Melanoma is a common cutaneous tumour. It is of great importance due to its increasing incidence and aggressive behaviour, with metastasis to lymph nodes and internal organs. When suspecting melanoma, excisional biopsy should be performed to obtain complete histological information in order to determine the adverse factors such as ulceration, mitosis rate, and Breslow depth, which influence preoperative staging and provide data for sentinel lymph biopsy decision making. The indicated management for melanoma is wide local excision, observing recommended and well-established excision margins, depending on Breslow depth and anatomical location of the tumour. Therapeutic lymphadenectomy is recommended for patients with clinically or radiologically positive lymph nodes.
This article reviews surgical treatment of melanoma, adverse histological factors, sentinel lymph node biopsy, and radical lymphadenectomy.
Details are presented on special situations in which management of melanoma is different due to the anatomical location (plantar, subungual, lentigo maligna), or pregnancy.
El melanoma es una neoplasia cutánea común que ha alcanzado gran importancia en las últimas décadas debido al aumento en su incidencia y a su comportamiento agresivo, con metástasis ganglionares y a distancia frecuente. La biopsia, en caso de sospecharse melanoma, debe ser escisional, con el objetivo de obtener información histológica completa y analizar factores de mal pronóstico, como ulceración, número de mitosis y el Breslow, que influyen en la estadificación preoperatoria del paciente y en la decisión de realizar biopsia de ganglio centinela o no. La escisión local amplia es el manejo indicado para el melanoma con márgenes periféricos de piel normal ya establecidos de acuerdo al Breslow y a la localización del tumor. La linfadenectomía terapéutica es el tratamiento recomendado de los pacientes con melanoma que tienen ganglios linfáticos clínica o radiológicamente positivos.
En este artículo se realiza una revisión del tratamiento quirúrgico del melanoma, la toma adecuada de biopsia de lesiones sospechosas, los factores histológicos adversos, las indicaciones de biopsia del ganglio centinela y de linfadenectomía radical. Además se revisan situaciones especiales en las cuales el manejo del melanoma difiere por su localización (acral plantar, subungueal, lentigo maligno) o diagnóstico durante el embarazo.
Primary cutaneous melanoma is one of the most common skin cancers. It is the fifth most common malignant neoplasm in men and the sixth most common in women; it is associated with high morbimortality due to its aggressive behaviour, its high risk of regional and distant lymph node metastases.1 It is estimated that in the United States approximately 76,000 people will have been diagnosed with melanoma in 2014, and 9710 deaths will be attributed to this cancer.2 Seventy-five percent of skin cancer-related deaths are due to melanoma. However, it is believed that these figures are an underestimation of reality, as a considerable number of in situ or superficial melanomas are not reported. The risk during life of acquiring an in situ or superficial melanoma has considerably increased, at 1 in 30 from 1 in 1500 in 1935.3
EpidemiologyAlthough melanoma has a peak of presentation between the fifth and sixth decades of life,4 its incidence in people aged between 25 and 29 has increased to become the most common cancer in this age group. Ninety-five percent of cases start on the skin, the remainder originate from the eyes and mucosa (oral, vagina or anus),5 and from 3% to 10% of people present with metastatic disease with no clinically evident primary lesion.6
Diagnostic approachIf a melanoma is suspected a complete physical examination of all of the skin should be made, including the oral and anogenital mucosa, the palms of the hands, and the soles of the feet. There is increasing interest in dermatoscopy7 as a diagnostic technique in the study of skin tumours, especially pigmented tumours. Advanced digital computed imaging techniques are also used.
Once the pigmented lesions suspicious of melanoma have been detected, an excisional biopsy should be performed (margin of 1–3mm),8 ideally with negative margins. On the limbs, it should be directed longitudinally in order not to subsequently alter the sentinel node result.9
An appropriate biopsy should enable the Breslow's depth to be assessed, since the extension tests that are required, the final surgical margin, and the patient's prognosis will depend on this Breslow's depth, which is the depth of the melanoma measured in millimetres from the most superficial layer of the epidermis to the deepest point of penetration. The greater the Breslow depth the poorer the patient's prognosis, and the lower the cure rates.
