Monocytes play an important role in atherosclerotic progression having both pro and anti-inflammatory effects depending on different circulating monocyte subpopulations. The objective of this study is to characterise these subpopulations and their association with cardiovascular risk factors.
MethodsTransversal study including 102 selected patients, mean age: 65 years-old (range 41–86), 69% males. A set of specific antibodies against classical monocytes (Mon1, CD14+CD16− CD300e+HLADR+), intermediate (Mon2, CD14+CD16+CD300e+HLADR+) and non-classical (Mon3, CD14−CD16+CD300e+HLADR+) was assayed.
Three groups of patients were included: 17 asymptomatic with more than one cardiovascular risk factor (group 1), 56 subjects asymptomatic but with vascular pathology assessed by ultrasound or microalbuminuria (group 2) and 19 patients with a previous atherothrombotic event (group 3). The cardiovascular risk was determined by Framingham and REGICOR scores.
ResultsAn association between study groups and the percentage of Mon1 and Mon2 was observed (ANOVA, p<0.05), being independent of age and sex for Mon2. Likewise Mon1 and Mon2 subpopulations were associated with cardiovascular adverse events (β=0.86, p=0.02 and β=0.1 p=0.002, respectively), independently of age and sex in the case of Mon2.
Moreover the percentage of Mon3 was associated with the presence of several cardiovascular risk factors (β=0.21, p=0.04) in the univariate analysis. In addition, there was a correlation between the levels of Mon1 and Mon2 and leukocytes (r=0.7, p<0.001 and r=0.26, p=0.01, respectively).
ConclusionsThe analysis of monocyte subpopulations may be clinically useful to stratify the inflammatory profile related to the different cardiovascular risk groups.
La participación monocitaria en la progresión aterosclerótica y sus efectos pro- o antiinflamatorios dependen de las subpoblaciones circulantes. El objetivo de este estudio es la caracterización de dichas subpoblaciones y su asociación con los factores de riesgo cardiovascular.
MétodosEstudio transversal que incluye 102 pacientes seleccionados; edad media: 65 años (rango 41–86 años), 69% varones. Se utilizó un panel de anticuerpos específicos frente a monocitos clásicos (Mon1, CD14+CD16− CD300e+HLADR+), intermedios (Mon2, CD14+CD16+CD300e+HLADR+) y no clásicos (Mon3, CD14−CD16+CD300e+HLADR+).
Se establecieron tres grupos de estudio; grupo 1: sujetos asintomáticos con más de un factor de riesgo cardiovascular (n=17); grupo 2: sujetos asintomáticos, pero con patología vascular por ecografía o microalbuminuria (n=56); y grupo 3: pacientes con algún evento vascular aterotrombótico previo (n=19). Asimismo, se calculó el riesgo cardiovascular mediante las escalas Framingham y REGICOR.
ResultadosSe observó una asociación entre las subpoblaciones Mon1 y Mon2 y los grupos del estudio (ANOVA, p<0,05), independiente de la edad y el sexo para los Mon2. Asimismo, las subpoblaciones Mon1 y Mon 2 se asociaron con eventos vasculares adversos (β=0,86, p=0,02 y β=0,1 p=0,002, respectivamente), siendo la asociación de Mon2 independiente de la edad y el sexo. Además, el porcentaje de Mon3 se asoció con la presencia de más de 2 factores de riesgo cardiovascular (β=0,21, p=0,04) en el análisis univariante. Finalmente, se halló una correlación entre los niveles de Mon1 y Mon2 con el número de leucocitos (r=0,7, p<0,001 y r=0,26 p<0,01, respectivamente).
ConclusionesEl análisis de subpoblaciones monocitarias es de gran interés clínico, ya que permite establecer un diferente perfil inflamatorio según los grupos de riesgo cardiovascular establecidos.
