol-rich lipoproteins varies, and it is greatest in small dense LDL. Triglyceride-rich lipoproteins and their residual derived particles are also considered to be atherogenic, given that they are able to pass through the endothelium and reach the arterial wall.20,23 The prognostic value of the size, number and composition of lipoproteins has been evaluated in multiple clinical studies.17,31,33,34
A study undertaken in the Framingham study cohort showed that a low concentration of p-LDL (under percentile 25) was a more sensitive indicator of lower risk of ACVD than an equivalent concentration of c-LDL and c-non-HDL.33 It was also observed that plasma concentrations of cholesterol transported by sdLDL are strongly correlated with an atherogenic lipid profile and are significantly associated with the incidence of coronary disease, even in individuals at low CVR according to their c-LDL concentration.34 Similar results were obtained in another broad study by the Samia Mora group, which found that a high p-LDL number was a better predictor of coronary disease among women with a low plasma concentration of c-LDL than the concentration of c-LDL.31 Likewise, in women with a high c-LDL concentration, the presence of a low concentration of p-LDL was a better predictor of CVR.31 Even treatment based on p-LDL concentration has shown better results in terms of the incidence of ACVD than treatment based on concentrations of c-LDL.17
Recent studies have corroborated that smaller LDL particles are an independent CVR factor,35 and that particle size within the VLDL-LDL spectrum is also associated with residual risk in patients treated with statins.36 The proportion of subfractions of smaller and larger size LDL particles is also associated with CVR, so that a lower proportion of large subfractions and a higher proportion of small subfractions has been associated with increased CVR. Even the change in the composition pattern with an increase in the relative quantity of small particles and a reduction in the quantity of large particles is associated with increased CVR.37 To summarise, the amount and size of particles has been shown to have a more robust association with vascular disease than traditional lipid biomarkers (c-LDL, c-HDL and TG).38
On the other hand, the cholesterol associated with residual particles of chylomicrons and VLDL, which is defined as the cholesterol transported by lipoprotein particles that are neither HDL nor LDL, has been identified as a predictive factor for coronary disease that is independent of c-HDL and c-LDL concentration,39,40 with a risk increase of 2.8 times for every 39 mg/dl increase,39 and as a predictive factor for mortality due to any cause.41 Inversely, a low concentration of residual cholesterol is associated with a reduction in the risk of ischemic disease.42 Nevertheless, and in spite of its prognostic importance, the residual cholesterol concentration cannot be reliably obtained using the most usual laboratory techniques for determination, although this is possible using NMR imaging techniques.24
In the light of the above considerations and given that the atherogenicity of cholesterol and TG depends on the lipoproteins which contain them, determining the number, size and composition of these particles may offer highly valuable information for more precise assessment of CVR in clinical practice.
Methods available in current clinical practice for studying the lipid profile and lipoproteins in plasmaStandard lipid profileThe standard lipid profile is performed by biochemical analysis of a serum sample, measuring the concentration of cholesterol, triglycerides, and the cholesterol of HDL lipoproteins.
The concentration of c-LDL is estimated indirectly in usual practice using Friedewald’s formula (c-LDL = CT−c-HDL−TG/5 [or TG/2.21 in SI]).43 This method is not free of limitations deriving from its basic principles, as the result of the formula also includes the cholesterol of the IDL and lipoprotein (a) [Lp(a)]. The formula assumes that there is a constant proportion of cholesterol vs. TG in VLDL particles, and it ignores the chylomicrons as well as any possible excess in residual particles. In practice, this means that the formula under-estimates c-LDL as the TG increase, so that above a TG concentration of 200 mg/dl the result of the concentration of c-LDL calculated starts to become under-estimated. According to the most relevant publications, the formula should not be used when the triglyceride concentration is higher than 400 mg/dl, as a total error greater than is permissible is found to exist when it is compared to the reference method. Moreover, in patients with plasma concentrations of c-LDL below 70 mg/dl a calculated value of c-LDL less than zero may be obtained.44,45 Nevertheless, the majority of studies and reference values in a range of populations were undertaken using this formula, applying it when possible and taking its limitations into account.
Other methods also exist for directly measuring c-LDL in serum samples without previous separation, although they have certain limitations. These limitations include high concentrations of triglycerides and the discrepancy between the results obtained using reagents by different manufacturers.