Excisional biopsy is not appropriate on: the palms of the hands, the soles of the feet, the face, fingers, subungual region, outer ear or on very large lesions; and in these cases it is indicated that an incisional biopsy is acceptable, taking the portion which has been clinically shown to be deeper. If the incisional biopsy does not allow accurate microstaging of the patient – which is frequent due to underestimating the thickness of the lesion – it is appropriate to repeat the procedure, and preferably go on to perform an excisional biopsy.10
Preoperative stagingWhen the diagnosis of melanoma has been confirmed, the patient needs to be staged. This is determined by the thickness, the histological features of the melanoma and the locoregional spread of the disease. Staging enables the risk of lymph node and systemic metastasis of the melanoma to be evaluated, which increases according to the thickness of the lesions. The recommendation, according to NCCN guidelines (National Comprehensive Cancer Network), 2014,10 is that routine testing for spread should not be undertaken in patients with stages I and II, unless the patient presents symptoms or signs of disease distant from the primary tumour. By contrast, they do stress that there should be a complete physical examination of the skin, the regional lymphatic pathways, and of the nodal basin. If there are any doubts on physical examination of the lymph nodes, it is suggested that an ultrasound should be performed of the nodal basin before sentinel node biopsy. If a suspicious lesion is found on ultrasound, this should be confirmed histologically. For stage III patients with positive sentinel nodes (clinically negative) the panellists leave the decision to treating physician whether to undertake a computed tomography (CT) scan or a positron emissions scan (PET/CT). They consider that histological confirmation of lymph node spread is appropriate by fine needle aspiration, core needle biopsy or open biopsy, and imaging studies for the purpose of staging, and evaluating specific signs and symptoms in patients with stage III melanoma with clinically positive lymph nodes. For patients with stage IV melanoma with distant metastasis, the consensus recommends confirming the metastasis histologically, and ideally perform a genetic study (BRAF or c-Kit mutation) to start targeted therapy, lactic dehydrogenase (prognostic marker) in addition to imaging studies (CT with or without PE/CT), including magnetic resonance (MRI) or contrasted CT of the central nervous system due to the high incidence of brain metastasis in stage IV patients.
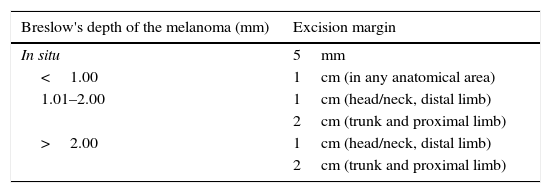
Surgical managementThe surgery recommended to remove this tumour, also known as wide radical excision, is the appropriate way to manage primary cutaneous melanoma in stages I–III, and including cases with regional nodal metastasis. The fundamental objective is to excise both the visible and microscopic tumour, and micro- and macroscopic satellites. This type of surgery must meet 2 criteria: the resection of the primary tumour should include a peripheral margin of normal skin measured from the visible edge of any residual pigmentation, tumour tissue or biopsy scar, and the deep margin of the excision should extend to the muscular fascia.11 However, it has not been demonstrated that including the muscular fascia in the resection is sufficient for the procedure to be successful.12 The appropriate excision margins have been widely investigated in randomised clinical studies,13,14 and it has been found necessary to widen the margins as the Breslow depth of the melanoma increases10 (Table 1). It is considered unlikely that margins greater than 2cm have a significant impact on local recurrence (12%) and the poor 5-year survival rate (55%) of patients with melanomas with a Breslow depth greater than 4mm, therefore offering appropriate management which, functionally and aesthetically, is more acceptable for the patient.15 The evidence shows that the failure of the most radical procedures, such as margins of 3–5cm and limb amputation, is due to the melanoma's intrinsic aggressive behaviour and not to inadequate primary surgical management. Persistence of melanoma-positive margins, on histological examination of the excision, requires a second excision to be made. In cases when it is not possible to achieve tumour-free negative margins, complementary radiotherapy has been suggested, which has demonstrated a decrease in local recurrence rates of some histological types of melanoma.16,17
Excision margins according to Breslow's depth.
| Breslow's depth of the melanoma (mm) | Excision margin |
|---|---|
| In situ | 5mm |
| <1.00 | 1cm (in any anatomical area) |
| 1.01–2.00 | 1cm (head/neck, distal limb) |
| 2cm (trunk and proximal limb) | |
| >2.00 | 1cm (head/neck, distal limb) |
| 2cm (trunk and proximal limb) |
Adapted from NCCN guidelines 2014.4.
