Clinical evidence has a more significant role in medical specialties than in surgery. Rectal cancer (CR) is no exception. This paper explores what CR-related subjects are being investigated at the present time in a quantitative and qualitative way and analyses this information to know what possible answers clinical research could give us in the future.
MethodsThe data collection was carried out in April 2014 and was based on 3 sources: 2 institutional clinical trials registries – American (clinicaltrials.gov) and European (EU Clinical Trials Register) – and a survey given to members of the Asociación Española de Coloproctología (AECP). The obtained studies were exported to a database designed especially for this review, which included a number of descriptive elements that would allow the cataloguing of the different studies. The AECP survey results were analysed separately.
ResultsThere are currently 216 clinical trials ongoing related to CR. Two-thirds are primarily conducted by oncologists. Nearly a third are surgical. The research focuses on improving preoperative treatment: new drugs, new schemes of chemo-radiotherapy (usually induction or consolidation schemes), or optimisation of radiotherapy and its effects. Surgical clinical trials are related to robotics, laparoscopy, stoma, low colorectal anastomosis, distal CR, and local treatment.
ConclusionMost of the current clinical trials ongoing on CR are analysing aspects of chemo-radiotherapy and its effects. A third focus on purely surgical issues.
La evidencia clínica tiene más peso en las especialidades médicas que en las quirúrgicas. El cáncer de recto (CR) no es una excepción. En este artículo, nos hemos planteado explorar de forma cuantitativa y cualitativa, qué cuestiones y materias relacionadas con el CR están siendo investigadas en el momento actual y, posteriormente, analizar esta información para conocer qué respuestas podrá darnos la investigación clínica en el futuro.
MétodosLa obtención de datos se realizó en abril de 2014 y se basó en 3 fuentes: 2 registros institucionales de ensayos clínicos, –el registro americano (clinicaltrials.gov) y el registro europeo (EU Clinical Trials Register)– y una encuesta realizada a través de la Asociación Española de Coloproctología (AECP). Los estudios obtenidos fueron exportados a una base de datos diseñada especialmente para esta revisión, en la que se incluyeron además una serie de elementos descriptivos que permitieran la catalogación de los estudios. Los resultados de la encuesta AECP fueron analizados de forma separada.
ResultadosHay actualmente en marcha 216 estudios referidos al CR. Dos tercios son fundamentalmente oncológicos. Casi un tercio son quirúrgicos. Las líneas de investigación se centran en la mejora del tratamiento preoperatorio: nuevos fármacos, nuevos esquemas de quimiorradioterapia (generalmente de inducción o consolidación) u optimización de la radioterapia y sus efectos. Los ensayos clínicos quirúrgicos estudian aspectos relacionados con robótica, laparoscopia, estomas, anastomosis bajas, CR distal y tratamiento local.
ConclusionesLa mayoría de los estudios actuales sobre CR analizan aspectos relacionados con la quimiorradioterapia y sus efectos. Un tercio se centran en temas especialmente quirúrgicos.
There is a perception that clinical studies do not represent a relevant role in surgery, and the surgeon does not represent a relevant role in the design of clinical studies.1 Such perception can fit reality only if we take into account the percentage of the surgical clinical practice that is based on clinical trials, especially if we focus on randomised and controlled trials (RCT). Howes et al.2 considered that, in surgical departments, only 24% of the medical procedures in admitted patients were supported by RCT, while this percentage increased up to 53% in the case of general medicine. Therefore, it is unquestionable that, from a strictly quantitative point of view, the clinical evidence is stronger in medical specialities than in surgical specialities. However, this fact should not be surprising, since there are some obstacles inherent to the surgical practice that hamper the performance of methodologically correct RCTs, especially if we analyse surgical techniques. The difficulty in the randomisation of patients, the variability in the surgical practice (particularly regarding new techniques and the learning curve), the assessment of surgical treatments against non-surgical treatments, and the difficulties to design trials, among other factors, make it especially hard for surgeons to pose methodologically flawless clinical studies.3
Rectal cancer (RC) is not an exception to these considerations. Until the decade of 1970s of the last century, RC treatment was not included in clinical studies. In the decade of 1980s and, mainly, 1990s, RC treatment started to provide a stronger scientific framework,1,4 with the development of adjuvant/neoadjuvant treatment and a standardised surgical technique, such as total mesorectal excision (TME). In more recent times, with the development of the laparoscopic approach5 and better technical options for local treatment, surgeons have gained a more active participation in the design and development of RCTs related to RC.
This article has 2 objectives: first of all, to quantitatively and qualitatively explore which facts and subjects related to RC are currently being investigated; and secondly, to analyse and compress this in principle heterogeneous information in order to know, based on evidence, which answers can be brought by clinical investigation in the more or less near future.
Materials and MethodsThe data collection was carried out in April 2014, and was based on 3 sources: 2 institutional registries of clinical trials– the American registry (NCT)6 and the European registry (EUDRACT)7—and a survey designed by the Spanish Association of Coloproctology (Asociación Española de Coloproctología, AECP) with which we attempted to perform an assessment of our closest environment.