An alternative to calculating c-LDL is to obtain the cholesterol of lipoproteins excluding the HDL cholesterol, which is commonly known as non-HDL cholesterol. This is calculated by subtracting the cholesterol concentration from the HDL cholesterol obtained. This calculation gives the concentration of cholesterol transported by all of the atherogenic particles.24,44 Its predictive value has been established in several studies36,46–48 and it is considered to be a residual risk indicator as it includes the cholesterol of residual particles.24,44 C-non HDL has been included as a therapeutic target in several clinical guides,2,3,49,50 although it has to be taken into account that hypertriglyceridaemia may also affect the precision of methods of measuring c-HDL that are necessary calculating it.
The basic lipid profile may be considered to offer information on the concentration of total and non-HDL cholesterol, as well as HDL and LDL lipoproteins and total triglycerides. However, this is not the case for other aspects such as the number of particles, their composition or their size, and these characteristics are associated with their differing atherogenic potential.
Apo B and apo A1Measurement of the mass concentration of apo B-100 is now considered to be a possible alternative to calculating c-non HDL. It is analysed by immunoturbidometric methods that do not require fasting and which are traceable using a calibration standard (WHO/IFCC SP3-07).44 For each VLDL lipoprotein initially secreted by the liver, there is only one molecule of apo B, and this also remains in the lipoprotein throughout its metabolic lifespan, so that the concentration of apo B is considered to be a direct measurement of the total number of atherogenic lipoprotein particles. The latest guides on the management of dyslipidaemia published by the European Societies of Cardiology and Atherosclerosis3 state that this marker is important in the evaluation of lipid metabolism. In a situation of normotriglyceridaemia, the majority of the mass concentration of apo B corresponds to the LDL content. This is because it has a longer average lifespan (days) in comparison with VLDL (hours).24 It has proven value as a predictive factor for CVR and is superior to that of c-LDL.44 One of the limitations of measuring apo B is that it is not used as a therapeutic target in clinical guides.44 However, several clinical practice guides recommend determining it and setting therapeutic targets secondary to those using c-LDL. It has to be taken into account that although this measurement is associated with the number of atherogenic particles, it offers no information on particle type or size, or the distribution of the lipoproteins that contain them.24,51 In other words, an apo B molecule may indicate the presence of a large VLDL or a sdLDL.
Apo A1 mass concentration is also analysed using immunoturbidimetry, and current methods have calibrators that are traceable to international standards WHO/IFCC SP1-01 without the need to obtain the sample while fasting. The ApoB:ApoA1 index may be used to evaluate AD and CVR.6,44 Some studies have shown that an increase in apo A1 but not in c-HDL is associated with a fall in CVR in patients treated with statins.52 Unlike what happens with apo B, each HDL particle may contain from 1 to 5 molecules of apo A1, so that its measurement cannot be considered to be equivalent to a quantity of HDL lipoproteins. Moreover, measuring apo A1 does not show the functionality of HDL.
Study of lipoprotein particlesSeveral analytical techniques make it possible to separate and characterise lipoprotein particles.
Ultra-centrifuging with a density gradient is the standard technique for the separation and quantification of plasma lipoproteins. Essentially, a discontinuous density gradient is created during centrifuging that permits lipoproteins to separate and accumulate in layers according to their densities, so that the concentration of the lipids in each type can be determined. However, the main limitation of this technique is that it is very laborious and analysis is very time consuming, leading to a high cost and lack of feasibility for clinical analysis laboratories with a large volume of samples.53,54
Several chromatographic techniques such as high performance liquid chromatography in gel (GP-HPLC) permit the separation of lipoproteins based on particle diameter, as the small ones have longer elution times than the large ones. This technique can be used to fraction lipoproteins according to their size, but it requires a combination of enzymatic methods to quantify the lipids, and these laborious techniques also depend strongly on the operator.55
Electrophoretic methods make it possible to separate and analyse lipoproteins based on their size. Although they permit an approximation to the sizes of LDL, HDL and VLDL particles and their subclasses, it requires an ad hoc preparation of the gels, and their the results of different laboratories cannot be interchanged as there are no international standards,54,56 which is clearly a drawback for their clinical use.
