Patients under 45 years with gastric cancer are associated with a poor prognosis. Recent studies report that the 5-year survival is better in younger patients after curative resection.
ObjectiveTo determine if prognostic factors such as age under 45 years old, anaemia, weight loss, tumour differentiation, histological sub-type, depth of invasion, and lymph node involvement, reduce the survival of patients with resectable advanced gastric adenocarcinoma undergoing gastrectomy with limited and extended lymphadenectomy.
Materials and methodsThis study included a cohort of consecutive cases treated in the Sarcomas Department of the Oncology Hospital of the Centro Médico Nacional Siglo XXI, of the Instituto Mexicano del Seguro Social, during the period between January 2000 and December 2006.
ResultsOf the total of 588 patients evaluated, 112 (19%) were under 45 years, 43% classified as Borrmann IV, and 36% as Borrmann III. Metastatic disease was present in 39.3%, localised diffuse in 12.5%; lower resectability 52.7 vs. 61.3% in older than 45 years.
At the end of the study 29.5% of patients under 45 years were alive; no recurrence in 26.8%, with an overall survival of 58.6±4.3 months, compared with 18.3% of patients alive over 45 years, 17.9% disease-free, and with overall survival 35.2±4.3 months resectable disease.
ConclusionsPatients under 45 years have a better survival after a two-year disease-free period.
Los pacientes menores de 45 años con cáncer gástrico tienen un pronóstico desfavorable. Estudios recientes refieren que la sobrevida a 5 años es mejor en jóvenes posterior a la resección curativa.
ObjetivoDeterminar si los factores pronóstico, como: edad menor de 45 años, anaemia, pérdida de peso, grado de diferenciación, subtipo histológico, tumour palpable, profundidad de la invasión y afección ganglionar, reducen la sobrevida en pacientes con adenocarcinoma gástrico avanzado resecable, tratados mediante gastrectomía con linfadenectomía limitada y extendida.
Material y métodosEstudio de cohorte histórica de casos consecutivos de adenocarcinoma gástrico, atendidos y tratados en el Servicio de Sarcomas del Hospital de Oncología de Centro Médico Nacional Siglo XXI, del Instituto Mexicano del Seguro Social, durante el período comprendido entre enero de 2000 y diciembre de 2006.
ResultadosSe evaluó a 588 pacientes; el 19% (n=112) fueron menores de 45 años, el 43% clasificados como Borrmann IV y el 36% como Borrmann III. Tuvieron enfermedad metastásica un 39.3%, localización difusa un 12.5% y menor resecabilidad en 52.7 vs. 61.3% en mayores de 45 años.
Al finalizar el estudio, un 29.5% de los pacientes menores de 45 años estaban vivos; el 26.8% sin recurrencia, con una sobrevida global de 58.6±4.3 meses, comparado con el 18.3% de los pacientes vivos mayores de 45 años, de los cuales el 17.9% estaba sin enfermedad y con una sobrevida global de 35.2±4.3 meses con enfermedad resecable.
ConclusionesLos pacientes menores de 45 años tienen mejor sobrevida después de los 2 años de período libre de enfermedad.
Gastric cancer is the most common gastrointestinal neoplasm worldwide, it ranks second in global cancer mortality and represented 3% of cancers diagnosed in Mexico in 2000.1–3 The incidence of stomach cancer is highest in Asia, where early stage diagnosis (stage IA) is established in up to 30%, It is identified in advanced stages in 70% due to screening programmes4 and 88% present at stage III or IV at time of diagnosis.5
It is resectable in 60–80%, and post-operative mortality ranges from 6% to 14%. The rate of survival at 5 years remains at 8–26%, which contrasts strongly with survival rates in Japan, which reach 52% in some series.6
A retrospective study performed in our country reported that 80.2% of patients with this neoplasm were diagnosed in stages IIIB and IV, with survival at 2 years of 13.8%. The mean age of the patients was 58.6 years.7,8
To date surgical treatment is the only procedure which has curative potential. The presence or absence of distal lymph node metastases in gastric adenocarcinoma is still the most important indicator of survival, after curative resection.9
Being aged under 40 years has been associated with increased frequency of poorly-differentiated tumours (55.5%, p=0.02) or with histology of seal ring cells (25.9%, p=0.01). By contrast, a frequency of 11.3% p=0.01) and 34.6% (p=0.02), respectively, has been associated for patients aged over 40. This implies poorer clinical and pathological features which, therefore, indicate an unfavourable survival prognosis for elderly patients and tumours with the same anatomic location.6,10 However, recent studies contradict this: despite the histological features in young people, in addition to the prevalence in females, survival at 5 years is better in people aged below 50 (54 vs 46%, p=0.035), than in those aged over 50.11
Other recent studies reflect that the frequency of gastric cancer has increased from 2 to 8% to 19% in Eastern countries in recent years and that, despite the lower curative resection rate in young patients (84 vs 92%, p<0.001), survival at 5 years for resected tumours is greater in young people (80 vs. 75%, p=0.002).12 Weight loss prior to diagnosis results in poorer tolerance to 5-fluorouracil-based treatment, with increased mucositis toxicity and palmo-plantar syndrome, secondary to nutritional deficits of glutamine and vitamin B6 respectively.13–15
