Obtaining tumor-free margins during breast conservative surgery (BCS) is essential to avoid local recurrence and frequently requires reoperation. Radiofrequency ablation (RFA) of surgical margins after lumpectomy seems to be a helpful tool to avoid reoperations, but evidence is insufficient. This study analyzes the efficacy and safety of RFA after BCS to obtain free surgical margins.
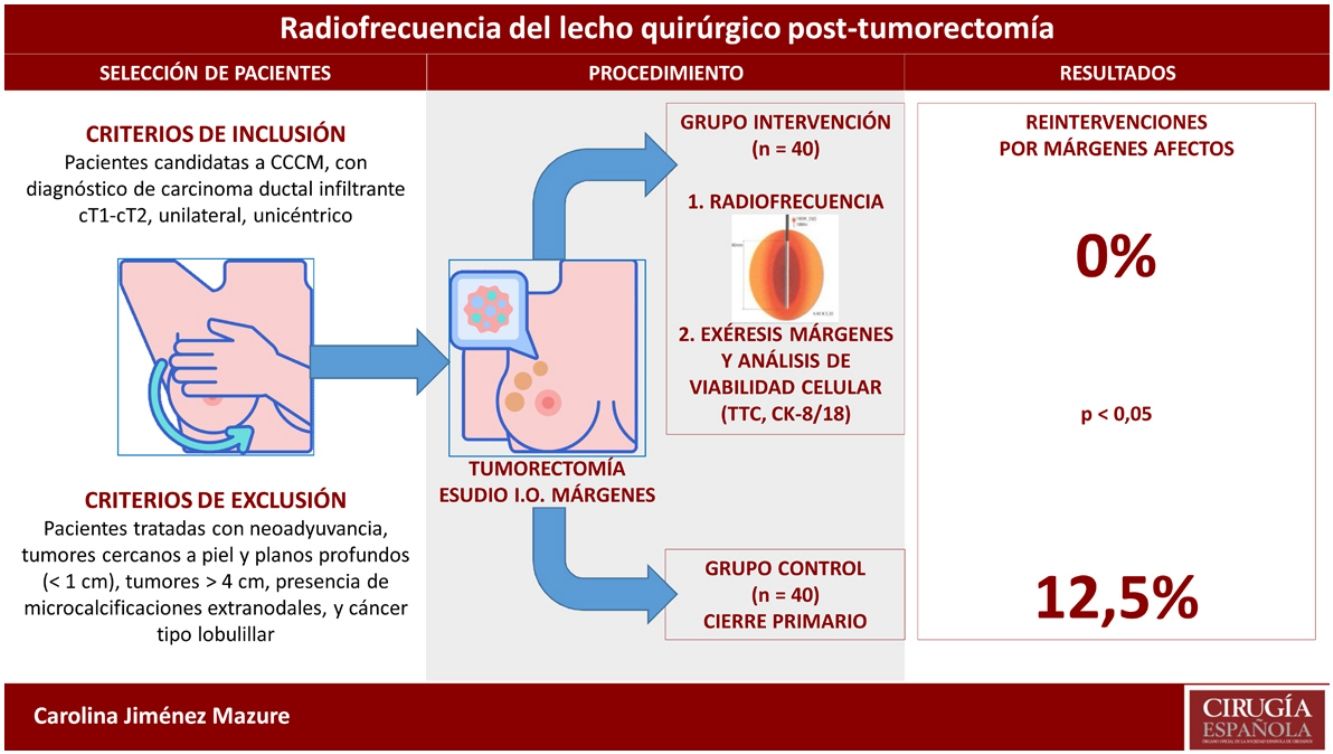
MethodsNon-randomized experimental study performed in an intervention group of 40 patients assigned to receive RFA after lumpectomy and successive resection of surgical margins, and a historical control group of 40 patients treated with BCS alone. In the intervention group, the RFA effect on tumor cell viability in the surgical margins was analyzed. Also, reoperation rate, complications and cosmetic results were compared in both groups.
ResultsA total of 240 excised margins were analyzed after RFA, obtaining a high number of tumor-free margins. Compared to the control group, the reoperation rate decreased significantly (0% vs 12%; P = .02), without differences in terms of postoperative complications (10% vs 5%; P = .67) or cosmetic results (excellent or good 92.5% vs 95%; P = .3).
