The abdominal approach for the treatment of rectal tumours is associated with considerable morbidity. Transanal endoscopic microsurgery (TEM) is a technical alternative, and less invasive than radical surgery, and thus, with a lower associated morbidity. Also, with the correct selection of patients, TEM shows similar oncological results to radical surgery. The objective of this study is to review our results with TEM and discuss its indications in the treatment of rectal tumours.
Patients and methodAn observational, retrospective study with prospective collection of data conducted from July 2008 to January 2011. TEM indications were: benign rectal tumours non-resectable using colonoscopy; early malignant rectal tumours (T1N0M0) with good prognostic factors: neoplastic tumours in more advanced stages in selected patients (high surgical risk, refused radical surgery or stoma and palliative care).
ResultsA resection was performed using TEM on 52 patients (35 benign and 17 malignant tumours). The mean hospital stay was 4.9 days, with an associated morbidity of 15.3%. The R0 resection in adenomas and carcinomas was 97.1% and 88.8%, respectively. During a follow-up of 15 (3–31) months, one recurrence of an adenoma was observed which was re-operated on using TEM.
ConclusionsTEM is a safe and effective procedure for the treatment of benign and selected early malignant rectal tumours, and is associated with a low morbidity. However, it is a therapeutic strategy based on a multidisciplinary team, basically with careful selection of patients, a validated technique and a strict follow-up protocol.
El abordaje abdominal para el tratamiento de los tumores rectales se asocia a una morbilidad considerable. La microcirugía endoscópica transanal (TEM) supone una técnica alternativa, menos invasiva que la cirugía radical, y por tanto, con una menor morbilidad asociada. Además, con una correcta selección de pacientes, la TEM presenta resultados oncológicos equiparables a la cirugía radical. El objetivo de este estudio es revisar nuestros resultados con TEM y discutir sus indicaciones en el tratamiento de los tumores rectales.
Pacientes y métodoEstudio observacional con recogida prospectiva de datos desde julio de 2008 hasta enero de 2011. Las indicaciones de TEM fueron: lesiones benignas rectales no susceptibles de resección mediante colonoscopia; lesiones rectales neoplásicas precoces (T1N0M0) con factores de buen pronóstico; lesiones neoplásicas con estadios más avanzados en pacientes seleccionados (alto riego quirúrgico, negación de cirugía radical o estoma e intención paliativa).
ResultadosSe realizó resección mediante TEM a 52 pacientes (35 lesiones benignas y 17 malignas). La estancia media hospitalaria ha sido de 4,9 días con una morbilidad asociada del 15,3%. La resección R0 en adenomas y carcinomas fue del 97,1% y 88,8% respectivamente. Durante el seguimiento de 15 (3-31) meses, se ha evidenciado una recidiva de un adenoma que ha vuelto a ser intervenido mediante TEM.
ConclusionesLa TEM es un procedimiento seguro y efectivo para el tratamiento de lesiones rectales benignas y malignas precoces seleccionadas, asociada a una baja morbilidad. No obstante, se trata de una estrategia terapéutica, basada en un equipo multidisciplinario, fundamentada en una cuidadosa selección de pacientes, una técnica quirúrgica auditada y un estricto protocolo de seguimiento.
The surgical treatment of rectal tumours has traditionally involved an abdominal approach, which includes interventions with sphincter preservation (low or ultra-low anterior resection) or abdominoperineal resections. In addition to occasionally requiring the formation of a stoma (permanent or temporary), these radical interventions are also associated with an appreciable mortality rate and a considerable rate of complications, which include urogenital alterations, sexual dysfunction, and altered defecation.1–3
One alternative for the treatment of certain rectal lesions has been called “classic” endoanal resection, described by Parks4 in 1970, which preserves sphincter function and is associated with low rates of morbidity and mortality. The limitation for this procedure is the location of the lesion (within 7–8cm of the anal margin), as well as technical difficulties associated with reduced control of the margins of the resection.5,6
As a solution to these problems, Buess et al.7 in the 1980s described the method for transanal endoscopic microsurgery (TEM). This technique allowed for the resection of lesions up to 18–20cm, which is the maximum reach of a specifically designed rectoscope. This tool includes an incorporated CO2 insufflation–aspiration system that maintains a stable pneumorectum, providing better visualisation of the rectal ampulla. For these reasons, TEM was heralded as both an oncologically and surgically safe technique, associated with a low mortality rate (4%–24%) that in many cases is irrelevant.8 Ramirez et al.9 published the first study related to the use of TEM in Spain.