The presence of a palpable mass at the time of diagnosis is considered a sign of unresectibility, and therefore poor prognosis for survival at 5 years (0–20%), with extremely poor average life expectancy (<4 months).6,16,17
Many studies have demonstrated that involvement of the serosa is the negative prognostic factor which has the greatest effect on survival, since if the neoplasm has infiltrated the serosa, the impact of lymph node dissection on survival is reduced because surgery cannot control transcoelomic spread which is the main cause of recurrence and death in post gastrectomy cancer patients.18 A survival rate of 62.7% has been reported for tumours that invade the muscularis propria, 42.2% when there is infiltration of the subserosa and 30.1% with involvement of the serosa (p≤0.0001).9
Furthermore, it has been indicated that the prognosis of patients presenting invasion of the serosa is more favourable when there is no lymph node metastasis (N0), and the rate of survival at five years progressively drops when there is lymphatic involvement (67.9 vs 32.8%, p<0.01).20
Thus, it is currently thought that the depth of tumour invasion and lymph node involvement are 2 of the most important independent prognostic factors of gastric carcinoma.21
On comparing the type of surgery, total or partial gastrectomy, for distal gastric cancer we found similar results in terms of morbidity, general mortality and global survival; therefore, a conservative surgical approach is preferable, providing that this enables complete resection of the tumour.22
ObjectiveTo identify the main survival prognostic factors of patients with advanced resectable gastric cancer, treated by gastrectomy with limited and extended lymphadenectomy, in the Sarcomo Department of the Oncology Hospital of Centro Médico Nacional Siglo XXI, of the Instituto Mexicano del Seguro Social.
Material and methodsA historical cohort study identified at time of diagnosis and implemented from the day of surgery. The clinical histories of the patients attended and treated in the Sarcoma Department of the Oncology Department of the Centro Médico Nacional Siglo XXI, of the Instituto Mexicano del Seguro Social were reviewed retrospectively, in the period between 1 January 2000 and 31 December 2006; with a follow-up period of 6 years.
Patients aged from 16 to 72 years, who had undergone endoscopy with biopsy in the unit (the sample was reviewed by our hospital's Pathology Department) with a histological diagnosis of advanced clinical stage gastric adenocarcinoma. These patients had undergone surgical treatment with curative intent. Limited dissection was considered dissection D1, which included the lymph node relays from 1 to 6; extended dissection was considered dissection D2 comprising lymph node relays from 1 to 11 plus dissection of the serosa of the transcavity of the omentum from the transverse mesocolon.
Depending on the anatomic location of the tumour, subtotal gastrectomy was performed if the distal 2/3 of the stomach were removed (antrum and body) and total gastrectomy if the whole stomach was resected.
The patients underwent contrasted computed axial tomography of the abdomen and pelvis in the Radiology and Imaging Department of the hospital to assess resectability. Their haemoglobin levels were measured by haematic biometry.
Any involuntary weight loss, related to the current disease and not attributable to another cause was considered for the study. The abdominal tumour corresponding to the gastric tumour under study was referred to as a palpable mass.
Staging was carried out according to the TNM classification of the American Joint Committee on Cancer (AJCC), 7th edition 2009. The performance status of the patient was determined according to the Eastern Cooperative Oncology Group (ECOG) classification, and was taken from the information in the clinical record.
Lauren's classification was also used, which divides the gastric cancer into 2 subtypes (intestinal and diffuse), each with their own histopathological, clinical and epidemiological features.
With regard to the variables based on cellular morphology, information was obtained in line with that documented in the histopathology report on the surgical specimen (papillary, colloid, medullary and seal ring cells), and with regard to the degree of differentiation, which went from G1 to G4.
The tumour size and metastasis were obtained from the Pathology Department report.
The statistical analysis was recorded on a database and subsequently, on SPSS version 13.0 for Windows. Descriptive analysis was performed recording mean, standard deviation and frequency table. The Student's t-test was used for independent groups for differences in means. The chi-square test was used for factors associated with complications and the odds ratio for quantifying risk factors. Survival was analysed using the Kaplan–Meier method. The long rank test (Mantel–Cox) or Breslow's test was used to compare survival curves. A p value of <0.05 was considered statistically significant with a confidence interval of 95%.