Melanomas located on the palms of the hands, soles of the feet, head, neck and those which are histologically associated with ulceration, angiolymphatic invasion, satellitosis or high Breslow depth have a greater risk of local recurrence after wide radical excision. In Balch et al. study of 200118 it was demonstrated that for melanomas of 1–4mm thickness, local recurrence is associated with high mortality, and that ulceration on the primary melanoma is the most important prognostic factor which should alert us to the high risk of local recurrence and metastasis.
For the majority of cases it is recommended that the residual defect should be reconstructed with primary closure or a total or partial thickness graft. Flaps are only indicated in cases where the primary defects are too large to perform the abovementioned procedures. If a graft is the best option for reconstructing a defect on a limb, it should not be collected from the proximal limb, as this could potentially reintroduce tumour cells into the reconstructed wound.
Most guidelines recommend managing melanomas with margins of 0.5cm in situ.10 However, applying these margins has been demonstrated to be insufficient for managing lentigo maligna located in the head and neck due to the principally radial growth of this melanoma subtype. For this reason, it is better approached if a method with control of the margin is used, deferring reconstruction of the defect until complete excision of the tumour has been confirmed.19 For melanoma in situ in anatomical areas other than the head and neck, it is useful to make a wide radical excision with margins of 0.5–1cm, better cure rates have been reported with margins of 1cm, without considerable differences in morbidity.11
Approach to the patient with clinically negative metastasis-sentinel lymph node biopsyAfter surgical management of the melanoma, the next step is to stage the regional lymph nodes. Melanoma in situ has a metastatic potential that is not significant, as do melanomas with a Breslow depth of less than 1mm which are not associated with other histological factors with a poor prognosis (<5%).20 For melanomas with a Breslow depth of between 1 and 4mm the risk of micrometastasis to regional lymph nodes is 20–25%, and 3–5% for distant metastasis,21,22 and considered as the principal prognostic factor for long-term survival in patients with stage I and III melanoma. These are not easily detectable with imaging techniques such as ultrasound,23 or even PET/TC,24 which is one of the diagnostic tools suggested to define the presence or otherwise of metastasis in melanoma patients. Sentinel lymph node biopsy is a minimally invasive procedure which is highly accurate in detecting lymph node micrometastasis, and it has replaced elective lymphadenectomy in staging patients with clinically negative lymph nodes. A randomised study revealed that sentinel lymph node biopsy provides important prognostic information, in identifying patients with primary melanomas with an intermediate or thick Breslow depth, with nodal metastasis who would benefit from immediate completion lymphadenectomy, which prolongs disease-free survival and distant spread of the