Even so, the key point of TEM is appropriate patient selection, or in other words, precise indications for its use. Currently, the indications for this technique are not limited to benign lesions. TEM is also the technique of choice for early malignant lesions that are susceptible to local treatment, and in selected cases, can be used to treat more advanced malignant lesions. Recently, the indications for TEM have been extended to include other pathologies than rectal tumours, such as in the treatment of stenosis, fistulas, and retrorectal tumours, among others.10,11
Here we present our results from the treatment of malignant and benign lesions using TEM.
Patients and MethodsOurs was an observational study with prospective data collection including all patients operated upon using TEM for the treatment of rectal lesions between June 2008 and January 2011. The patients transferred to our care were examined in detail, with a complete clinical history, rectal examination, rigid rectoscopy, colonoscopy, pelvic MRI, endoanal ultrasound, and abdominal CT scan in the case of malignant lesions. In our study, the indications for utilising TEM were: benign rectal lesions not susceptible to colonoscopic resection, early neoplastic (T1N0M0) rectal lesions with good prognostic factors, and more advanced neoplastic lesions in selected patients (high surgical risk, refusal of radical surgery or stoma, and palliative intent).
Surgical TechniqueWe performed a mechanical preparation of each patient the day prior to the intervention and administered antibiotic and antithrombotic prophylaxis, normally used in colorectal surgery. The procedure was done under general anaesthesia and a urethral catheter. The position of the patient depended on the location of the tumour, since TEM must be performed such that the lesion faces the inferior part of the rectoscope.
A systematic rigid rectoscopy was performed in the operating room to confirm the characteristics of the lesion. The dissection commenced by marking the theoretical margins of resection using electrocautery at 5–10mm, depending on the characteristics of the lesion. The resection is performed with an ultrasonic scalpel. Once the lesion is resected, the zone was irrigated with povidone–iodine solution, diluted in saline at 1%. The defect was closed whenever possible using a continuous suture secured with silver clips.
We required an adequate pneumorectum for proper visualisation of the lesion. To this end, once the intervention was completed, the pneumorectum was evaluated on a scale of 1 (very bad) to 10 (optimal). The pneumorectum was categorised into: optimal (8–10), acceptable (5–7), or suboptimal (≤4).
The pathological analysis is a crucial piece of information in this type of surgery. Following excision of the lesion, it is mounted to a cork using needles to avoid retraction, and then evaluated in the operating room by a histopathologist before undergoing a definitive analysis. In the case of malignant lesions, the Kikuchi classification12 is applied for sessile lesions, and the Haggitt13 for polyploid lesions.
Postoperative Care and Follow-upThe patient commences liquid tolerance the following day, with progressive increases if tolerance continues.
After the anatomopathological study, those patients with T2 or T1 lesions with unfavourable criteria (vascular or lymphatic invasion, poorly differentiated lesions, Sm3 on the Kikuchi scale, or positive resection margins) are proposed to the oncological committee for radical surgery or, in selected cases, adjuvant therapy. The follow-up with these patients will depend on the characteristics of the lesion. During the first two years, rectoscopy and tumour markers (CEA and Ca19-9) are taken every four months. From the third to the fifth year, these controls are performed every six months. In the case of malignant lesions, the examinations are accompanied by an annual colonoscopy and abdominal CT scan.