Ion mobility analysis is based on the principle that lipoproteins behave differently depending on their size when they are transported by a laminar airflow subjected to an electric field. These electrophoretic mobility differences of lipoprotein particles in gas phase make it possible to determine their size and quantify them into subfractions.57,58 This technique requires complex pre-treatment of the sample, and this leads to a high level of variability in the results from different laboratories, as well as requiring important technical infrastructure, so that this technique is hardly suitable for clinical use.
In any case, and apart from the intrinsic limitations of each technique, the biomarkers measured and procedures for sample preparation and handling mean that laboratory personnel have to be highly qualified, and there is very heavy consumption of time and resources, among other aspects.
Liposcale® and lipoprotein profilesTechnical basis for directly measuring lipoprotein particlesNMR imaging has been used for years to determine the concentration in serum or plasma of lipoprotein particles, as well as their size and to infer their composition.
It is based on the fact that the molecular structure of the esterified cholesterol and triglycerides which are transported inside lipoproteins contains methyl groups that, under the influence of radiofrequency pulses, resonate at frequencies that differ slightly depending on the size of the lipoprotein that transports them, thereby generating a spectrum of the said frequencies. The smaller the lipoprotein, the lower the resonance frequency of the lipids in its core. Thus by using NMR-based analytical techniques, it is possible to directly measure the size of the lipoproteins.
The Liposcale® test is a method based on two-dimensional NMR spectroscopy, which analyses the attenuation of the lipid peak signals when they are subjected to a known gradient of magnetic field. The difference in signal attenuation is associated with a different diffusion of lipoprotein particles (Diffusion Ordered Nuclear Magnetic Resonance Spectroscopy [DOSY-NMR]). The two-dimensional DOSY-NMR study makes it possible to know the hydrodynamic characteristics of the molecules, as well as the diffusion coefficient associated with each lipoprotein subclass. Different lipoprotein subclass sizes are calculated directly on the basis of the diffusion coefficients using the Stokes-Einstein equation.11,59 Direct measurement of size is of particular importance, as it is used to finally calculate the number of lipoprotein particles by dividing the spatial volume of the total lipid molecules by the average volume.
Liposcale® is the result of a project developed in Spain that offers a new generation of technology based on the use of NMR to study lipoproteins.
The spectrum generated by Liposcale® is translated into information on the number and size of lipoprotein particles distributed arbitrarily in three fractions (VLDL, LDL and HDL) each of which contains three subfractions. The cholesterol and TG content of each type of lipoproteins are also determined.11 The c-LDL concentration as well as the number of LDL particles, including Lp(a) cannot be quantified individually using this technique.
Liposcale® validation studies have shown its superiority to Lipoprofile® in terms of the correlation between the number of lipoprotein particles and the concentration of apoproteins in the subfractions isolated by centrifuging. It has also shown an excellent correlated with data on circulating particle size as determined by electron microscope.11
Lipoprotein levels and the lipid profileLiposcale® analysis produces more than 25 variables associated with lipoprotein particles:
- •
the concentration of VLDL, LDL and HDL particles and their respective subclasses (large, medium and small);
- •
average size (diameter) of VLDL, LDL and HDL;
- •
cholesterol and triglycerides concentration of the main types of lipoproteins (VLDL, IDL, LDL and HDL).