Cardiovascular diseases (CVD) are responsible for 3.9 million deaths in Europe every year, making them the main cause of mortality worldwide.1
Atherosclerosis represents the main substrate of CVDs and chronic inflammation of the vascular wall has a central role in its pathogenesis. Classical cardiovascular risk factors (CVRFs) are associated with the activation of immune system cells that are present in atherosclerotic lesions and promote their progression.2 Monocytes are a critical component of the immune response to inflammation, since once they have been recruited by the endothelium, thanks to the activity of cytokines and adhesion molecules, they adopt a change of phenotype to an inflammatory profile that plays an important role in the progression of atherosclerotic plaque and myocardial remodelling.3,4
Traditionally, monocytes have been divided into two populations according to the expression of CD14 and CD16, with clearly differentiated functions: type 1 or classical monocytes, the role of which was inflammatory and type 2 or non-classical monocytes, which had an anti-inflammatory and patrol function eliminating damaged endothelial cells and maintaining the integrity of the vessel. However, in 2010, a new classification was established with three subpopulations of monocytes: classical (Mon1, CD14+CD16−), intermediate (Mon2, CD14+CD16+) and non-classical (Mon3, CD14+CD16+)5,6 with clear differences in phenotype, function and genetic expression and also in responses to stimuli among the groups of CD16 monocytes.5 Intermediate monocytes have been proposed as a monocyte in transition from a classical monocyte to a non-classical monocyte.4 Based on their surface antigens and cell function, intermediate monocytes have both a phagocyte function and anti-inflammatory effects such as high levels of IL-1β and tumour necrosis factor (TNF)α.7 However, recent studies indicate that CD16+ monocytes, i.e. types 2 and 3, are involved in inflammatory and infectious processes,7 have the ability to secrete proinflammatory molecules such as IL-6, MMP-9 and CCR2 and present high affinity for the activated endothelium,8 with type 3 monocytes (CD14+) being those that have the greatest inflammatory activity and production of cytokines in response to toll-like receptor (TLR) ligands and the first to reach the site of the lesion.7
In recent years, strategies have been developed in order to be able to identify patients at greater risk of developing CVD at an early stage with the aim of being able to carry out better prevention.9 Due to the different inflammatory profile expressed by the different monocyte populations and their relationship with cardiovascular risk, the objective of the study was to analyse the monocyte subpopulations in peripheral blood in different groups of patients established based on CVRFs.
Subjects and methodsStudy design and patientsBetween April 2016 and December 2017 a total of 102 patients aged between 45 and 86 were included with CVRFs and subclinical and clinical atherosclerosis recruited in Internal Medicine consultations, Vascular Medicine and Occupational Medicine Area of the Clínica Universidad de Navarra [Navarre University Clinic] (CUN) and of the department of Internal Medicine of the Complejo Hospitalario de Navarra [Navarre Hospital Complex] (CHN).
Patients over the age of 45 with two or more CVRFs were included. The exclusion criteria were: presenting with an active, acute or chronic inflammatory tumour disease of any aetiology and having received treatment with non-steroidal anti-inflammatory drugs (NSAIDs) or steroids in the two weeks prior to the analytical extraction.
The Biobank of the Universidad de Navarra supplied the data for the patients included in the study which were processed following the standard operating procedures approved by the Ethics and Scientific Committees.
Risk factors and risk groupsIn general, those patients who had systolic blood pressure >140 or diastolic blood pressure >90 and/or who were taking antihypertensive drugs were considered as hypertensive; those who had total cholesterol >200, LDL >130 and HDL <59 or who were taking lipid-lowering drugs were considered as dyslipidaemic; those who had triglycerides >150 were considered as having hypertriglyceridaemia; if the patients’ diagnosis was established by the glucose curve or they were taking oral antidiabetic drugs or insulin then they were considered as diabetic. Obesity was assessed according to body mass index (BMI) or according to waist circumference.
Those patients who continued to smoke or who had given it up less than a year before inclusion in the study were recorded as smokers, according to what was reported in the questionnaires.
Patients were divided up into three groups according to the clinical data collected:
- -
Group 1: asymptomatic patients with two or more CVRFs (n=17).
- -
Group 2: asymptomatic patients in whom vascular pathology had been detected by imaging test or microalbuminuria (subclinical atherosclerosis, n=56).
- -
Group 3: patients with clinical manifestations of CVD (n=19).
Likewise, the cardiovascular risk was calculated according to the Framingham and REGICOR risk scales.
Medical history and physical examinationA clinical assessment was carried out by means of a medical interview and physical examination taking blood pressure and heart rate. Anthropometric data was collected by calculating the BMI.
A collection was then carried out of demographic and anthropometric parameters (age, gender, BMI), previous pathologies (stroke, coronary heart disease, peripheral arterial disease and kidney failure), CVRFs (hypertension, dyslipidaemia, diabetes mellitus and smoking), routine medication and family history. The presence of atherosclerotic plaques was determined by carotid, abdominal and femoral ultrasound and the carotid intima-media thickness was measured using the Siemens S2000 and S3000 ultrasound system.
Analytical studyAn analytical study was performed (after fasting for at least 8h) within the routine care process of CVRF assessment: lipid profile (total cholesterol, LDL, HDL and triglycerides), serum and urine creatinine levels, urine albumin, liver function tests, glucidic profile (glycated haemoglobin and baseline fasting blood glucose), as well as inflammatory parameters such as C-reactive protein.