ConclusionsRFA after lumpectomy is a reliable, safe and successful procedure to obtain tumor-free surgical margins and to decrease the reoperation rate without affecting complications or compromising cosmetic results.
Obtener márgenes libres en cirugía conservadora del cáncer de mama (CCCM) es esencial para evitar la recurrencia local, precisando para ello la reintervención en múltiples ocasiones. La ablación por radiofrecuencia (RFA) de los márgenes tras tumorectomía parece ser una herramienta útil para evitar las reintervenciones, aunque con insuficiente evidencia. En este estudio se analiza la eficacia y seguridad de la RFA tras la CCCM para obtener márgenes libres.
MétodosEstudio experimental, no aleatorizado, realizado en un grupo intervención de 40 pacientes al que se aplicó RFA tras tumorectomía y exéresis posterior de los márgenes, y otro grupo control histórico de 40 pacientes al que se realizó CCCM. En el grupo intervención, se analizó el efecto de la RFA sobre la viabilidad de las células tumorales en los márgenes extirpados. Se realizó además un análisis comparativo sobre el porcentaje de reintervenciones, las complicaciones y el resultado estético en ambos grupos.
ResultadosSe estudiaron 240 márgenes extirpados tras RFA, evidenciando un elevado número de márgenes libres. Comparado con el grupo control, disminuyó significativamente el número de reintervenciones (0% vs. 12%; p = 0,02), sin hallar diferencias respecto a las complicaciones (5% vs. 10%; p = 0,67) ni al resultado estético (excelente o bueno 92,5% vs. 95%; p = 0,3).
ConclusionesLa RFA tras tumorectomía es una técnica sencilla, segura y eficaz para la obtención de márgenes libres, y permite reducir las reintervenciones sin afectar a las complicaciones ni al resultado estético.
The main mission of breast-conserving surgery (BCS) is to obtain resection margins that are free of cancer cells in order to reduce local recurrence.1 Ideally, this procedure should be carried out in a single operation, providing the best aesthetic results possible with fewer and less severe complications. However, the rate of reoperations due to margin involvement is 25%-40% in certain studies.2 This creates greater stress for the patient, delayed treatments, a high number of unnecessary reoperations, worse cosmetic results, higher costs, and is in itself a risk factor for local recurrence.3,4
Furthermore, 63% of tumors that are candidates for BCS present cancer cell foci in the tumor area, which remain in the surgical bed and justify treatment with postoperative adjuvant therapies (mainly radiotherapy) after surgery.5 In fact, a large part of local recurrence, which is currently estimated at between 2.5% and 5% within 10 years, is the result of insufficient treatment of this residual disease.6 Obtaining a negative margin predicts that the residual tumor is minimal and potentially controllable with adjuvant therapies.
Radiofrequency ablation (RFA), which had already been used regularly in tumors located in other organs, was described for the first time in the breast in 1999.7 Since then, it has been gaining prominence as it provides additional treatment on the tumor and a good part of the residual peritumoral foci, without the need to remove excess glandular tissue.8 Its efficacy has been demonstrated in invasive ductal carcinoma, although its role has been much less consistent in ductal carcinoma in situ and in lobular neoplasia.9
To date, however, few studies have sought to demonstrate its benefit in reducing reoperations in BCS,10,11 in which intraoperative RFA has been presented as a potentially safe and effective tool in controlling margins during BCS, although more evidence is still needed regarding the efficacy and clinical safety of this technique.9
The aims of this study are to describe the effects of intraoperative RFA on surgical margins and to evaluate its efficacy in terms of reducing reoperations as well as its safety in terms of the number of complications and cosmetic results.
MethodsStudy designWe conducted an experimental, single-center, non-randomized study with a prospective intervention group, in which the intervention was applied, and a retrospective control group that had been treated according to standard hospital procedure.
Patient selectionInclusion criteria: patients who were candidates for BCS and had a diagnosis of unilateral, unicentric, invasive ductal carcinoma (cT1-cT2).
Exclusion criteria: patients treated with neoadjuvant therapy, tumors close to the skin and deep planes (<1 cm), tumors >4 cm, presence of extranodal microcalcifications, and lobular cancer.