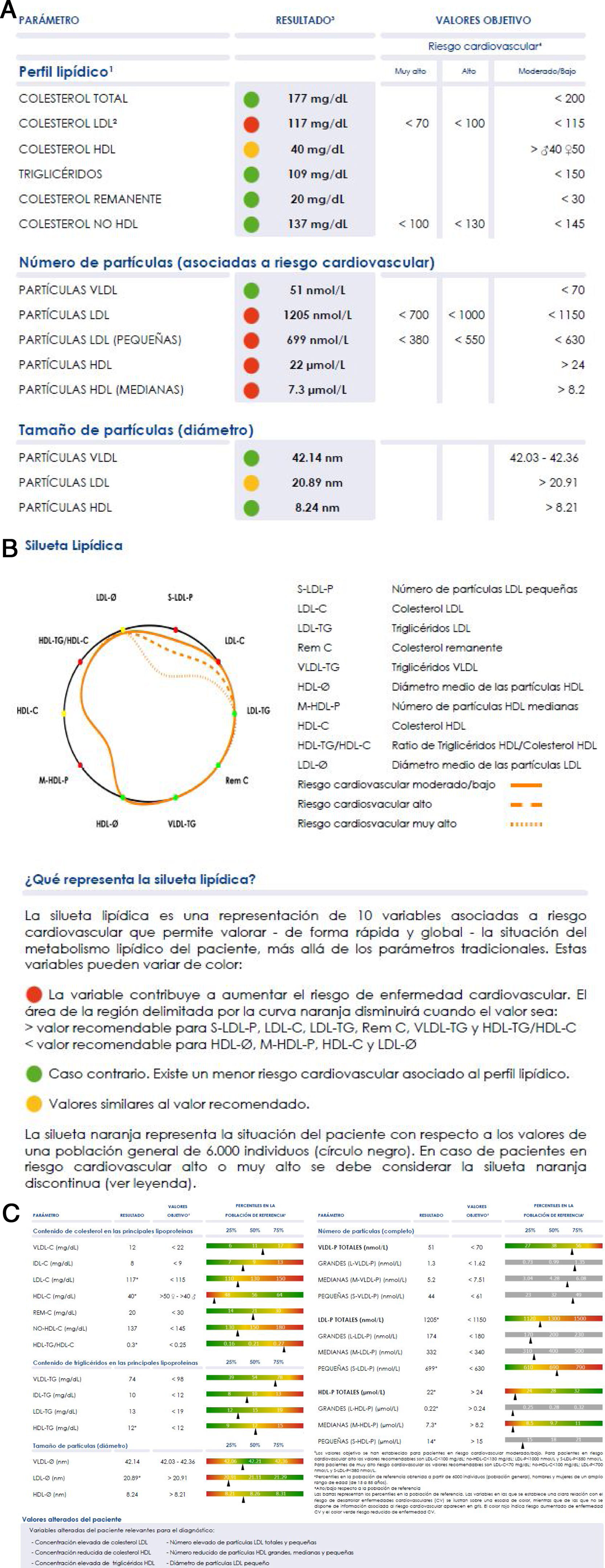
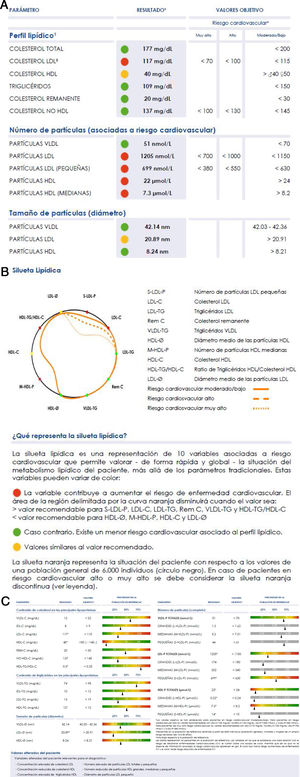
The large number of data, although it underlines the enormous potential of the technique, hinders its clinical use, as this requires simplicity of interpretation. A graphic display of the main findings has therefore been designed, to simply inform about the lipoprotein profile of the patient in comparison with the standard profile we show in Fig. 2. The orange profile represents the situation of the patient respecting the values of a general population of 6000 individuals (black circle). The individual profile delimits a smaller area when the variables show values associated with a higher risk of developing cardiovascular diseases (values above the 70 percentile in VLDL-C, VLDL-TG, VLDL-P, LDL-C, LDL-TG, S-LDL-P and HDL-TG, or under the 30 percentile in LDL-Ø, HDL-Ø, HDL-C and M-HDL-P); and a larger area in the contrary case. If the variables contribute to clearly reducing the area delimited by the curve, they are shown in red; and if the opposite holds they are shown in green. If a value is similar to the 70 or 30 percentiles, according to the classification of variables described above, it is shown in yellow.
The Liposcale® test characterises plasma lipoprotein profiles and detects alterations that predispose to ACVD, enabling preventive clinical action to be implemented37,60–62 over and above atherogenic cholesterol levels (c-LDL or c-non HDL) or total triglyceride levels.