disease, in patients with melanomas of intermediate thickness.25 The ideal candidate for sentinel lymph node biopsy is a patient with a melanoma of a Breslow depth of at least 1mm and with no clinical or radiological regional lymph node metastases. The indications for sentinel lymph node biopsy were recently broadened, and it is recommended in patients with a Breslow depth of 1–4mm in any anatomical location, in staging regional disease in patients with a Breslow >4mm, and in patients with a Breslow depth of 0.75–1mm associated with adverse histological factors such as ulceration, mitotic rate>1, angiolymphatic invasion or sattelitosis.26 It is also recommended that a sentinel lymph node biopsy should be routine in paediatric patients with a melanoma of a Breslow depth of 1mm or larger, because these patients have a greater risk of lymph node metastasis than adults, despite their better prognosis. Atypical melanocytic nevi in children and adolescents have a high rate of positive sentinel lymph nodes. Therefore, sentinel lymph node biopsy could be indicated in paediatric patients in whom a melanoma is included as a differential diagnosis.27
The use of sentinel lymph node biopsy in some melanoma subtypes is controversial; in pure desmoplasic melanoma, for example, a low incidence of nodal metastases has been demonstrated (0–4%)28,29; however in others, rates of regional metastases of up to 14% have been found, and for mixed desmoplasic melanoma the incidence is higher (25%) than for pure desmoplasic melanoma, similar to that of non-desmoplasic melanoma.30,31 Some authors, however, consider that the risk of lymph node metastasis of desmoplasic melanoma is sufficient justification for undertaking a sentinel lymph node biopsy if a Breslow depth of 1mm or more is found in these tumours.10
Despite the high precision of sentinel lymph node biopsy in detecting lymph node micrometastasis, there are some cases where the use of this technique is suboptimal, in patients who have already undergone wide radical excision and defect closure, for example, when lymphogammagraphy has revealed more than 2 lymph node drainage basins, in melanomas near or on the lymph node drainage basin, melanomas of the head and neck where lymphogammagraphy has mapped an intraparotid sentinel lymph node, when there are confirmed distant lymph node metastases, when lymphogammagraphy is negative, and when life expectancy is limited, due to advanced melanoma, or other comorbidities.9
Although sentinel lymph node biopsy is not a very invasive procedure, it is not complication-free. Complications include those of the surgical wound (infections, dehiscence, etc.) lymphoedema (<5%), the formation of seroma, reactions to the contrast medium (<1%) and false-negative results (5–15%).32
Approach to the patient with clinically positive lymph node metastasisWhen patients present with a primary melanoma, and with clinically palpable lymph nodes, lymph node metastasis of the melanoma should be staged and confirmed by fine needle aspiration biopsy (FNAB).33 FNAB is a fast, precise and clinically useful technique for evaluating patients with a suspected metastatic melanoma. In the event that the tissue obtained with FNAB is not sufficient for a diagnosis or that the resource is not available, excisional lymph node biopsy is suggested.