Description of procedures and interventionPreoperative evaluation and lumpectomyAll patients who were candidates for BCS underwent preoperative evaluation with mammography, breast and axillary ultrasound, core needle biopsy (CNB), and nuclear magnetic resonance imaging. Subsequently, lumpectomy was performed as day surgery, together with either selective biopsy of the sentinel node or axillary lymph node dissection, depending on the case. The lumpectomy specimen surgical margins were studied intraoperatively.
Intervention: radiofrequency application procedureIntraoperative RFA after lumpectomy involved placing an electrode in the surgical bed that, by means of a high-frequency generator, produces an alternating current that causes ionic agitation, consequently creating friction and generating heat. When 45°-50 °C is reached, protein denaturation and coagulative necrosis occur. The degenerative changes produced by RFA can gradually increase, achieving complete disappearance of the cells over several months.
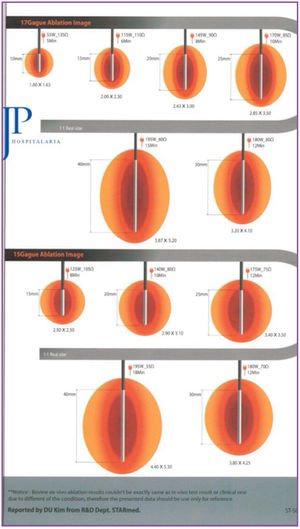
The system used was the VIVA RF (STARmed Co., Ltd., JJP Hospitalaria SL, Seville, Spain), with a VIVA RF coagulation electrode (reference 17-20V05-30) at a power of 115 W. This monopolar device, with an active tip and variable length, allows for better control of ablation, as well as less dispersion of energy. The intensity and time of application were estimated based on the length of the active tip selected and the desired ablation area, following the manufacturer’s instructions (Fig. 1).
The RFA application technique depended on the size of the cavity and the surgeon’s criteria. In small cavities, the terminal was placed and directed under ultrasound control in the surgical bed, after creating a tobacco pouch and closing the skin. In large cavities, ablation was performed margin to margin under direct vision.
Margin resectionIn the intervention group, after completing RFA, the 6 surgical margins were resected (anterior, posterior, external, internal, superior, inferior) to evaluate signs of cell viability. In the control group, only the affected margins were resected in the intraoperative analysis.
Pathology studyFor the analysis of cell viability in the samples that received RFA, staining with 2,3,5-triphenyltetrazolium chloride (TTC) was done by immersion for 1 h at 37 °C. TTC is a staining technique that uses the reduction of tetrazolium salts as an indicator of cellular respiratory activity.11 In case of persistence of viable tumor cells, the TTC salts are reduced and precipitated, forming a complex that is insoluble in water and deep red in color, which is macroscopically identifiable. In addition, in some patients, at the discretion of the pathologist, the cytokeratin 8/18 (CK-8/18) immunohistochemical technique was added, which is a very specific but not very sensitive marker based on the detection of intermediate filament keratin that is found in the intracytoplasmic cytoskeleton of epithelial tissue and disappears in an early phase of apoptosis.12
Finally, and as was done in the control group, we subsequently stained with hematoxylin-eosin (H-E) for conventional study.
Reoperation criteriaThe criteria were the presence of viable invasive ductal carcinoma, demonstrated by any of the techniques used (TTC, CK-8/18 or HE), which was in contact with the surgical margin (no-ink) of the lumpectomy specimen or, where appropriate, the extended margin.
Collection and measurement of variablesThe following descriptive variables were collected: age (years), menopause (yes/no), tumor size (mm), axillary involvement (yes/no), tumor histology (invasive ductal carcinoma ± ductal carcinoma in situ), clinical staging (I/II), pathological staging (I/II/III), and phenotype (luminal A/B, HER2+, triple negative).
The outcome variables were:
- -
Presence of cell viability, according to the pathological techniques described, in the resection margins after applying RFA
- -
Percentage of reoperations
- -
Number of complications during surgery or in subsequent follow-up (hematoma, surgical site infection, etc) and their form of treatment (conservative, surgical, etc)
- -
Aesthetic results after one month and 6 months (before and after receiving radiotherapy), classified from poor to excellent using the Harvard/NSABO/RTOG13 breast cosmesis scale
To calculate the sample size, we considered a power of 80% using a bilateral chi-square test for 2 independent samples, taking into account that the level of significance is 5% and assuming, (according to previous studies11) that the rate of reoperation in the control group would be 30% and 5% in the intervention group, which resulted in the need for 36 patients in each group. When we estimated a 10% loss, the number necessary was 40 patients per group.