The venous blood samples were obtained during fasting in tubes which contained EDTA 1g/l and heparin sodium (17U/ml), as well as a sample of urine from an isolated urination. Plasma was separated from whole blood by centrifugation at 1500×g for 15min at 4°C.
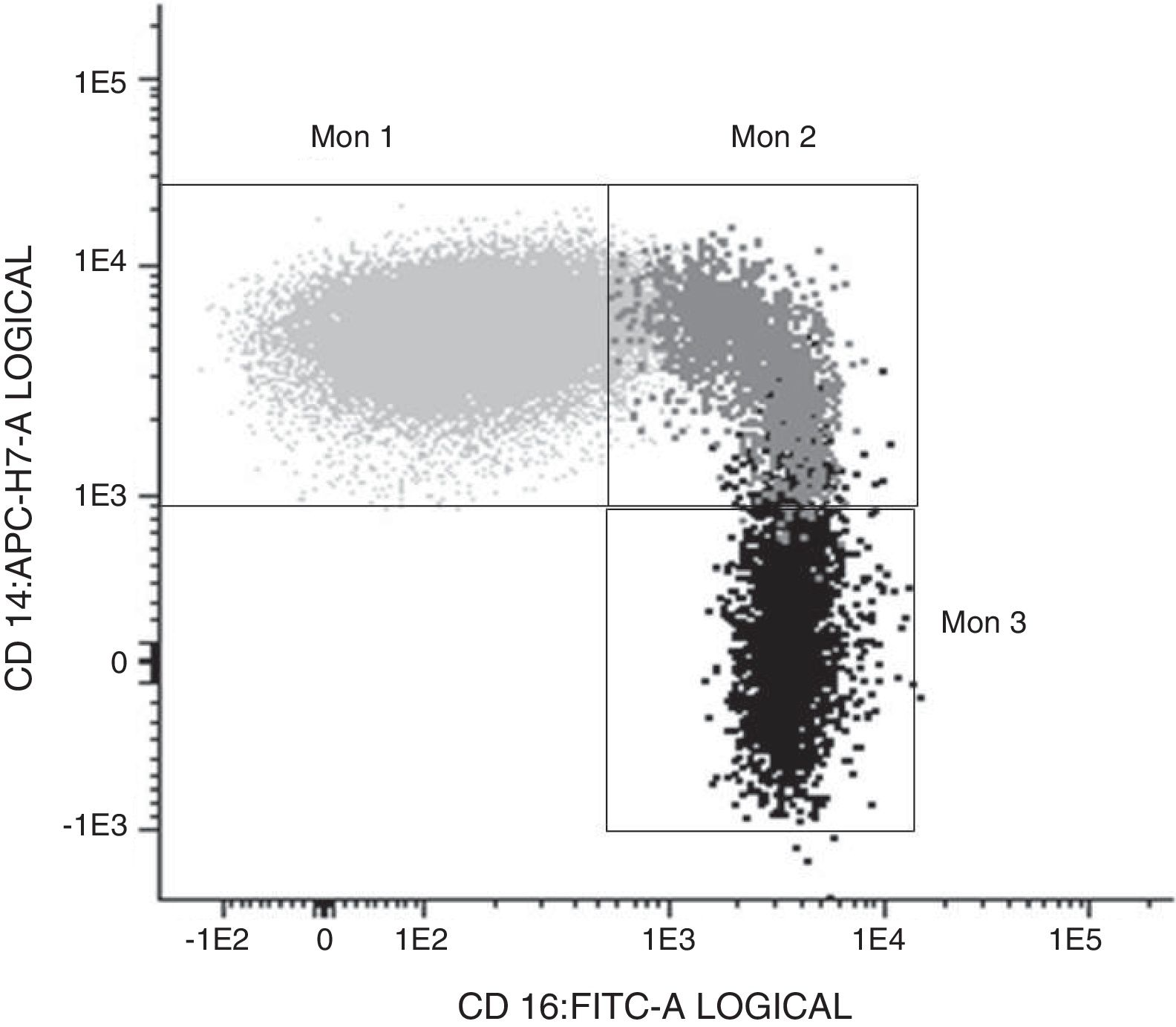
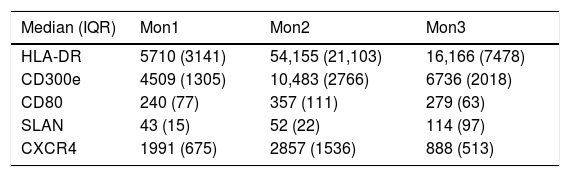
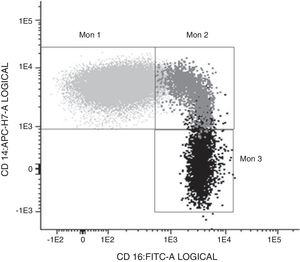
Determination of monocyte subpopulations by flow cytometryThe characterisation of monocytes was performed using flow cytometry in a sample of fresh anticoagulated peripheral blood in EDTA, acquiring the samples in a FACS Canto II cytometer and using the FACSDiva software (Becton Dickinson Bioscience, San José, CA). The data were analysed with the Infinicyt software (Cytognos, Salamanca). The classification established in the consensus document of the European Society of Cardiology5 was used in the study, using a panel of antibodies common to the antigen-presenting cells (HLA-DR), others specific against monocyte/macrophage behaviour (CD14, CD16, CD80, CD163, CD300e, SLAN), as well as the chemokine receptor 4 (CXCR4). Classical monocytes (Mon1) were characterised as being CD14++CD16−, CD300e+HLADR+, intermediate (Mon2) as being D14+CD16+CD300e+HLADR+ and non-classical (Mon3) were CD14loCD16++CD300e+HLADR+, as can be seen in Table 1 and in Fig. 1.