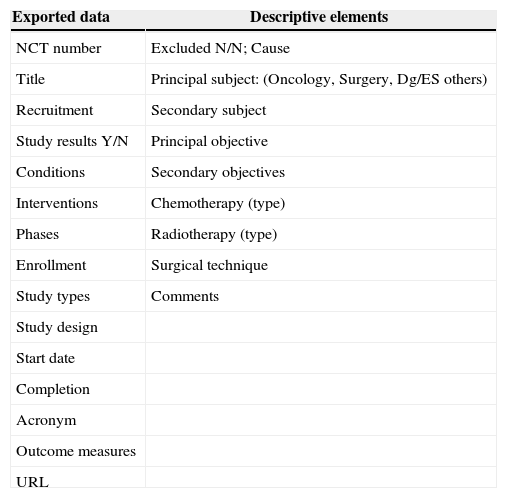
The NCT registry includes world-wide studies. At the moment of the search, 51% out of the total amount of registered studies were outside U.S.A., 43% were only American studies, and 6% included other countries. The search was performed with the words “rectal neoplasm”, and afterwards the box “include only open studies” was clicked. The obtained studies were exported to a Microsoft Office Excel© 2003 page and, from there, to a FileMaker pro© 6 database, specially designed for this revision, in which, besides the fields referred to the exported data, a series of descriptive elements (Table 1) were included. Such descriptive elements allowed the classification of various studies, as well as the exclusion of those that were not specifically referred to RC.
Set of Data Exported from clinicaltrials.com and Descriptive Elements Added to Each Clinical Trial.
| Exported data | Descriptive elements |
|---|---|
| NCT number | Excluded N/N; Cause |
| Title | Principal subject: (Oncology, Surgery, Dg/ES others) |
| Recruitment | Secondary subject |
| Study results Y/N | Principal objective |
| Conditions | Secondary objectives |
| Interventions | Chemotherapy (type) |
| Phases | Radiotherapy (type) |
| Enrollment | Surgical technique |
| Study types | Comments |
| Study design | |
| Start date | |
| Completion | |
| Acronym | |
| Outcome measures | |
| URL |
Dg/ES: diagnosis or extension study.
The EUDRACT registry includes studies of the whole European continent. The search was also performed with the words “rectal neoplasm”, and the box “only open studies” was checked. In EUDRACT, there are studies already registered in NCT, not always under the same heading or in the original language, which made us revise them one by one, except for those already registered in NCT.
The survey was carried out by the AECP. All the members received a questionnaire by e-mail. These were sent 3 times (November 2013, January and March 2014). The clinical studies and trials obtained were revised and included in the database. Researchers provided the codes of those studies already included in the NCT and EUDRACT to avoid duplication of registries.
Our analysis is based on data obtained from the NCT and EUDRACT. The data obtained from the survey are analysed separately.
Clinical trials were analysed according to the main subject of the study (Table 1) and its design (observational or experimental). Experimental studies were classified as per their methodological phase (phases I, II, III, IV, or without any assigned phase), and the primary and secondary objectives of the studies included in each phase were investigated. Those studies classified in 2 phases were included for the analyses of the 2 phases that they belonged to.
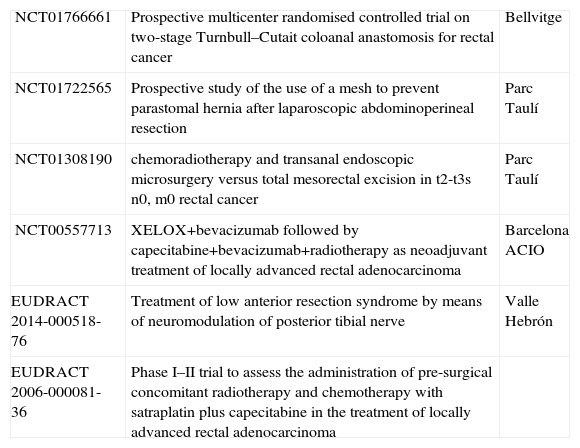
ResultsThe search in NCT provided a total of 952 studies. Out of them, 257 were excluded as they were studies related to multiple tumours (39%), not related at all with RC (22%), or focused on prostate cancer (10%) or anal cancer (9%). From the remaining 695, 499 were also excluded because they generally referred to colorectal cancer (CRC). Most of these studies were mainly oncological (61%), and many of them (236) were related to metastatic CRC. Only 13% of them could be classified as surgical studies. In light of the above, the total amount of clinical trials from the NCT registry included in our study was 196. The consultation made in EUDRACT provided a total of 162 studies. After revising them, we found 20 specific studies for RC (not included in NCT). Therefore, the total amount of clinical trials specifically about RC currently in process is 216, according to both institutional registries consulted. From these, 6 (3%) are Spanish (4 in NCT and 2 in EUDRACT) (Table 2).