The survival rates of patients who present with clinically palpable lymph node metastasis reduce significantly (10–50%), according to the number of lymph nodes affected, the extent of spread to the lymph nodes, and the Breslow depth of the primary melanoma.34
Surgical treatment of lymph node metastasisAfter regional lymph node metastasis of the melanoma has been confirmed, the standard treatment is radical lymphadenectomy. This in turn is known by 3 different terms according to the method used for diagnosis, and whether or not it has been confirmed histologically. Thus, completion lymphadenectomy refers to surgery after a positive sentinel lymph node biopsy: elective lymphadenectomy when surgery is performed on clinically negative lymph node basins, and when nodal involvement has not been confirmed histologically, and finally, the procedure performed on clinically positive lymph node basins after histological confirmation, which is known as therapeutic lymphadenectomy.11
Therapeutic lymphadenectomy is indicated in all patients with clinically evident lymph node metastases, and should not be replaced by radiotherapy or systemic adjuvant therapy, although they can be used as coadjuvant treatment.35 Therapeutic lymphadenectomy is not indicated for patients in whom extensive distant metastasis and/or large lymph node metastasis, fixed to adjacent structures, has been confirmed. These patients have a poor prognosis and might benefit from other treatments, such as palliative radiotherapy or systemic therapy. Elective lymphadenectomy is not routinely performed, and as mentioned above, has been substituted by sentinel lymph node biopsy. There is some controversy with regard to the function and indications of completion lymphadenectomy after positive sentinel lymph node biopsy. To date, it has not been confirmed that completion lympadenectomy improves patient survival compared with observation after positive sentinel lymph node biopsy, or that all patients with a positive sentinel lymph node biopsy would benefit from completion lymphadenectomy, as they do not all develop clinically evident lymph node metastasis, and sentinel lymph node biopsy might have resected the only focus of nodal metastasis. Furthermore, for the moment, there is no evidence from patients with positive sentinel lymph node biopsy who do not have at least a 5% probability of other non-sentinel lymph node involvement.26
At present, the conclusion of the guidelines from the American Society of Clinical Oncology-Society of Surgical Oncology (ASCO-SSO), is to perform completion lymphadenectomy on all patients with a positive sentinel lymph node biopsy, and if the patient refuses lymphadenectomy, strict follow-up is recommended to enable the detection and early treatment of lymph node recurrence.26