Characterisation of the monocyte subpopulations according to surface markers.
| Median (IQR) | Mon1 | Mon2 | Mon3 |
|---|---|---|---|
| HLA-DR | 5710 (3141) | 54,155 (21,103) | 16,166 (7478) |
| CD300e | 4509 (1305) | 10,483 (2766) | 6736 (2018) |
| CD80 | 240 (77) | 357 (111) | 279 (63) |
| SLAN | 43 (15) | 52 (22) | 114 (97) |
| CXCR4 | 1991 (675) | 2857 (1536) | 888 (513) |
Fluorescence intensity values expressed as median and interquartile range (IQR).
The data were collected and analysed with the statistical package STATA version 13.0. The Kolmogorov–Smirnov test was conducted to evaluate the distribution normality of the research parameters. The data were expressed as mean±standard deviation. The Student's t-test was performed for the comparison between means of two groups and analysis of variance (ANOVA) was performed on one factor for the comparison of variables with respect to the established risk group, followed by Bonferroni correction for multiple comparisons. Analysis of covariance (ANCOVA) was used to correct confounding factors such as age and gender. The linear regression test adjusted for age and gender was used to determine the association of each one of the monocyte subpopulations with risk factors. In addition, Pearson's correlation coefficient was calculated to determine the association between continuous numeric variables and χ2 was used for categorical variables. Differences with p<0.05 were considered significant.
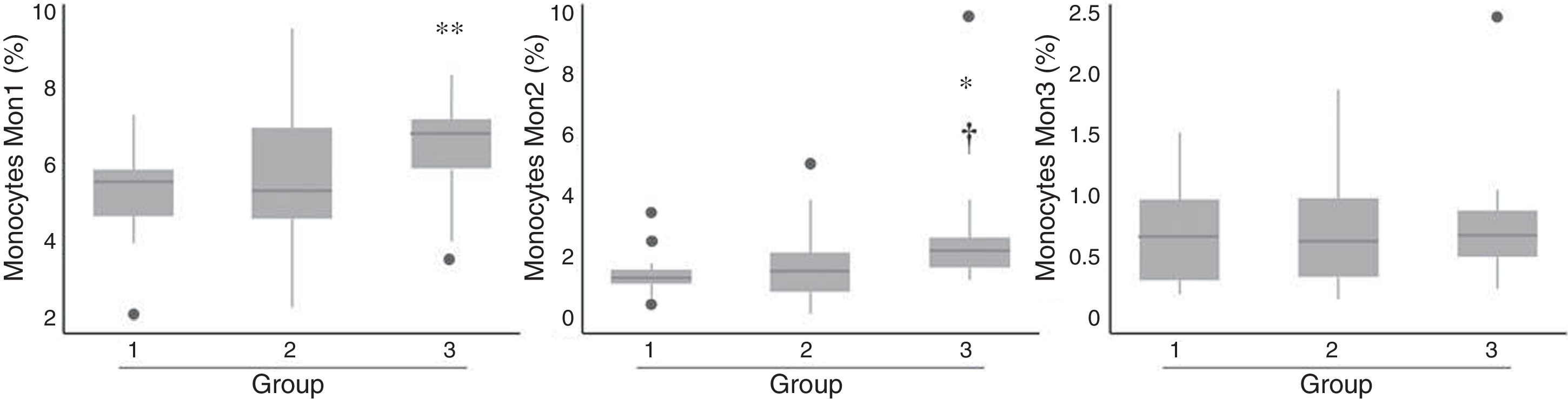