Indications for complete lipoprotein study using NMRIt is a notorious fact that ACVD still appears in patients who keep c-LDL concentrations within target levels. This is because of what is known as residual risk,5 in which AD plays a major role.5–7
AD is frequent in patients with diabetes mellitus, obesity, metabolic syndrome, chronic kidney disease and familial hyperlipidaemia combined with polycystic ovary syndrome, among other pathologies, all of which are associated with higher CVR.6 In these patients, measuring c-LDL concentrations is considered insufficient to evaluate the CVR associated with alterations in the lipid metabolism, so that it is recommended to determine other biomarkers as well, such as c-non HDL or apo B.6,44 Nevertheless, as was remarked above, c-non HDL concentrations are directly influenced by TG concentrations, to the degree that the correlation between c-LDL and c-non HDL falls as the TG increase,63 so that it does not offer us information on the type of lipoproteins that are present. Apo B is a biomarker that directly informs about the number of lipoprotein particles, although it does not distinguish the type of atherogenic particles or their sizes.
Although Liposcale® offers information that is clinically useful, about the number, composition and size of lipoprotein particles, its cost, which is higher than that of standard parameters, means it is necessary to define the patient groups in which it is most useful in clinical terms, to contribute to diagnostic and therapeutic decision-making.
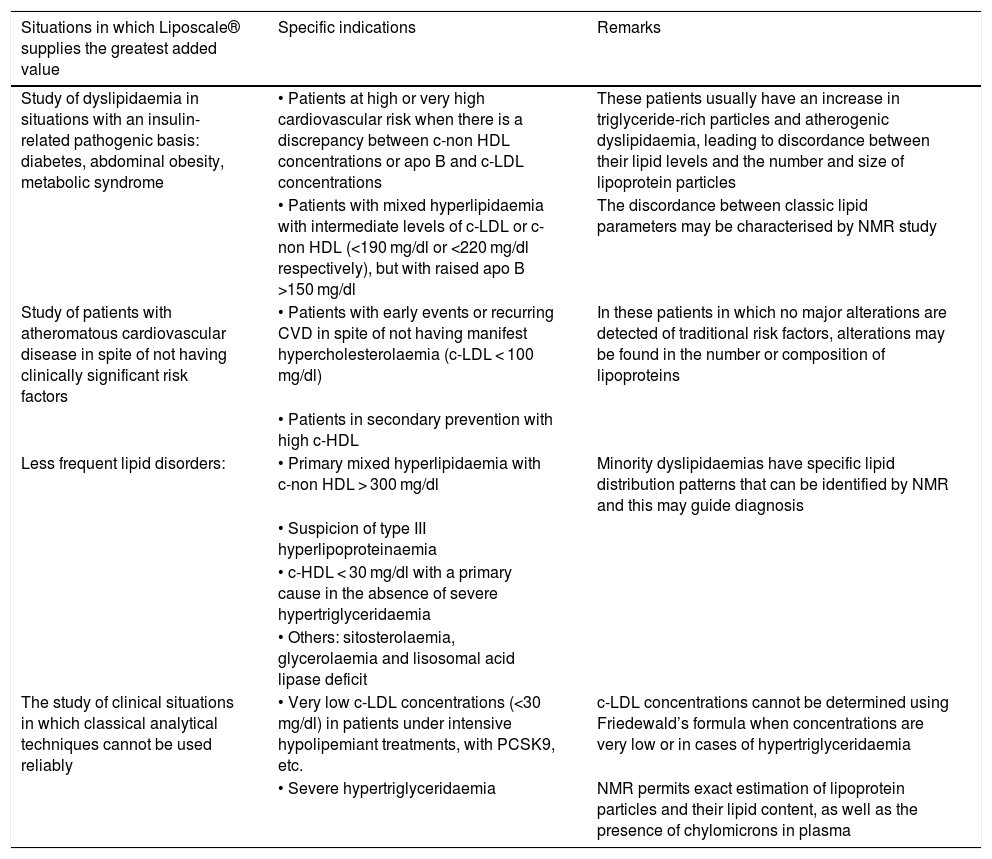
Table 1 shows the groups of patients in which the advanced lipoprotein test (Liposcale®) may be of the greatest clinical utility.