Treatment of local recurrenceLocally recurring melanoma is associated in most cases with systemic metastasis, which dramatically reduces these patients’ 10 year survival (5%). The initial Breslow thickness is the greatest prognostic indicator of local recurrence, and death in patients with melanoma, associated with other adverse histological factors such as ulceration and mitosis.9 Clinically it presents as a blue subcutaneous nodule, of variable size but usually from 2 to 5cm in diameter, which commonly presents in the neighbourhood of the excision of the primary melanoma (satellite metastasis) or en route to the regional lymphatic drainage basin (in-transit metastasis).9 In these cases, diagnosis should be made by FNAB or with excisional biopsy under local anaesthesia. When a diagnosis of recurrent melanoma is confirmed, the next step is to undertake further imaging studies (CT, MRI or PET/CT), and sentinel lymph node biopsy, if the patient is a candidate, for re-staging, to evaluate symptoms, and to define management.10
Ideally, in patients with recurrent melanoma (local, satellite and/or in-transit), tissue should be taken for genetic analysis of the tumour, which is particularly important in order to assess the use of targeted therapies, or plan their inclusion in a clinical study. If the absence is confirmed of regional nodal disease, surgical excision is recommended with negative margins, and primary closure of the defect, where possible. Patients with resectable in-transit recurrence might benefit from sentinel lymph-node biopsy, in addition to wide radical excision, and reconstruction of the defect with graft or flap. Although it is still not clear whether resection margins should be wide in recurrences, it is clear that a margin of normal skin should be left.10
Special clinical situationsThere are still questions as to the correct management of patients with primary melanoma in some clinical situations, such as subungual melanoma, acral melanoma, or the appropriate management of pregnant patients with a diagnosis of melanoma. However, expert recommendations are a tool on which management of these cases can be based.
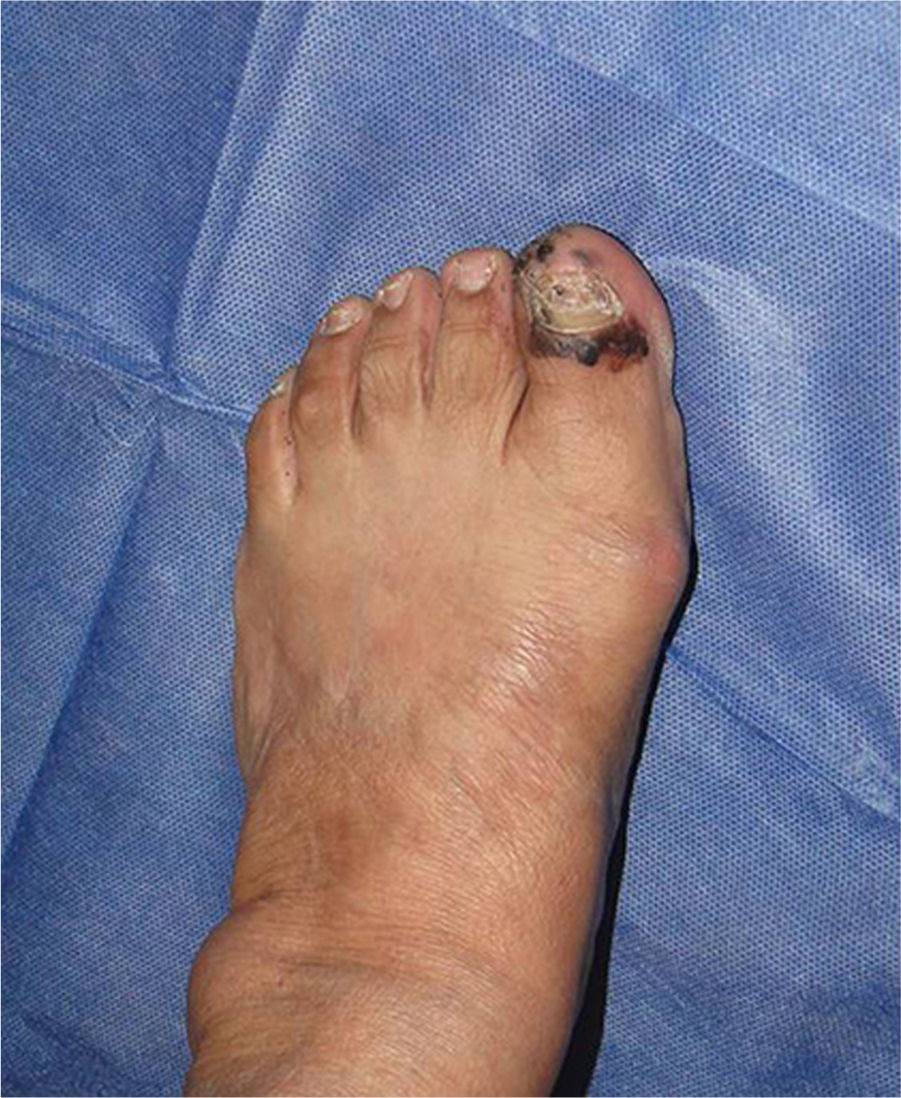
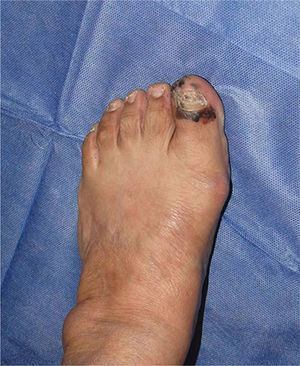
Subungual melanomaThis is a rare variant of melanoma in the white population, with a prevalence of 3%, in contrast to its prevalence in black patients, of 15–35%.36 The most common location of this melanoma is the first finger and the first toe (75%). It presents clinically as a longitudinal melanonychia, de novo or pre-existing with recent changes. Hutchinson's sign – pigmentation of the periungual skin – is highly suggestive of melanoma (Fig. 1). Amelanic melanoma presents atypically to conventional melanoma, as an erythmatose, frequently ulcerative nodule in the subungual region associated with onycholysis, and dystrophy of the nail plate with an absence of pigment. If there are any of these signs a biopsy should be taken for histological confirmation. Longitudinal biopsy which includes where possible all the pigmentation (excisional), as with other types of melanoma, is the ideal technique for studying a lesion for which melanoma is the differential diagnosis. Biopsy should include tissue from the nail bed, and reach the periosteum in depth.37