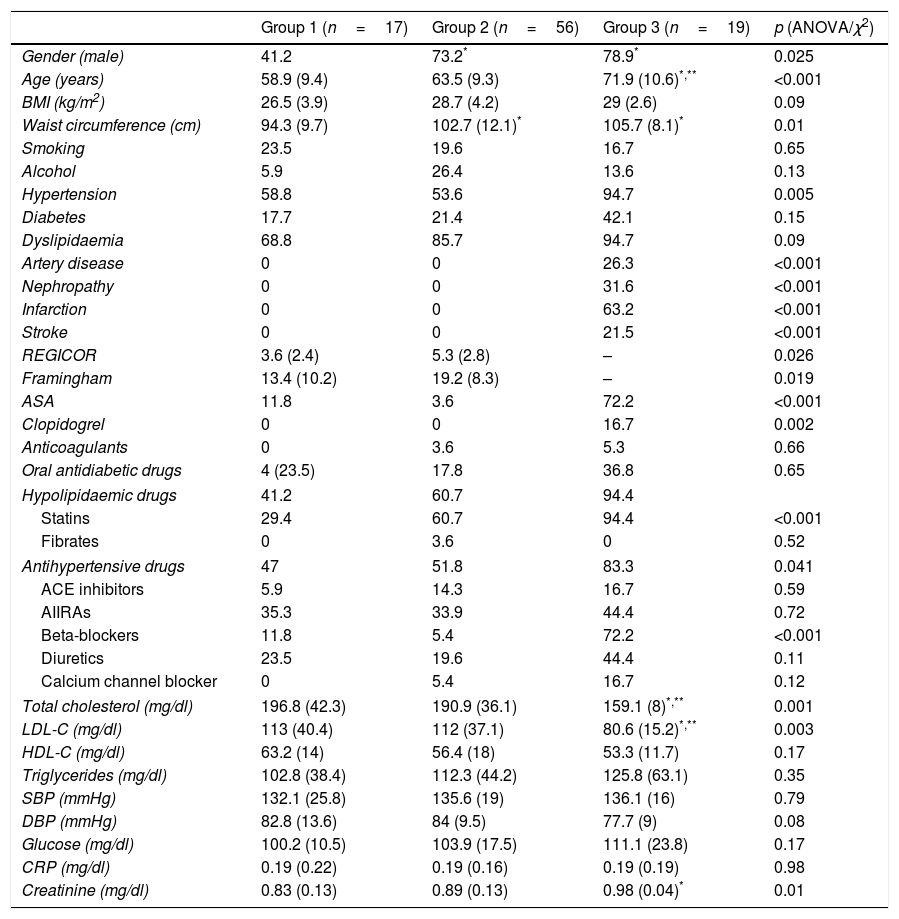
ResultsA total of 102 patients were included, with a mean age of 64.4 (range 45–86); 68.5% were male. They were classified into three risk groups based on clinical data; group 1 (n=17, 18%): asymptomatic subjects with more than one CVRF; group 2 (n=56, 61%): asymptomatic subjects but with vascular pathology by imaging test or microalbuminuria; and group 3 (n=19, 21%): patients with an atherothrombotic vascular event. Ten patients were excluded from the study as they did not have a blood count or cytometry results available. Their demographic characteristics, risk factors, concomitant medication and analytical parameters are described in Table 2. Differences were observed between risk groups in the percentage of males, age, waist circumference, cholesterol levels and creatinine. Patients from groups 2 and 3 had a higher frequency of males and a larger waist circumference compared to group 1. Patients from group 3 were older and had higher levels of total cholesterol and LDL-C compared to the two previous groups, and they had a higher level of creatinine compared to group 1. No differences were found in the remainder of the factors studied. In terms of medication, it was observed that patients from group 3 took more antiplatelet drugs (both aspirin and clopidogrel), statins and beta-blockers than patients from the other groups. The Framingham risk score and REGICOR index were significantly higher in group 2 compared to group 1 (p=0.019 and p=0.026, respectively).