Patients in which the advanced lipoproteins test (Liposcale®) may be of greatest clinical utility.
| Situations in which Liposcale® supplies the greatest added value | Specific indications | Remarks |
|---|---|---|
| Study of dyslipidaemia in situations with an insulin-related pathogenic basis: diabetes, abdominal obesity, metabolic syndrome | • Patients at high or very high cardiovascular risk when there is a discrepancy between c-non HDL concentrations or apo B and c-LDL concentrations | These patients usually have an increase in triglyceride-rich particles and atherogenic dyslipidaemia, leading to discordance between their lipid levels and the number and size of lipoprotein particles |
| • Patients with mixed hyperlipidaemia with intermediate levels of c-LDL or c-non HDL (<190 mg/dl or <220 mg/dl respectively), but with raised apo B >150 mg/dl | The discordance between classic lipid parameters may be characterised by NMR study | |
| Study of patients with atheromatous cardiovascular disease in spite of not having clinically significant risk factors | • Patients with early events or recurring CVD in spite of not having manifest hypercholesterolaemia (c-LDL < 100 mg/dl) | In these patients in which no major alterations are detected of traditional risk factors, alterations may be found in the number or composition of lipoproteins |
| • Patients in secondary prevention with high c-HDL | ||
| Less frequent lipid disorders: | • Primary mixed hyperlipidaemia with c-non HDL > 300 mg/dl | Minority dyslipidaemias have specific lipid distribution patterns that can be identified by NMR and this may guide diagnosis |
| • Suspicion of type III hyperlipoproteinaemia | ||
| • c-HDL < 30 mg/dl with a primary cause in the absence of severe hypertriglyceridaemia | ||
| • Others: sitosterolaemia, glycerolaemia and lisosomal acid lipase deficit | ||
| The study of clinical situations in which classical analytical techniques cannot be used reliably | • Very low c-LDL concentrations (<30 mg/dl) in patients under intensive hypolipemiant treatments, with PCSK9, etc. | c-LDL concentrations cannot be determined using Friedewald’s formula when concentrations are very low or in cases of hypertriglyceridaemia |
| • Severe hypertriglyceridaemia | NMR permits exact estimation of lipoprotein particles and their lipid content, as well as the presence of chylomicrons in plasma |
Lastly, it should be underlined that lipid profile study using NMR may be highly useful in situations of cardiovascular risk or dyslipidaemia that has been poorly defined by conventional clinical studies and laboratory tests. In these situations, the data supplied by NMR make it possible to define alterations in lipoprotein structure and composition more precisely, as well as the associated CVR.
Conclusions2D NMR spectrometry which is the basis of Liposcale® permits the direct analysis of lipid metabolism, over and above the usual clinical parameters. This is because it makes it possible to differentiate the properties of the different lipoproteins and their respective subfractions, characterising them according to their composition and size, as well as quantifying the number of each one of these particles. This more complete and detailed information about lipoprotein metabolism alterations and the associated CVR can be of special benefit for certain patient profiles, including those with: a) suspicion of discordance between their lipid concentrations and the number of particles, such as in cases of diabetes, obesity, metabolic syndrome and hypertriglyceridaemia; b) early onset or recurring ACVD without CVR factors that justify this; c) rare or complex lipid disorders, such as extreme concentrations of c-HDL, and d) clinical situations in which classical analytic techniques cannot be used, such as when c-LDL levels are very low.
FinancingWe would like to thank Laboratorios Rubió for financing the meeting in which the debate that originated this paper started, as well as the work of the medical editor. Laboratorios Rubió has not influenced the contents of the manuscript, and the authors have undertaken their scientific work with total independence.
Conflict of interestsXP, LM, FC, JR, DI, JP, JLD and PV: have received fees for congresses and/or consultancy work for several pharmaceutical companies involved in hypolipemiant therapies. NA and LM are shareholders in the Universidad Rovira i Virgili spin-off: BiosferTeslab; CB and EM have no conflict of interests to declare.
Please cite this article as: Pintó X, Masana L, Civeira F, Real J, Ibarretxe D, Candas B, et al. Documento de consenso de un grupo de expertos de la Sociedad Española de Arteriosclerosis (SEA) sobre el uso clínico de la resonancia magnética nuclear en el estudio del metabolismo lipoproteico (Liposcale). Clin Investig Arterioscler. 2020;32:219–229.