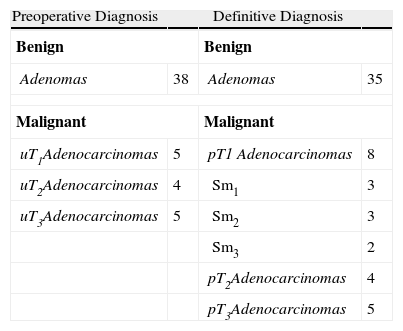
ResultsBetween June 2008 and January 2011, a total of 52 patients were operated on, 24 of which were referred from different hospitals in Andalusia. They included 19 women and 33 men, with a mean age of 68 years (33–89 years). The preoperative diagnosis was adenoma in 38 patients and carcinoma in 14 patients. Of the carcinomas, five were preoperatively categorised as urT3 and four as urT2 (Table 1). TEM was indicated for different reasons: as palliative care in six cases due to the high surgical risk associated with a radical resection, and three patients because they refused radical resection due to the high associated morbidity and mortality rates and to the possibility of a stoma.
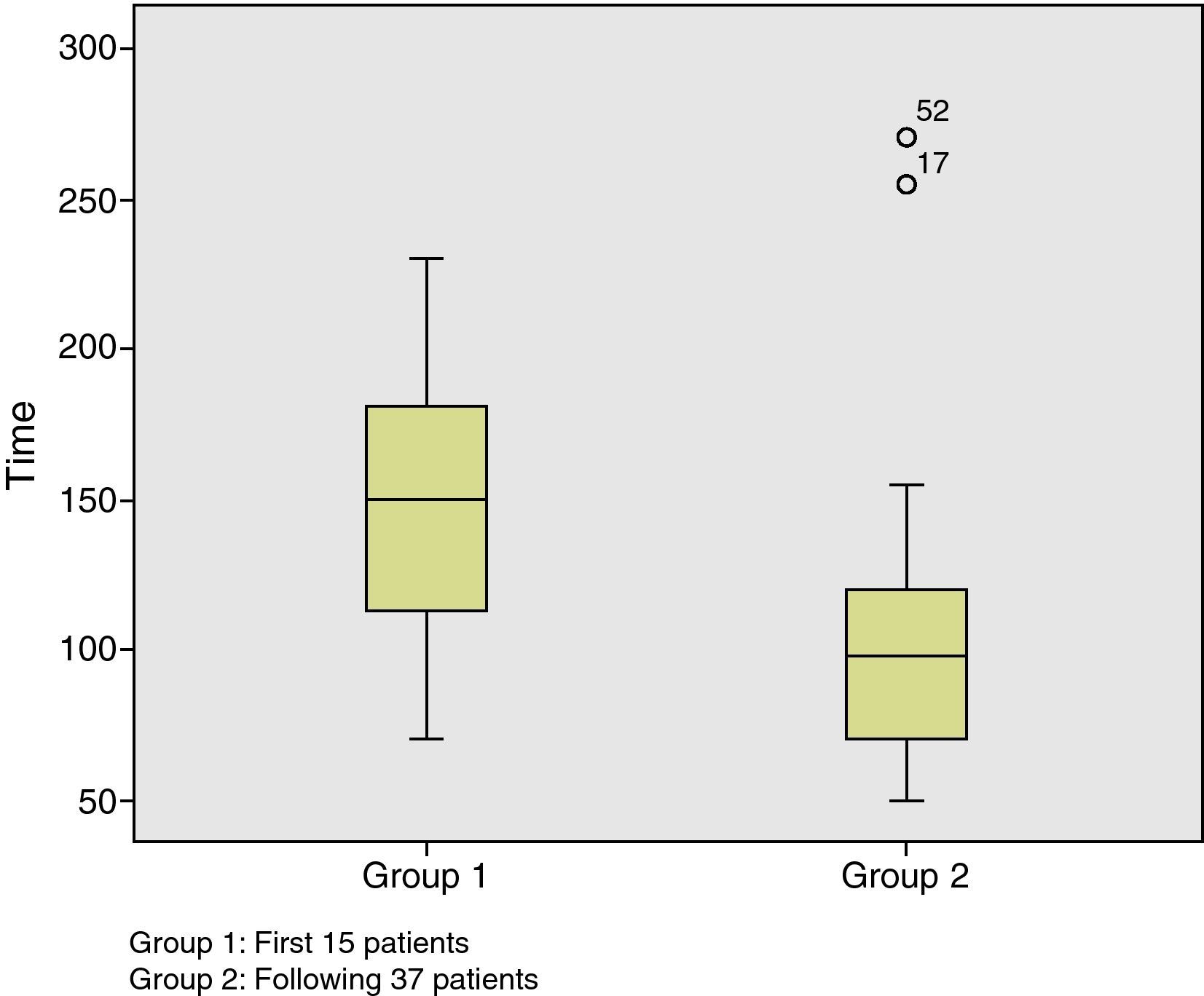
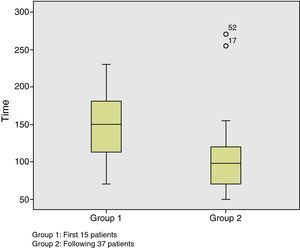
All patients were operated on by two surgeons. The mean duration of the procedure was 123±56min (50–270min). Taking into account that the first cases are part of the learning curve, we can observe that starting with patient number 15, the mean duration of the procedure began to significantly decrease (P=.004) (Fig. 1). The mean height of the lesion was 7.6±4cm (1–16cm). The location was posterior in 17 cases (32.6%), anterior in 11 cases (21.2%), right lateral in 10 cases (19.2%), and left lateral in 14 cases (27%). The pneumorectum was considered optimal in 29 cases (55.8%), acceptable in 18 cases (34.6%), and suboptimal in 5 cases (9.6%). Although there were no statistically significant differences, the lesions associated with an optimal pneumorectum were on average higher than those associated with a suboptimal pneumorectum (7.8cm vs 5.2cm). We observed no relationship between the position of the patient during the procedure and the quality of the pneumorectum.