For the analysis of the results, the SSPS version 20 program was used. The results for the categorical variables are expressed as frequencies and percentages, and the continuous variables as mean and standard deviation (SD). For the comparative analysis of categorical variables, the chi-squared test was used. Statistical significance was established at P < .05.
Ethical considerationsThe study followed the rules of the Declaration of Helsinki and was approved by the Research Ethics Committee. An informed consent form was signed by all participating patients.
ResultsA total of 80 patients were included in the study, 40 in each group, and there were no losses to study. The clinical characteristics and tumor data of these patients are presented in Table 1. No significant differences were found in any of the variables collected, therefore the groups can be considered homogeneous in terms of clinical and tumor characteristics.
General patient characteristics and tumor data.
| Variable | Intervention group (n = 40) | Control group (n = 40) | P |
|---|---|---|---|
| Age (yrs) | 60.9 ± 9.7 | 62 ± 11.6 | 0.45 |
| Menopause | 37 (92.5%) | 33 (82.5%) | 0.17 |
| Tumor size (mm) | 14.8 ± 5.4 | 15.4 ± 5.7 | 0.45 |
| Axillary involvement | 10 (25%) | 15 (37.5%) | 0.23 |
| Tumor histology | 1 | ||
| Invasive ductal carcinoma | 20 (50%) | 20 (50%) | |
| Invasive ductal carcinoma + DCIS | 20 (50%) | 20 (50%) | |
| Clinical stage (TNM) | 0.8 | ||
| I | 29 (72.5%) | 28 (70%) | |
| II | 11 (27.5%) | 12 (30%) | |
| Pathology stage (TNM) | 0.1 | ||
| I | 23 (57.5%) | 17 (42.5%) | |
| II | 16 (40%) | 17 (42.5%) | |
| III | 1 (2.5%) | 6 (15%) | |
| Phenotype | 0.55 | ||
| Luminal A | 18 (45%) | 16 (40%) | |
| Luminal B | 18 (45%) | 22 (55%) | |
| HER2, positive | 0 (0%) | 0 (0%) | |
| Triple negative | 4 (10%) | 2 (5%) |
RFA: radiofrequency ablation; DCIS: ductal carcinoma in situ; HER2: human epidermal growth factor receptor 2.
In the intervention group, RFA was applied in 70% of cases in small cavities with the tobacco pouch technique, using a mean ablation time of 6.36 min, and in 30% in large cavities, margin to margin, with an average time of 22 min.
Resections were performed and the margins of all patients (240 margins) were studied. The TTC staining technique was used in all, showing no data for cell viability. The immunohistochemical technique using CK-8/18 staining was performed in 17 patients (102 margins) and revealed weak positivity in 5 margins (4.9% of the total margins analyzed with this technique; 2.1% of the total margins), although there was no involvement of the contralateral side of the tumor in any of them. In 4 of these cases, the RFA was applied in small cavities after making a tobacco pouch, and in the remaining case of a large cavity, it was applied margin to margin. The subsequent pathological analysis using H-E staining also did not show tumor involvement of the excised margins (Table 2).
Analysis of resected margins in the intervention group.
| Pathology study | Small cavity (tabaco pouch) (n = 25) | Large cavity (margin to margin) (n = 15) | P |
|---|---|---|---|
| TTC stain (+) | 0/25 (0%) | 0/15 (0%) | 1 |
| CK-8/18 technique (+) | 4/11 (36,4%) | 1/6 (16.7%) | 0.39 |
| H-E stain (+) | 0/25 (0%) | 0/15 (0%) | 1 |
CK-8/18: cytokeratin 8/18; H-E: hematoxylin-eosin; TTC: 2,3,5-triphenyltetrazolium chloride.
In the pathological study of the lumpectomy specimen, at least one affected margin was found in 14 patients (35%), and involvement was found close to the margin (<2 mm) in another 16 (40%).