Spanish Clinical Trials Registered in NCT and EUDRACT.
| NCT01766661 | Prospective multicenter randomised controlled trial on two-stage Turnbull–Cutait coloanal anastomosis for rectal cancer | Bellvitge |
| NCT01722565 | Prospective study of the use of a mesh to prevent parastomal hernia after laparoscopic abdominoperineal resection | Parc Taulí |
| NCT01308190 | chemoradiotherapy and transanal endoscopic microsurgery versus total mesorectal excision in t2-t3s n0, m0 rectal cancer | Parc Taulí |
| NCT00557713 | XELOX+bevacizumab followed by capecitabine+bevacizumab+radiotherapy as neoadjuvant treatment of locally advanced rectal adenocarcinoma | Barcelona ACIO |
| EUDRACT 2014-000518-76 | Treatment of low anterior resection syndrome by means of neuromodulation of posterior tibial nerve | Valle Hebrón |
| EUDRACT 2006-000081-36 | Phase I–II trial to assess the administration of pre-surgical concomitant radiotherapy and chemotherapy with satraplatin plus capecitabine in the treatment of locally advanced rectal adenocarcinoma |
ACIO: Catalan Association of Oncology Research (Asociación Catalana de Investigación Oncológica).
With regard to the main subject of the study, 61% of the trials were mainly oncological (131 studies), 30% were surgical (66 studies), 6% (13 studies) were related to diagnostic or extension study methods, and the rest were related to other subjects, for example, 4 studies related to quality of life (trial QoLiRECT [NCT01477229]; NCT00712751; NCT00648635; NCT01216189) or 2 related to pathology (trial TRaMA [NCT01887509]; NCT01097265).6
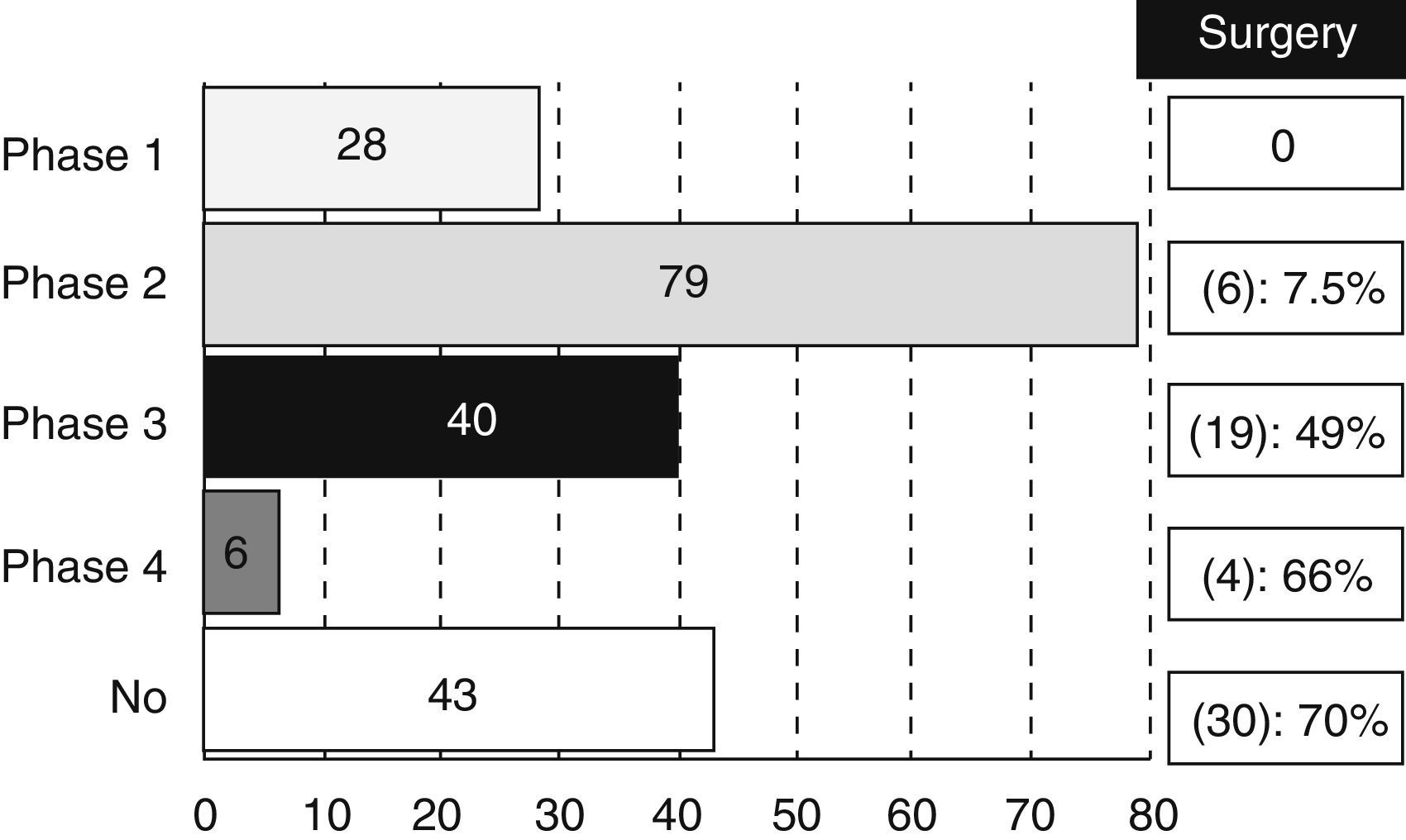
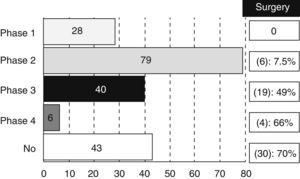
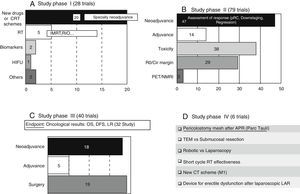
Most of the revised studies (179 trials, 83%) were experimental and 14% (30 trials) were observational. The type of trial did not appear in 7 registries. There were 28 studies in phase I, 79 in phase II, 40 in phase III and 6 trials in phase IV, even though 17 studies were methodologically registered in 2 phases (13 were considered as phase I/II and 4 as phase II/III). We also analysed the percentage of studies that could be classified as “surgical” according to the different phases (Fig. 1).