A full-thickness resection was performed in 90.4% of cases (47 patients), and a submucosal resection was performed in the rest (5 patients): in two cases for anterior benign lesions in women, two cases for benign voluminous lesions above the reflection, and another for a benign lesion situated low and proximal to the sphincter. During the dissection, the pieces were fragmented in three patients, and pulled out as a complete surgical specimen in all others. The defect was sutured in 41 cases (78.8%, 85% of which were complete sutures and 15% were partial). The mean surface area of the lesion was 19.5±13.8cm2 (2–100cm2).
There were two perforations of the abdominal cavity that necessitated conversion to a laparotomy, with a low anterior resection in one case and a Hartmann procedure in another. These two cases were palliative patients with elevated surgical risk and defects that would not allow for a safe repair using TEM. Thus, the percentage of patients requiring conversion to laparotomy was 3.8%. One of the patients, with a very low lesion, was converted to a conventional transanal resection due to a continuous loss of pneumorectum that impeded a proper resection of the tumour.
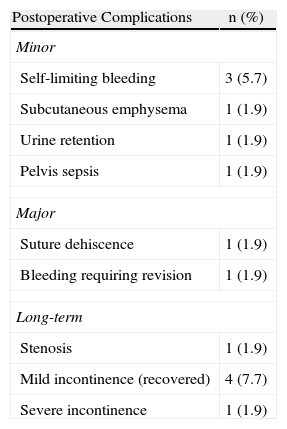
The mean duration of hospital stay was 4.9 days (2–33 days). The morbidity rate was 15.3%, with six minor complications and two major complications requiring a second intervention (Table 2). One patient was readmitted after one week due to bleeding that required surgical exploration and control with haemostatic suture. This was a patient with high surgical risk who underwent TEM after preoperative radiation therapy who also had severe actinic proctitis. Another patient developed suture dehiscence who required a second operation.