The results of the comparative analysis with the control group are shown in Table 3. No patient was reoperated due to margin involvement in the intervention group (0%), while in the control group it was necessary in 5 patients (12.5%); this difference was statistically significant. In contrast, the data on cosmetic results (obtaining an excellent or good result) and on the number and type of complications did not reflect significant differences. All the complications described in both groups evolved satisfactorily with conservative treatment. The patient in the intervention group who presented surgical site infection had acceptable cosmetic results with some asymmetry of the nipple-areola complex.
DiscussionObtaining free margins, together with the treatment of residual tumor disease, is key to preventing local recurrence after BCS.6 Despite all efforts, today the rate of reoperations due to margin involvement remains unacceptably high.2 This study confirms the hypothesis that the application of RFA is effective in treating residual tumor cells in breast tissue after lumpectomy; it achieves a greater number of free margins and, therefore, reduces the rate of reoperations.
As for the efficacy of RFA, when it is applied directly to the tumor, its ablative capacity drops from 92% in small tumors to 30% in tumors >2 cm.9 Therefore, when performing lumpectomy prior to the application of RFA, it is reasonable to think that it increases the efficacy on small persistent residual peritumoral foci. Another circumstance that can compromise the effectiveness of RFA is elevated impedance, which is why the tobacco pouch should be created and the cavity collapsed. In large cavities, the persistence of air is more probable after the creation of the tobacco pouch, leading to greater impedance. Thus, in a novel manner, we propose applying RFA margin to margin under direct vision. The results of the study cell viability analysis do not find differences between the new technique and the standard technique, even though the cavities are larger.
To date, it has not well established which pathology technique can unequivocally confirm complete ablation when analyzing breast tissue after applying RFA,14 so we chose TTC stain, which has been used in previous studies,11 and the CK-8/18 technique, to provide a newer approach. The analysis of the lumpectomy specimen from the intervention group not subjected to RFA showed involvement of at least one margin in 14 patients and involvement close to the margin (<2 mm) in another 16. In contrast, in the surgical resection margins after RFA, no margin involvement was found after staining with TTC (out of 240 analyzed). In addition, tumor remains were only detected in 5 cases using the CK-8/18 technique (5 margins out of 102 analyzed), all of them weakly. Furthermore, they were shown to be non-viable according to the TTC staining technique.
As in other studies,15 the meaning of the weak positivity of CK-8/18 is not clear, although it should not be ignored since it could show that apoptosis of tumor cell remains had occurred incompletely. Another possible explanation is that the analysis was carried out too close to the application of RFA, as the degenerative changes caused by heat last for several months.
Obtaining free margins after applying RFA has made it possible to avoid reoperations in the intervention group, with a statistically significant difference compared to the control group (0% vs 12%; P = .02). Moreover, this was done without compromising the aesthetic results or increasing the number and severity of complications. The evidence in this regard is very limited, and our results concur with those of other studies in which RFA was applied after lumpectomy.10,11 We provide a greater number of patients, a control group, and we propose a novel technique both due to the terminal used as well as its manner of intraoperative application.
However, the study has certain limitations. On the one hand, the presence of a historical control group, created retrospectively, with all the implications that this entails for collecting data, although in the end the clinical and tumor characteristics of the patients were comparable. Furthermore, the absence of randomization and blinding may influence the analysis of the results, mainly the cosmetic results, which were also evaluated by the same team that performed the surgical procedure. Another important limitation of the study design is that it was necessary to remove all the surgical margins for the analysis of cell viability, therefore more treated tissue was removed than necessary, which could potentially underestimate the appearance of complications derived from RFA.
There are questions that remain unanswered, such as the efficacy of RFA to reduce local recurrence, the long-term complications of treated tissue remaining in situ, the best technique for histopathological study, and the patient profile that would most benefit from this procedure in order to optimize resources.
In conclusion, RFA after lumpectomy in BCS is a safe, effective, and easily reproducible technique that contributes towards obtaining free resection margins and, therefore, reduces the number of reoperations.
FundingThis study has been partially supported by JJP Hospitalaria, S.L., who provided the ablation terminals free of charge.
Conflict of interestsThere are no conflicts of interests to report.
Please cite this article as: Jiménez Mazure C, Ribeiro González M, Soto Aguilar C, Hidalgo Martín MT, Jiménez Fernández AJ, Ferrer González MA, et al. Ablación por radiofrecuencia del lecho quirúrgico tras tumorectomía en cirugía conservadora del cáncer de mama. Cir Esp. 2020;98:472–477.