Demographic characteristics of the population.
| Group 1 (n=17) | Group 2 (n=56) | Group 3 (n=19) | p (ANOVA/χ2) | |
|---|---|---|---|---|
| Gender (male) | 41.2 | 73.2* | 78.9* | 0.025 |
| Age (years) | 58.9 (9.4) | 63.5 (9.3) | 71.9 (10.6)*,** | <0.001 |
| BMI (kg/m2) | 26.5 (3.9) | 28.7 (4.2) | 29 (2.6) | 0.09 |
| Waist circumference (cm) | 94.3 (9.7) | 102.7 (12.1)* | 105.7 (8.1)* | 0.01 |
| Smoking | 23.5 | 19.6 | 16.7 | 0.65 |
| Alcohol | 5.9 | 26.4 | 13.6 | 0.13 |
| Hypertension | 58.8 | 53.6 | 94.7 | 0.005 |
| Diabetes | 17.7 | 21.4 | 42.1 | 0.15 |
| Dyslipidaemia | 68.8 | 85.7 | 94.7 | 0.09 |
| Artery disease | 0 | 0 | 26.3 | <0.001 |
| Nephropathy | 0 | 0 | 31.6 | <0.001 |
| Infarction | 0 | 0 | 63.2 | <0.001 |
| Stroke | 0 | 0 | 21.5 | <0.001 |
| REGICOR | 3.6 (2.4) | 5.3 (2.8) | – | 0.026 |
| Framingham | 13.4 (10.2) | 19.2 (8.3) | – | 0.019 |
| ASA | 11.8 | 3.6 | 72.2 | <0.001 |
| Clopidogrel | 0 | 0 | 16.7 | 0.002 |
| Anticoagulants | 0 | 3.6 | 5.3 | 0.66 |
| Oral antidiabetic drugs | 4 (23.5) | 17.8 | 36.8 | 0.65 |
| Hypolipidaemic drugs | 41.2 | 60.7 | 94.4 | |
| Statins | 29.4 | 60.7 | 94.4 | <0.001 |
| Fibrates | 0 | 3.6 | 0 | 0.52 |
| Antihypertensive drugs | 47 | 51.8 | 83.3 | 0.041 |
| ACE inhibitors | 5.9 | 14.3 | 16.7 | 0.59 |
| AIIRAs | 35.3 | 33.9 | 44.4 | 0.72 |
| Beta-blockers | 11.8 | 5.4 | 72.2 | <0.001 |
| Diuretics | 23.5 | 19.6 | 44.4 | 0.11 |
| Calcium channel blocker | 0 | 5.4 | 16.7 | 0.12 |
| Total cholesterol (mg/dl) | 196.8 (42.3) | 190.9 (36.1) | 159.1 (8)*,** | 0.001 |
| LDL-C (mg/dl) | 113 (40.4) | 112 (37.1) | 80.6 (15.2)*,** | 0.003 |
| HDL-C (mg/dl) | 63.2 (14) | 56.4 (18) | 53.3 (11.7) | 0.17 |
| Triglycerides (mg/dl) | 102.8 (38.4) | 112.3 (44.2) | 125.8 (63.1) | 0.35 |
| SBP (mmHg) | 132.1 (25.8) | 135.6 (19) | 136.1 (16) | 0.79 |
| DBP (mmHg) | 82.8 (13.6) | 84 (9.5) | 77.7 (9) | 0.08 |
| Glucose (mg/dl) | 100.2 (10.5) | 103.9 (17.5) | 111.1 (23.8) | 0.17 |
| CRP (mg/dl) | 0.19 (0.22) | 0.19 (0.16) | 0.19 (0.19) | 0.98 |
| Creatinine (mg/dl) | 0.83 (0.13) | 0.89 (0.13) | 0.98 (0.04)* | 0.01 |
ACE inhibitors: angiotensin converting enzyme inhibitors; ARAII: angiotensin II receptor antagonists; ASA: acetylsalicylic acid; BMI: body mass index; CRP: C-reactive protein; DBP: diastolic blood pressure; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SBP: systolic blood pressure.
Continuous variables are presented as mean and standard deviation. Qualitative variables are presented in percentages.
Bonferroni test:
Patients from group 3 had a significant increase in the percentage of cells with both Mon1 and Mon2 phenotype compared to group 1, as well as Mon2 compared to group 2 (Fig. 2), while no differences were observed in terms of the distribution of Mon3 cells. After adjusting for age and gender, only Mon2 cells were associated independently with the established risk groups (p=0.029).