Postoperative Complications.
| Postoperative Complications | n (%) |
| Minor | |
| Self-limiting bleeding | 3 (5.7) |
| Subcutaneous emphysema | 1 (1.9) |
| Urine retention | 1 (1.9) |
| Pelvis sepsis | 1 (1.9) |
| Major | |
| Suture dehiscence | 1 (1.9) |
| Bleeding requiring revision | 1 (1.9) |
| Long-term | |
| Stenosis | 1 (1.9) |
| Mild incontinence (recovered) | 4 (7.7) |
| Severe incontinence | 1 (1.9) |
Mean patient follow-up lasted 15 months (3–31 months). The events registered during this time period were: one stenosis in a patient with a voluminous lesion in which the defect had not been sutured and that is currently improving with endoscopic dilations, four patients with mild gas incontinence who recovered three months after surgery, and one patient who had severe defecation urgency is currently under treatment with biofeedback.
Benign LesionsThe histological study revealed adenomas in 35 patients. A R0 resection was confirmed in 34 patients (97.1%). In three patients who were preoperatively diagnosed with benign lesions, the definitive histological analysis revealed a pT1 carcinoma (Sm1 on the Kikuchi scale). One patient with a giant tubulovillous adenoma with highly multifocal epithelial dysplasia underwent an R1 resection and had a recurrence after seven months. The patient was again operated on using TEM and the histopathological study indicated a villous adenoma with moderate dysplasia and free resection margins.
Malignant LesionsThe postoperative diagnosis revealed a carcinoma in 17 patients. The staging of the tumour was pT1 in eight patients (three patients Sm1, three patients Sm2, and two patients Sm3), pT2 in four, and pT3 in five (Table 1). The R0 resection was confirmed in 15 patients (88.2%). The two patients with R1 resections were preoperatively diagnosed as T3 and treated with TEM as a palliative measure. Among the patients with a definitive pT2 diagnosis, two received postoperative RT and another two were treated with radical resection (intraoperative conversion due to entry in cavity). Among the patients with a pT3 diagnosis, three received postoperative radiation therapy and another two were palliative patients who died during the follow-up period due to external causes. During the mean follow-up time of 18 months (5–31 months), there was no evidence of local recurrence.
DiscussionIt is important to point out that TEM does not change the indication criteria for the resection of rectal lesions. In our judgement, the indications for TEM are: (a) elective surgical treatment of benign rectal tumours; (b) malignant tumours in initial stages (T1N0) with good prognostic criteria; (c) in association with adjuvant or neo-adjuvant treatment in selected cases of small rectal carcinomas that are well or moderately differentiated, superficial urT2/T3 urN0, in elderly patients and those with important risk factors, or in the context of a controlled clinical trial; and (d) palliative treatment in patients with more advanced stages of disease and with high surgical risk or who refuse radical surgery. Additionally, in N0 rectal lesions with discrepancies regarding the T stage, a complete excisional biopsy can be indicated, with posterior radical surgery in the case of T1 lesions with a poor prognosis, or in the case of T2–T3 lesions.14,15
One relevant aspect of the surgical technique used is if, once the lesion is resected, the defect should be systematically sutured (whenever possible). There is a certain amount of controversy surrounding this point. Ramirez et al.16 in a randomised study, concluded that their results were not affected by the decision to suture the defect or not. On the contrary, other authors defend a systematic closing of the defect to avoid problems of postoperative bleeding and stenosis.8 In our opinion, a systematic suturing of the defect is convenient, in addition to the reasons put forth by the Parc Tauli group,8 because we consider it a fundamental skill to be able to suture the defect when closing is required (entry into the cavity).