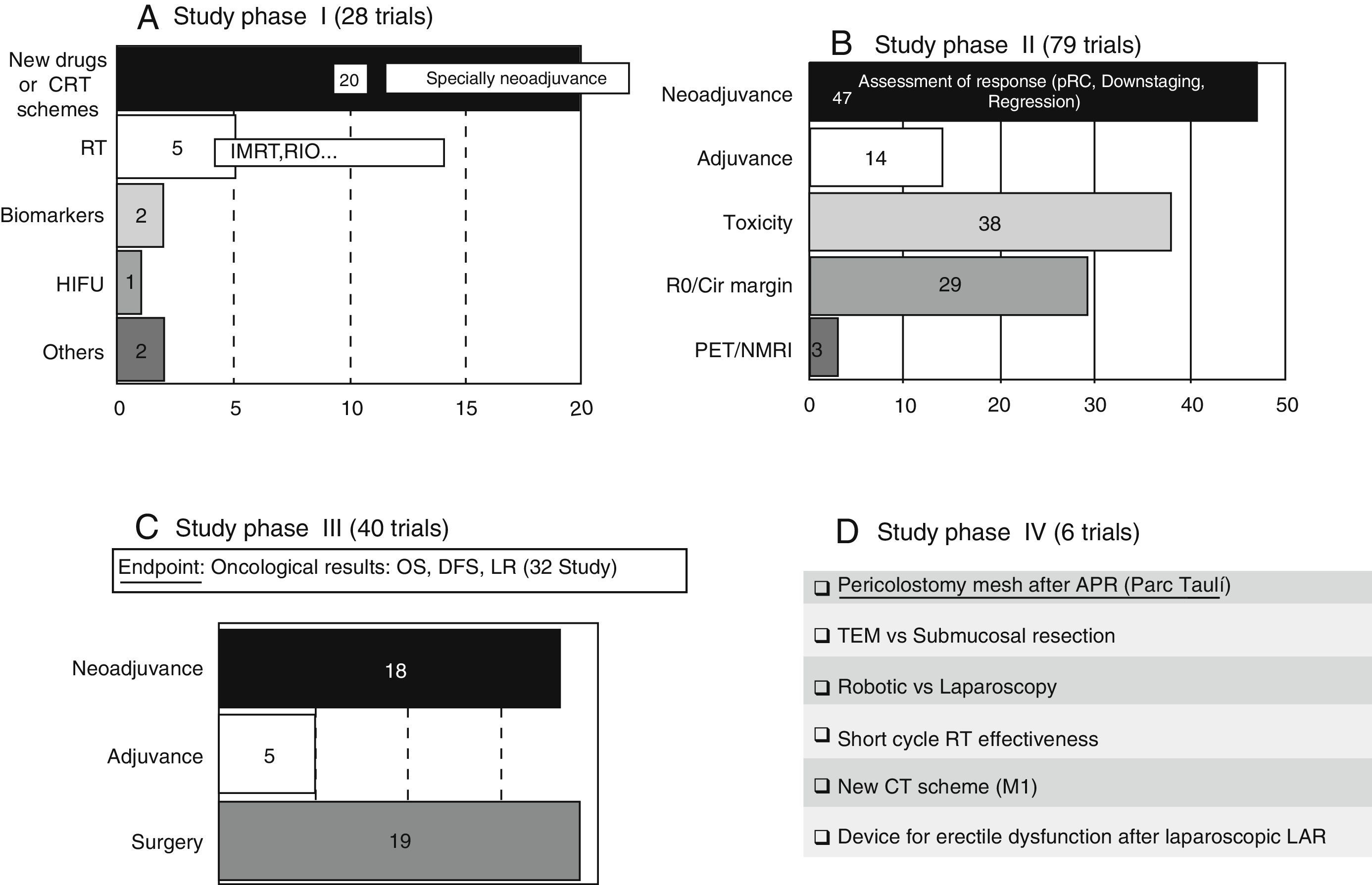
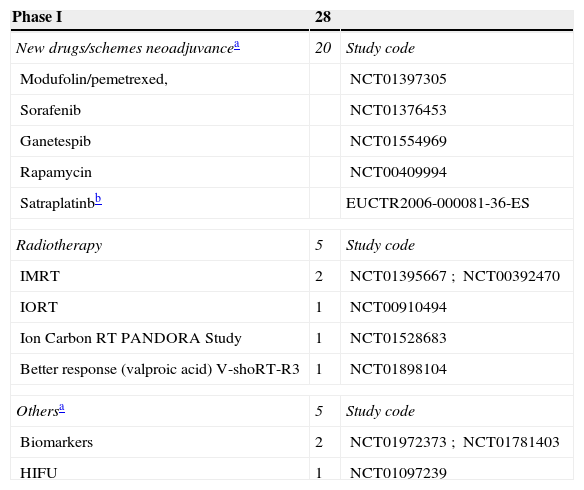
These trials entail the first tests in human beings and provide information about dosing and adverse effects.8 A large number of phase I clinical trials study new therapeutic schemes or new drugs, generally related to neoadjuvance. There are also studies related to the improvement in radiotherapy treatment (RT). The content and codes of some phase I studies are detailed in Table 3.