The margins for wide radical excision are based on the guidelines according to Breslow thickness, and adverse histological factors. If the melanoma is in situ, the recommended margins are 5mm, including the bed and proximal matrix, and reconstruction with partial thickness graft. The most appropriate management of an invasive subungual melanoma of a lower limb is amputation at the level of the metatarsophalangeal joint. However, for an invasive subungual melanoma affecting an upper limb, amputation at the level of the joint distal to the lesion is preferred, using margins of 1cm, with the objective of giving the patient more conservative management, enabling better function of the hand.38
Indications for sentinel lymph node biopsy in subungual melanoma are based on the guidelines for managing conventional melanoma based on the thickness of the melanoma, and the presence or otherwise of palpable lymph nodes.26
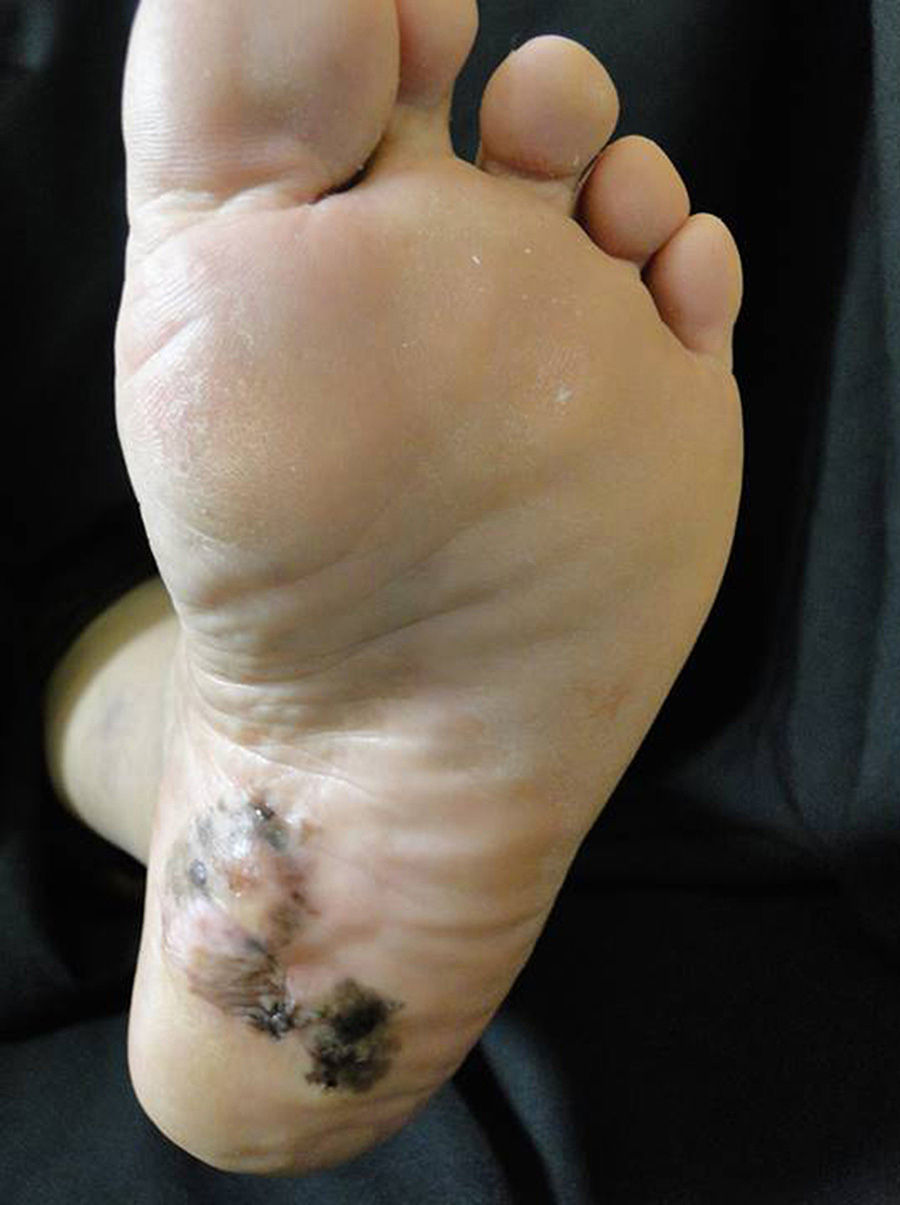
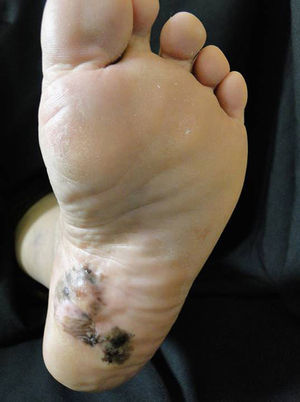
Plantar acral melanomaThe importance of this clinical subtype of melanoma is the poor prognosis associated with it due to late diagnosis.39 High clinical suspicion on the part of the treating physician is required in addition to training in dermatoscopy, as this tool is very useful in deciding whether to take a biopsy from a plantar melanocytic lesion(Fig. 2).9
Biopsy in these patients should be excisional. However, incisional biopsy can be used when the lesions are very large. Definitive management is based on the stage of the disease and the management recommended by the established guidelines for melanoma for equivalents in thickness and histology in other skin locations.10
The decision to perform a sentinel lymph-node biopsy is based on the above-mentioned ASCO-SSO indications according to the stage of the melanoma.26 Therapeutic lymphadenectomy is recommended for primary melanoma with clinically positive lymph nodes.
Reconstruction of defects on the sole of the foot depends on their location on the plantar surface, the disease stage, comorbidities, and the patient's lifestyle. In patients with defects in non-weight bearing sites, sedentary patients, comorbidities and/or associated metastatic disease a primary closure of the defect is preferred with either partial or total skin graft. For defects on weight-bearing sites on the plantar surface, the reconstruction options are rotation or advance skin flaps or free musculocutaneous flaps, preferably performed by a reconstructive plastic surgeon.9
Lentigo malignaWith a lesion on the face suggestive of lentigo where malignant changes are suspected, a biopsy should be taken to confirm the diagnosis. However, the appropriate method for biopsy is a challenge, as these are usually lesions with poorly defined edges, which are large for such an aesthetically sensitive area as the face. According to the guidelines for the management of melanoma, the most appropriate method is excisional biopsy; incisional or shave biopsy are often suboptimal. However, an acceptable option is a deep incisional biopsy, or punch biopsy of the area which is seen clinically to be the deepest.10 Shave biopsy can compromise complete histological evaluation of the tumour, and appropriate Breslow's depth measurement, therefore we do not use this technique for diagnosing melanoma in our patients.