Our study confirms that this is a safe procedure with low rates of morbidity and mortality. Our results fall within the range published in other series (2%–30%),17–22 with the majority of complications being minor and resolved using conservative treatment.Benign lesions are the primary indication for TEM. They represent 73% of all lesions present in our study. The studies that compare TEM with conventional transanal surgery show the many advantages of TEM, such as a safer and more reliable resection of the margins, reduced fragmentation of the lesion, and reduced rate of recurrence.22 Additionally, TEM can reach lesions in the middle and upper rectum that are impossible to reach using classical endoanal resection techniques. In our study, tumours at 16cm were included, and the distance has reached 20cm in other studies.16 In the systematic review carried out by Middleton et al.23 the use of TEM to treat adenomas led to a 5.7% conversion rate, a range of complications of 3%–7%, and a 5% rate of recurrence. In our series, we observed a recurrence rate for benign lesions of 3.8%, which is comparable to the ranges reported in the medical literature (3%–16%).17,22–24
The primary factor limiting the effectiveness of local treatment for early rectal cancer is the level of lymph node invasion. The depth of the invasion into the rectal wall, level of differentiation, vascular invasion, lymphatic invasion, and neural invasion are all independent factors for lymph node metastases. The T-stage estimates the probability that lymph node involvement exists, which varies from 0% to 15% for T1 tumours and 16%–28% for T2. As such, strict selection criteria along with a surgical technique that includes the complete wall and free margins are fundamental for obtaining good results.
Many series have published excellent results using TEM for the treatment of T1 rectal cancer. Floyd et al.25 described 53 patients with T1 rectal cancer with a mean follow-up time of 2.8 years, observing a 7.5% recurrence rate. Similar results for low recurrence rates have also been mentioned in other series.14,26–30 In our study, we included eight patients with T1 rectal cancer who did not develop local recurrence during the follow-up period. In the histological analysis, we also support measuring the level of submucosal invasion (Kikuchi12), since patients with pT1 Sm3 lesions have a significantly higher risk of lymphatic dissemination than patients with minor submucosal invasion (23% Sm3 vs 3% and 8% for Sm1 and Sm2, respectively).31 In these patients, radical surgery is necessary.
More controversy surrounds the role of TEM in the treatment of superficial T3 or T2 rectal cancers, given their high probability of developing lymph node metastases. Currently, except for in the context of controlled clinical trials, the treatment of choice for these patients is radical surgery. However, some authors have demonstrated favourable results in selected T2 patients who receive adjuvant treatment following TEM.22,32
One alternative for the treatment of advanced adenocarcinomas may be neoadjuvant therapy followed by a local excision. Lezoche et al.33 group use preoperative radiation therapy followed by TEM in selected patients with T2 and T3 N0 lesions smaller than 3cm. The survival rate at 90 months was 89%, with only a 4.1% rate of local recurrence after 55 months of follow-up. In this same group, Guerrieri et al.34 analysed the results from 66 T2 patients and 24 T3 patients, resulting in a 4.1% rate of local recurrence and a 90% survival rate in T2 patients, 77% in T3. The efficacy of neoadjuvant treatment in T2N0 adenocarcinomas followed by local treatment with TEM is unknown, requiring more controlled studies that can show guaranteed and safe results. Currently, we are part of a multi-centre, prospective, controlled, and randomised clinical trial that attempts to throw light on this controversial subject. This study, organised by Dr. Serra Aracil, of the Parc Tauli group (Sabadell), has the primary objective of analysing local recurrence after two years of follow-up in patients with (superficial) T2–T3s N0M0 lesions, treated using preoperative chemo-radiation therapy and TEM as compared to conventional radical surgery (total excision of the mesorectum).
Recently, transanal resections have been proposed through a single port trocar (SILS) as a new possible treatment for certain rectal lesions (five patients with a mean follow-up of 12 weeks).35 New prospective, controlled, and randomised studies with a long-term follow-up period are needed to obtain conclusive results.
ConclusionsTEM is a minimally invasive, safe, and effective procedure for the treatment of selected benign and early malignant rectal lesions. It is associated with low morbidity and mortality rates, and in many cases avoids the consequences of radical surgery. TEM is not simply a local surgical technique, but a therapeutic strategy based on a multidisciplinary team (gastroenterologists, radiologists, pathologists, anaesthesiologists, oncologists, nursing staff, and colorectal surgeons), a careful patient selection process, an audited surgical technique, and strict follow-up protocol.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Ferrer Márquez M, et al. Indicaciones y resultados de la microcirugía endoscópica transanal en el tratamiento de los tumores rectales en una serie consecutiva de 52 pacientes. Cir Esp. 2011;89:505–10.