Content of Studies in Phases I, II, and III.
| Phase I | 28 | |
|---|---|---|
| New drugs/schemes neoadjuvancea | 20 | Study code |
| Modufolin/pemetrexed, | NCT01397305 | |
| Sorafenib | NCT01376453 | |
| Ganetespib | NCT01554969 | |
| Rapamycin | NCT00409994 | |
| Satraplatinbb | EUCTR2006-000081-36-ES | |
| Radiotherapy | 5 | Study code |
| IMRT | 2 | NCT01395667; NCT00392470 |
| IORT | 1 | NCT00910494 |
| Ion Carbon RT PANDORA Study | 1 | NCT01528683 |
| Better response (valproic acid) V-shoRT-R3 | 1 | NCT01898104 |
| Othersa | 5 | Study code |
| Biomarkers | 2 | NCT01972373; NCT01781403 |
| HIFU | 1 | NCT01097239 |
| Phase II | 79 | |
|---|---|---|
| Assessment of response to neoadjuvancea | 47 | Study code |
| Only CT: BACCHUS and FOWARC studies | NCT01650428; NCT01211210 | |
| Induction or consolidation CT: COPERNICUS | NCT01263171 | |
| Assessment of cCR: PET/NMRI | 3 | NCT00254683; NCT01525056; NCT00574353 |
| Phase III | 40 | |
|---|---|---|
| Neoadjuvance | 18 | Study code |
| TEM/lymphadenectomy | 3 | NCT01308190b; NCT00738790; NCT00154752 |
| RT (IMRT, boost, hyperfractionation, …) | 4 | NCT01064999; NCT01224392; NCT01653301; NCT01814969 |
| Short cycle (vs CRT) | 4 (2) | NCT00738790; NCT00833131 (NCT00597311; NCT01459328) |
| Time of neoadjuvance-surgery: GRECCAR6 study | 1 | NCT01648894 |
| CT ALONE vs CRT | 2 | NCT01515787; NCT01211210 |
| Induction/consolidation: RAPIDO and POLISH TRIAL | 4 | NCT01558921; NCT02031939; NCT00833131; NCT01804790 |
| Adjuvance | 5 | Study code |
| Adjuvance vs observation | 1 | NCT0194197 |
| New schemes | 2 | NCT00189657; NCT00749450 |
| New drugs | 2 | NCT01830621; NCT00497107 |
| Surgery | 19 | Study code |
| Laparoscopy/robotic | 6 | NCT00726622; NCT00601549; NCT01130233; NCT00007930; NCT01423214; NCT01899547 |
| TEM/Tt local | 2 | NCT01308190b; NCT00738790 |
| Pelvic floor reconstruction (APR): BIOPEX and NEAPE trials | 2 | NCT01347697; NCT01927497 |
| Lymphadenectomy | 2 | NCT00190541; NCT00154752 |
| Post endoscopic TME | 1 | NCT00531297 |
| Pouch | 1 | NCT00238381 |
| Others | 5 | NCT01269567; NCT01999634; NCT01786694; NCT01372007; NCT00773981 |
APR: abdominoperineal resection; cCR: complete clinical response; HIFU: high intensity focused ultrasound; IMRT: intensity modulated radiation therapy; PET: positron emission tomography; CRT: chemoradiotherapy; CT: chemotherapy; IORT: intraoperative radiation therapy; NMRI: nuclear magnetic resonance imaging; RT: radiotherapy; TEM: transanal endoscopic microsurgery; Tt: treatment.
These trials, which should have a control group and randomised assignment, try to establish the dose/answer relation, measure the efficiency, and broaden data on drug security.8 A large majority of the phase II studies (47 out of 79 trials) have as principal and secondary objectives the assessment of the response to various neoadjuvance schemes, either from the pathological, clinical, or radiological point of view. The response is assessed after the trial with new drugs or therapeutic schemes in a total of 37 studies. The content and codes of some phase II studies are also detailed in Table 3.