When a diagnosis of lentigo maligna has been confirmed the tumour is resected, 5mm margins for lentigo maligna of the head and neck are usually suboptimal, therefore a staged resection technique is recommended to enable thorough evaluation of the margins.19 On confirmation of the presence of an invasive lentigo maligna, it is staged according to Breslow's depth.40 Lymph gammagraphy with technetium sulphur colloid is used to stage lentigo maligna melanoma. This substance replaces the blue stains which are used routinely, as they are unnecessary in this site, and there is a remote risk of permanent dyschromia of the skin, necrosis and anaphylaxis.41 If micrometastasis is confirmed, a completion lymphadenectomy should be performed plus surface parotidectomy if the micrometastases are in the periparotid lymph nodes.9
Melanoma and pregnancyMelanoma in pregnancy has a prevalence of up to 31%, of all the cancers which present in this condition,42 and is a neoplasm with high morbimorbidity, and with a not insignificant risk of metastasis to the placenta and the foetus. It is known that pregnancy does not significantly affect the aggressivity of the melanoma in terms of metastasis and survival; however, it is appropriate to be aware that they can occur in pregnancy, and correct and fast management is required in this situation.42,43 Biopsy in pregnant patients with a suspected melanoma should be excisional as in all cases, and local anaesthetic is recommended with lidocaine without epinephrine. When the diagnosis has been confirmed, staging can be undertaken safely with a chest X-ray and lactic dehydrogenase or MRI, or abdominal ultrasound if the melanoma has a high Breslow's depth or palpable adenopathies. The excision margins are the same as those for a woman who is not pregnant.44 Sentinel lymph node biopsy is safe with technetium sulphur colloid.
ConclusionsLesions suspicious of melanoma should be biopsied excisionally where possible in order to enable the pathologist to report the histological characteristics of the tumour as completely as possible, which should include the presence or otherwise of ulceration, the number of mitosis, Breslow's depth and other adverse histological factors such as Clark level, the presence of lymphovascular invasion, satellitosis and regression of the melanoma. Of these adverse histological factors, the most associated with micrometastasis is ulceration, followed by the presence of one or more mitoses. Patients with stage I and II melanoma do not require routine testing. In patients with regional lymph node involvement or in stage III of the disease, the recommendation is histological confirmation by fine needle aspiration or open biopsy, and it is left to the criteria of the physicians whether to perform imaging studies to find distant metastases. Imaging studies such as CT and/or PET, plus brain MRI, are indicated in patients with stage IVmelanoma, and LDH level, which has prognostic significance for stage IV melanomas. Treatment for localised, and regionally metastatic melanoma is essentially surgical. The term local excision implies the use of peripheral margins of 1–2cm from any residual pigmentation or scar, according to the Breslow's depth and the anatomical location of the melanoma. Sentinel lymph node biopsy is a minimally invasive procedure which provides information on the patient's prognosis, and which also identifies the patients for whom completion lymphadenectomy is most useful, therefore it has replaced scheduled lymphadenectomy, which is currently not recommended routinely. The patients who benefit most from sentinel lymph node biopsy are those with melanomas of 1–4mm with no clinical or radiological evidence of regional lymph node involvement, although the indications have extended to further clinical scenarios.
Finally, melanoma should be considered as one of the most aggressive skin cancers due to its high rates of regional, and distant metastasis. This cancer requires early diagnosis so as to offer appropriate treatment, and reduce the morbimortality associated with it.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Zuluaga-Sepúlveda MA, Arellano-Mendoza I, Ocampo-Candiani J. Actualización en el tratamiento quirúrgico del melanoma cutáneo primario y metastásico. Cir Cir. 2016;84:77–84.