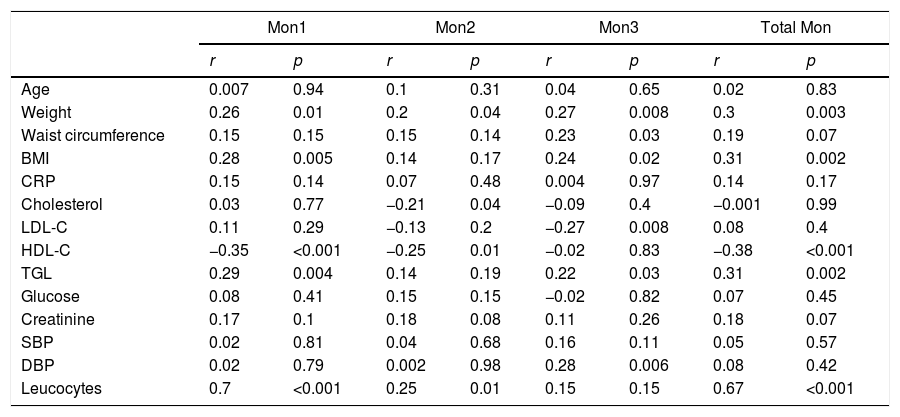
Association of the different monocyte subpopulations with cardiovascular risk factorsTable 3 shows the correlation between CVRFs and the different monocyte subpopulations in the study's total population.
Correlations of cardiovascular risk factors and number of monocytes of each subpopulation in the total of patients analysed (n=92).
| Mon1 | Mon2 | Mon3 | Total Mon | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age | 0.007 | 0.94 | 0.1 | 0.31 | 0.04 | 0.65 | 0.02 | 0.83 |
| Weight | 0.26 | 0.01 | 0.2 | 0.04 | 0.27 | 0.008 | 0.3 | 0.003 |
| Waist circumference | 0.15 | 0.15 | 0.15 | 0.14 | 0.23 | 0.03 | 0.19 | 0.07 |
| BMI | 0.28 | 0.005 | 0.14 | 0.17 | 0.24 | 0.02 | 0.31 | 0.002 |
| CRP | 0.15 | 0.14 | 0.07 | 0.48 | 0.004 | 0.97 | 0.14 | 0.17 |
| Cholesterol | 0.03 | 0.77 | −0.21 | 0.04 | −0.09 | 0.4 | −0.001 | 0.99 |
| LDL-C | 0.11 | 0.29 | −0.13 | 0.2 | −0.27 | 0.008 | 0.08 | 0.4 |
| HDL-C | −0.35 | <0.001 | −0.25 | 0.01 | −0.02 | 0.83 | −0.38 | <0.001 |
| TGL | 0.29 | 0.004 | 0.14 | 0.19 | 0.22 | 0.03 | 0.31 | 0.002 |
| Glucose | 0.08 | 0.41 | 0.15 | 0.15 | −0.02 | 0.82 | 0.07 | 0.45 |
| Creatinine | 0.17 | 0.1 | 0.18 | 0.08 | 0.11 | 0.26 | 0.18 | 0.07 |
| SBP | 0.02 | 0.81 | 0.04 | 0.68 | 0.16 | 0.11 | 0.05 | 0.57 |
| DBP | 0.02 | 0.79 | 0.002 | 0.98 | 0.28 | 0.006 | 0.08 | 0.42 |
| Leucocytes | 0.7 | <0.001 | 0.25 | 0.01 | 0.15 | 0.15 | 0.67 | <0.001 |
BMI: body mass index; CRP: C-reactive protein; DBP: diastolic blood pressure; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SBP: systolic blood pressure; TGL: triglycerides.
The number of Mon1 cells correlated positively with weight, BMI and triglycerides, and negatively with HDL-C. No association was found with diabetes, smoking, hypertension or the remaining CVRFs.
Mon2 cells presented a negative correlation with total cholesterol levels and HDL-C, but they were not associated with the remaining CVRFs.
Mon3 cells correlated positively with BMI, waist circumference, triglycerides and diastolic blood pressure, and negatively with LDL-C, but they were not associated with the remaining CVRFs.
Likewise, monocyte subpopulations were analysed in relation to the presence of more than two CVRFs (hypertension, diabetes, dyslipidaemia, obesity and smoking), and it was observed that Mon3 cells were associated with the presence of two or more factors (β=0.2; p=0.04), but this association was dependent on age.
Association of the different monocyte subpopulations with the presence of plaques and adverse vascular eventsMon1 and Mon2 subpopulations were correlated with the number of leucocytes (r=0.7 and r=0.26, respectively, p<0.01), but not with C-reaction protein or with creatinine levels.
An association was not observed between any monocyte population and the presence of atheromatous plaques.
Lastly, an association was found between adverse vascular events (defined as one single compound variable that included: myocardial infarction, acute stroke, peripheral arterial disease or nephropathy) and Mon1 percentage (β=0.86; p=0.02) and Mon2 (β=0.1; p=0.002); this association was maintained after adjusting for age and gender for Mon2 (β=0.1; p=0.013), but not for Mon1 (β=0.79; p=0.11). These results would indicate that patients with secondary events have a higher percentage of Mon2 than patients without events.