Phase III Trials (Fig. 2C)These studies are the complete therapeutic assessment. The regular use conditions should be reproduced and a wider and more representative sample is required. They should be controlled and with randomised assignment.8 From the 40 phase III clinical trials, 32 of them have global survival, free-disease survival, or local recurrence rates as principal objective. On that basis, we could classify the different phase III trials in 3 groups: neoadjuvance (18 studies), adjuvance (5 studies), and surgical (19 studies), even though some studies will be included in many categories. Some studies combine neoadjuvance with surgical techniques, such as transanal endoscopic microsurgery (TEM) (Spanish study TAU-TEM of the group Parc Taulí, Sabadell), or compare neoadjuvance with more radical surgical techniques, such as lymphadenectomy. Furthermore, optimised methods of RT application (intensity-modulated RT [IMRT], boost, …), the effectiveness of the short cycle of preoperative RT, the period between neoadjuvance and surgery, the omission of RT in preoperative treatment, and the usefulness of new schemes, such as induction chemotherapy (CT) or consolidation CT, are also studied. Regarding adjuvance, some studies are being carried out on, among others, the usefulness of postoperative CT in patients previously treated with chemoradiotherapy (CRT) and surgery.
The 19 current “surgical” studies are focused on laparoscopy, robotic, local treatment of RC, pelvic floor reconstruction after optimised abdominoperineal resection (APR), lymphadenectomy, and colonic reservoirs, among others, such as posterior endoscopic TME, placement of pelvic drains, microdialysis, use of somatuline, or endoanal vaccum (Table 3).
Phase IV Trials (Fig. 2D)These trials are designed to assess the security and effectiveness along the period of the product already commercialised.8 There are 6 studies registered in phase IV. Four of them are related to surgery. We highlight a Spanish study that is also of Parc Taulí, in which the prophylactic use of pericolostomy meshes after APR is investigated.
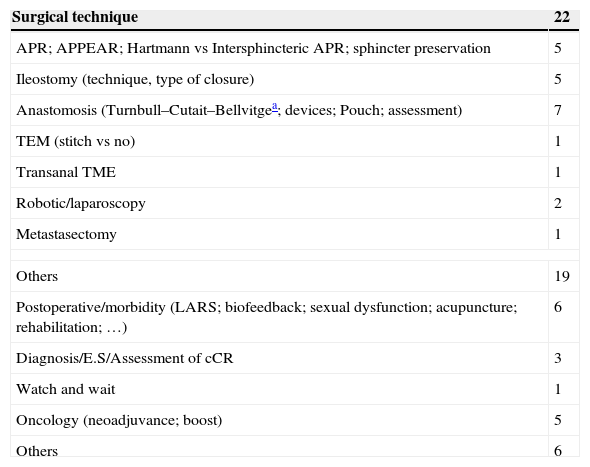
Experimental Trials Without Any Assigned Phase (Table 4)Within this type of experimental trials, there are 22 that investigate about surgical techniques and 19 related to more diverse features. Among the first ones, we highlight TURNBULL-BCN, a Spanish study of Hospital de Bellvitge, which investigates the Turnbull–Cutait delayed coloanal anastomosis (NCT01766661). There are also 6 studies related to the immediate postoperative period or functional results after low anterior resection (LAR), among which the work of the Valle de Hebrón group on neurostimulation in low anterior resection syndrome stands out (EUDRACT 2014-000518-76). There are 6 oncological studies that analyse the non-operative treatment or neoadjuvance-related subjects (oncological results or improvement of RT application), among others (Table 4).
Content of Experimental Clinical Trials Without Any Assigned Phase.
| Surgical technique | 22 |
|---|---|
| APR; APPEAR; Hartmann vs Intersphincteric APR; sphincter preservation | 5 |
| Ileostomy (technique, type of closure) | 5 |
| Anastomosis (Turnbull–Cutait–Bellvitgea; devices; Pouch; assessment) | 7 |
| TEM (stitch vs no) | 1 |
| Transanal TME | 1 |
| Robotic/laparoscopy | 2 |
| Metastasectomy | 1 |
| Others | 19 |
| Postoperative/morbidity (LARS; biofeedback; sexual dysfunction; acupuncture; rehabilitation; …) | 6 |
| Diagnosis/E.S/Assessment of cCR | 3 |
| Watch and wait | 1 |
| Oncology (neoadjuvance; boost) | 5 |
| Others | 6 |
APPEAR: Anterior Perineal PlanE for Ultra-low Anterior Resection; LARS: low anterior resection syndrome; TME: total mesorectal excision.
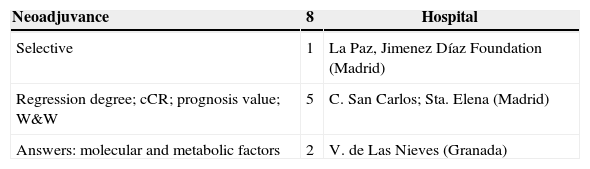
The response to the survey made by AECP was very scarce. Sixteen groups (Table 5) answered and provided a total of 25 clinical trials, 3 of which were already included in NCT and another one in EUDRACT. The content of the survey, with the exception of the trials already registered in NCT or EUDRACT, is illustrated in Table 6.
Research Groups and Hospitals That Answered the AECP Survey.
| Hospitals included in the AECP survey |
|---|
| • Hospital Valle de Hebrón (Barcelona)• Hospital de Bellvitge (Barcelona)• Parc Taulí Corporation (Sabadell)• Hospital La Fe (Valencia)• Hospital Arnau de Vilanova (Valencia)• Hospital de Torrevieja (Alicante)• Hospital U. La Paz (Madrid)• Clínica Santa Elena/Clínico San Carlos (Madrid)• Complejo Hospitalario de Ourense• Hospital U. Virgen del Rocío (Seville)• Hospital U. Virgen de las Nieves (Granada)• Hospital SAS of Jerez• Hospital San Juan de Dios (Seville)• Hospital U. de Álava (Vitoria)• Hospital Obispo Polanco (Teruel)• Complejo Hospitalario Torrecárdenas (Almería) |
AECP Survey: Content.
| Neoadjuvance | 8 | Hospital |
|---|---|---|
| Selective | 1 | La Paz, Jimenez Díaz Foundation (Madrid) |
| Regression degree; cCR; prognosis value; W&W | 5 | C. San Carlos; Sta. Elena (Madrid) |
| Answers: molecular and metabolic factors | 2 | V. de Las Nieves (Granada) |
| Extension study | 2 | Hospital |
|---|---|---|
| Assessment of mesorectal fascia by ERUS vs NMRI | 1 | La Fe (Valencia) |
| Wong classification: ERUSy NMRI | 1 | La Fe (Valencia) |
| Surgical techniques | 8 | Hospital |
|---|---|---|
| Local treatment | 1 | Hospital de Ourense |
| APR: prone vs supine; extended | 3 | La Fe (Valencia); Torrecárdenas (Almería) |
| Partial prostatectomy in T4 | 1 | La Fe (Valencia) |
| Transit reconstruction after TME | 1 | Valle de Hebrón (Barcelona) |
| Pelvic exenteration | 1 | Hospital de Ourense |
| Postoperative/sphincter function | 4 | Hospital |
|---|---|---|
| Perineal eventration | 1 | Hospital de Ourense |
| Anorectal function | 2 | C. San Carlos; Sta. Elena (Madrid) |
APR: abdominoperineal resection; cCR: complete clinical response; ERUS: endorectal ultrasound; NMRI: nuclear magnetic resonance imaging; TME: total mesorectal excision; W&W: Watch and Wait.
The revision of the current investigation on any clinical entity can provide very heterogeneous data that restrict its usefulness. In the particular case of RC, the inclusion of trials that analyse CRC in general led to unacceptable distortion in information that is already heterogeneous and varied. Therefore, these trials were excluded from our analysis.
It is not surprising that most of the trials are mainly oncological (61%), since CT and RT are currently playing a key role in the RC therapeutic scheme. However, it is important to highlight that a non-negligible amount of studies (30%) are classified as surgical. This figure increases up to 13% if we refer to the clinical trials related to CRC in general. That information confirms a greater involvement of surgeons in treatment of RC against CRC treatment in general. Moreover, it is still paradoxical that a high percentage of phase III or IV experimental studies can be considered as “surgical” (49% of phase III studies and 66% of phase IV studies). A possible explanation is the existing disagreement between Medicine and Surgery about the required amount of scientific evidence.1
In any case, the current investigation about RC is a complex, multifactorial, and very heterogeneous issue. Attempts to improve oncological results have led to a great variety of therapeutic schemes (Table 7) that hampers an adequate classification of studies. The analysis of clinical trials currently in process allows us to assert, in general, that nowadays there are 3 major lines of research: resectability improvement of locally advanced RC, especially in insufficient responses to preoperative treatments; remote monitoring of the disease; and a great variety of techniques and surgical approaches.
Current Treatment Schemes for Rectal Cancer.
| 1. Surgery alone—adjuvance2. Short cycle—immediate surgery—adjuvance3. CRT—deferred surgery—adjuvance4. Short cycle—deferred surgery—adjuvance5. Induction CT—CRT—deferred surgery—adjuvance6. Induction CT—short cycle—immediate surgery—adjuvance7. CRT—consolidation CT—surgery—adjuvance8. CT alone (induction)—surgery—adjuvance9. CRT—deferred surgery—observation |
Most of the phase I studies in RC are based on neoadjuvant therapy. New drugs are assessed not only to increase their own effectiveness, but also due to their radiosensitive effect. It is about typical phase I studies, where the maximum tolerated dose is determined and the adequate scheme to proceed with phase II studies is established. When the objective is radiosensitivity, a fix RT dose is usually used and the dose of the new drug is scaled. Among studied drugs, antiangiogenics stand out, such as E7820 (oral, used as second line in locally advanced RC) or sorafenib (approved for renal cancer treatment) and immunosuppressant drugs like rapamycin. These studies also assess how to increase the effectiveness of already accepted drugs, such as irinotecan, using higher doses selectively in patients with a determined genotype. Likewise, several phase I studies investigate the possibility to improve the RT, among others, with IMRT application (Table 3).
With regard to phase II studies, a large majority aims at assessing the response to neoadjuvance. Some of them investigate the possibility to use only chemotherapy in the preoperative treatment of clinical CRM− tumours, and exclude RT in order to avoid its adverse effects.9,10 This has already been highlighted by 2 preliminary studies from the Memorial Sloan-Kettering Centre (MSKC) presented in 2010. Such studies provide a pathological complete response (pCR) of 26% and 35% after neoadjuvance with FOLFOX and FOLFOX plus bevacizumab respectively, both cases without RT.11–13 In fact, there are studies that already question the value of RT (and even CRT) in tumours T3N0/N+ or T2N+ in which NMRI foresees a CRM− (>2mm), such as MERCURY study14 or a Spanish study, of the Hospital La Fe in Valencia, published in 2011.15 Nowadays, this fact is being investigated: the phase II trial BACCHUS from London University College, that analyses FOLFOX or FOLFOXIRI+bevacizumab and the Chinese phase II/III study FOWARC, which uses FOLFOX6 with and without RT.