DiscussionThis paper shows that the study of circulating monocyte subpopulations, Mon1, Mon2 and Mon3, using flow cytometry may be useful for assessing the different inflammatory profile of patients according to the groups established in this study based on CVRFs. In this way, an association, independent of age and gender, of the Mon2 monocyte subpopulation with risk groups established in the study, as well as having had a previous cardiovascular event, is observed. However, the Mon3 subpopulation was associated with the presence of two or more risk factors regardless of gender, but not of age.
The literature consistently reports that monocytes, regardless of subtype, are elevated in the face of inflammatory and/or infectious stimuli, as well as with obesity.10,11 Similarly, the Tromsø study12 demonstrated, in an extensive cohort of patients, that the elevation of monocytes was associated with the onset of new atheroscelorotic plaques detected by ultrasound. Likewise, in our cohort we found a different profile of monocyte subpopulations according to the different risk groups established in the study. Furthermore, those patients who had already had events (group 3) presented with higher Mon2 monocyte levels compared to patients from the remaining study groups.
Currently, in the era of personalised medicine, reliable markers which can individually predict residual inflammation of patients are being sought. They can therefore tell us which patients would benefit from more intensive treatments in order to prevent the onset of cardiovascular complications. In this context, monocytes have been proposed as possible markers of residual inflammation.9 To date, the C-reactive protein level has been used as a measurement of inflammation in several intervention studies (JUPITER, CANTOS).13–15 However, in our cohort of patients with CVRFs controlled with drugs, C-reactive protein levels were similar in the three risk groups.9 In this context, the assessment of monocyte subpopulations could provide additional information about the existing residual inflammation, being able to guide us on the need to optimise medical treatment.
Monocytes are involved in inflammatory processes and contribute to atherogenesis promoting the leucocyte recruitment in plaques.7 Of the different subpopulations, the Mon1 cells are the predominant subpopulation identified in atherosclerotic plaques and those that are considered inflammatory mediators.11 Some studies indicate that they are the first monocytes to appear in peripheral blood6 and their increase is linked to the onset of atherosclerotic plaques.16 However, in our study, we did not observe this association. As it was a cross-sectional study, it is not possible to establish an association with the progression of the atherosclerotic plaque.
In recent years, interest for the role of Mon2 monocytes in cardiovascular risk has increased considerably; it seems to be demonstrated in the literature that Mon2 monocytes are found to be repeatedly high in patients with previous cardiovascular events and that they could have a prognostic impact on patients who had already suffered from a previous myocardial infarction.17 As in our study, Marsh et al.17 had observed that patients who had had a previous cardiovascular event presented with a higher percentage of Mon2. These findings could be due to a greater expression in Mon2 of membrane receptors with the ability to adhere to the activated endothelium as shown in the studies by Rogacev et al.8 and, therefore, they could enhance the atherogenic process.5,8,16 Their determination could be of interest for reclassifying patients with residual inflammation, making it possible to identify those who require special care and follow-up.8
Recently, it has been suggested that Mon3 (non-classical), typically called patrollers, is the subpopulation which migrates to the lesion at an early stage, transforming itself locally into the other subpopulations and they seem to have an atheroprotective role, both in baseline situations and during inflammatory processes.5,18 These data would support the fact that in our study a different expression was observed in the number of Mon3 in those patients who had two or more CVRFs and that there was a significant correlation with the BMI, waist circumference, diastolic blood pressure and hypertriglyceridaemia values.
The study limitations are those typical of a cross-sectional study and the relatively low sample size with a non-homogenous distribution between groups, in which we can establish associations but not casual links. Similarly, a large heterogeneity is found in patients within the same group, predominantly in group 2, as in this group patients display a high variability of atheroscerlotic load, which includes the presence of plaques regardless of the number and the regions affected. However, these results open the door to new hypotheses for future prospective studies on the role of monocytes as predictors of major vascular events and possible therapeutic targets.
ConclusionsThe quantification of monocyte subpopulations, and specifically of Mon2, may be very useful in the study of patients with CVRFs, given that it could be indicative of residual inflammation, and, therefore, could contribute to elaborating a more personalised medicine, better identifying those patients who require a more intensive treatment.
FundingGrant from the Spanish Society of Arteriosclerosis (FEA clinical-Epidemiological 2017).
Conflicts of interestNone.
In particular, we would like to thank the patients for their participation and the Biobank of the Universidad de Navarra for its collaboration.
Please cite this article as: Marcos Jubilar M, Orbe J, Roncal C, Fernández Montero A, Colina I, Rodil R, et al. Análisis de subpoblaciones monocitarias en relación con los factores de riesgo cardiovascular. Clín Investig Arterioscler. 2019;31:152–159.