There are also several phase II and III studies that are testing various preoperative treatment schemes for RC, such as induction and consolidation therapies. With induction CT (CT previous to CRT administration), the intention is to treat, in a more efficient way, the remote dissemination of RC. Induction CT presents the following benefits: very early start, better treatment conclusion rates, “downstaging” possibility, and a reduction in micrometastases rates. The disadvantages are the delay in surgical resection and possible reduction of RT effect when tumour cell clones are selected.9,10 Induction CT has been studied in a phase II Spanish trial, published in 2010, which reached oncological results similar to those of the control group, but less toxicity and a higher rate of complete cycle conclusion.16 There are several studies in progress that analyse induction CT, such as COPERNICUS, GRECCAR-4, and the European EUDRACT 2011-003340-45 study, all of them at phase II (Table 3).
With consolidation CT, the gap between CRT and surgery is intended to be filled by adding another CT regime.10 The studies of the Habr-Gama group obtain more responses if the CT is prolonged after preoperative CRT, and inform a total complete response (cCR+pCR) of 65%.17 There are some studies in progress that analyse the consolidation CT. One of them is POLISH, a phase III study that tests the short cycle of RT+consolidation CT against the control group (large cycle CRT). RAPIDO is another phase III trial, coordinated by the University of Groningen and of similar design. There are 3 clinical trials, currently in progress, that assess induction and consolidation therapies in a combined way. One of trials is carried out by the MSKC group from New York (NCT02008656).
With regard to postoperative adjuvant therapy, there is no evidence to prove that adjuvant CT is equally efficient in the colon and in the rectum, especially when there are previously radiated tumours; however, such an idea is usually assumed.9,10 In this regard, there is an on-going phase III Brazilian trial that studies the possibility to skip postoperative CT in some patients treated with preoperative CRT and surgery.
Furthermore, the improvement and intensification of RC treatment with RT are also being studied, precisely by adding a concomitant boost (7.5–15 additional Gy in the last week of the treatment) or by means of IMRT, among others. The boost is analysed in 2 Belgian trials: RECTAL-BOOST and RECTUM-SIB. IMRT therapy allows a higher accuracy in RT application, which promotes the progressive increase of the dose, without any rise in the adverse effects rate.10 This therapy is being tested in several studies, all of them located at Asia.10,18
With respect to surgical studies, there are 3 RCT in progress that compare robotic surgery with laparoscopic surgery in the RC: ROLARR, coordinated by the University of California, COLRAR, from Korea, and ROLARR, from Shanghai. There are also trials that analyse issues related with stomas. The early closure of diverting ileostomies is the object of 2 European clinical trials: one is Danish, EASY, and another is from Odense. We highlight the phase IV Spanish study, conducted by Parc Taulí, which prospectively analyses the use of meshes as prevention of parastomal hernia after laparoscopic APR. Other trials are analysing the cylindrical APR, such as the multicentre RCT coordinated by the group of the Hospital La Fe in Valencia (lithotomy vs prone position), pending its registration in NCT. The pelvic floor reconstruction after APR is the objective of 3 trials: NEAPE and BIOPEX, both in phase III, and PRESSUR. Besides, techniques related to low anastomosis are analysed. Coloanal anastomosis using the 2-stage 2 Turnbull–Cutait is studied in the Spanish multicentre trial TURNBULL-BCN, conducted by the Bellvitge group (Barcelona) and the French study CASCADOR. Other studies compare the results of different types of anastomosis (colonic reservoirs or lateroterminal anastomosis), such as the German study SAVE. Regarding local treatment, TAU-TEM stands out, also from the Parc Taulí group. It is an RCT that compares neoadjuvance+TEM against TME in T2-3s tumours, N0 M0; the Dutch CARTS, not randomised, that analyses TEM after neoadjuvance, and a Canadian study that randomises patients, treated with TEM, with or without closure of the rectal wall.
In conclusion, according to the institutional registries of consulted clinical trials, there are currently 216 studies in progress specifically related to RC. A large majority of them are experimental and 2/3 are mainly oncological. Almost 1/3 of the clinical trials in progress can be classified as surgical. Investigation lines are focused on the improvement of preoperative treatment, either with trials of new drugs, excluding RT and its adverse effects in certain cases, or with the application of new CT schemes, generally of induction or consolidation. There is also a current investigation on how to improve the application of RT in the RC treatment. Most of the clinical trials related to surgery study the features related to robotic, laparoscopy, closure and confection of stomas, lower anastomosis, cylindrical APR, and local treatment.
Conflict of InterestThere are no conflicts of interest from any of the authors.
Please cite this article as: Reina Duarte Á, Ferrer Márquez M, Rubio Gil FA, Belda Lozano R, Álvarez García A, Blesa Sierra I, et al. ¿Qué se investiga en cáncer de recto? Cir Esp. 2015;93:381–389.