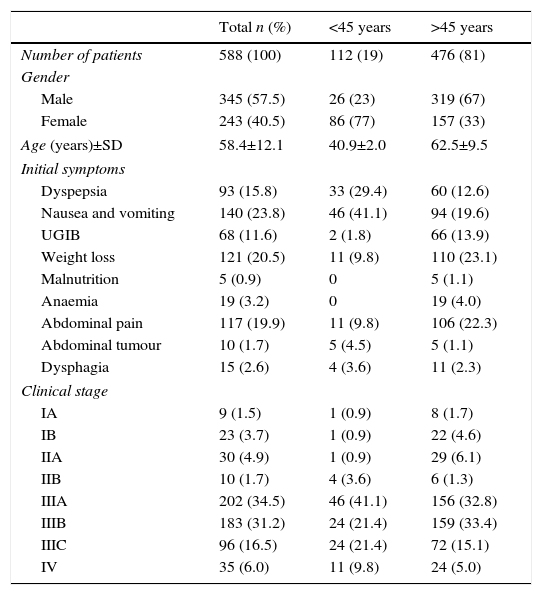
ResultsFive hundred and eighty-eight patients were evaluated, of whom 19% (n=112) were aged under 45 years and 81% (n=476) over 45. The demographic and clinical features are shown in Table 1. The table shows that females prevail (77%) in the group aged under 45 and males in the group aged over 45 (67%). The most common symptoms were nausea and vomiting in the group under 45 (41.1%), followed by dyspepsia (29%). By contrast, weight loss (23.1%) and abdominal pain (22.3%) were the most common symptoms in those over 45 years.
Clinical and demographic features of 588 patients with gastric adenocarcinoma treated with surgery.
| Total n (%) | <45 years | >45 years | |
|---|---|---|---|
| Number of patients | 588 (100) | 112 (19) | 476 (81) |
| Gender | |||
| Male | 345 (57.5) | 26 (23) | 319 (67) |
| Female | 243 (40.5) | 86 (77) | 157 (33) |
| Age (years)±SD | 58.4±12.1 | 40.9±2.0 | 62.5±9.5 |
| Initial symptoms | |||
| Dyspepsia | 93 (15.8) | 33 (29.4) | 60 (12.6) |
| Nausea and vomiting | 140 (23.8) | 46 (41.1) | 94 (19.6) |
| UGIB | 68 (11.6) | 2 (1.8) | 66 (13.9) |
| Weight loss | 121 (20.5) | 11 (9.8) | 110 (23.1) |
| Malnutrition | 5 (0.9) | 0 | 5 (1.1) |
| Anaemia | 19 (3.2) | 0 | 19 (4.0) |
| Abdominal pain | 117 (19.9) | 11 (9.8) | 106 (22.3) |
| Abdominal tumour | 10 (1.7) | 5 (4.5) | 5 (1.1) |
| Dysphagia | 15 (2.6) | 4 (3.6) | 11 (2.3) |
| Clinical stage | |||
| IA | 9 (1.5) | 1 (0.9) | 8 (1.7) |
| IB | 23 (3.7) | 1 (0.9) | 22 (4.6) |
| IIA | 30 (4.9) | 1 (0.9) | 29 (6.1) |
| IIB | 10 (1.7) | 4 (3.6) | 6 (1.3) |
| IIIA | 202 (34.5) | 46 (41.1) | 156 (32.8) |
| IIIB | 183 (31.2) | 24 (21.4) | 159 (33.4) |
| IIIC | 96 (16.5) | 24 (21.4) | 72 (15.1) |
| IV | 35 (6.0) | 11 (9.8) | 24 (5.0) |
SD: standard deviation; UGIB: upper gastrointestinal bleeding.
The clinical stage that predominated in the group aged under 45 years was IIIA (41.1%), and IIIB in the group over 45 (33.4%).
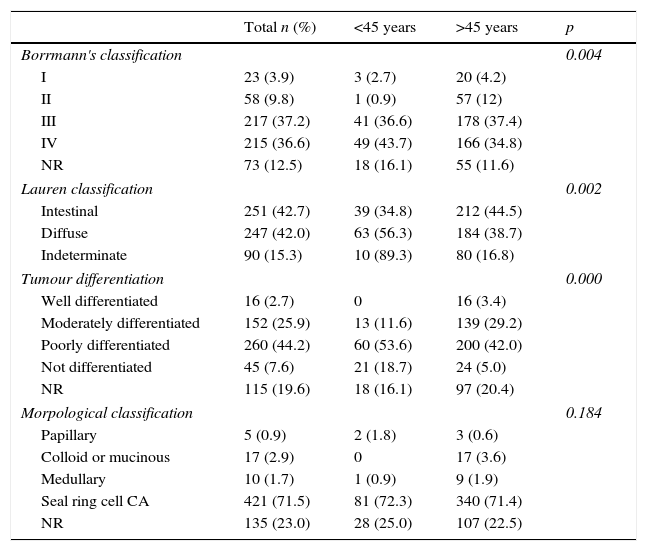
The presentation features of the tumour are shown in Table 2, Borrmann III (36.6%) predominated in those under 45, Borrmann III predominated (37.4%) in those aged over 45 (with regard to Lauren's classification, the diffuse type predominated in the under 45 group, rather than the intestinal type which predominated in those aged over 45). In both groups poorly differentiated tumours predominated. The prevailing histology was seal ring cell carcinoma.
Macroscopic and microscopic features of the tumour.
| Total n (%) | <45 years | >45 years | p | |
|---|---|---|---|---|
| Borrmann's classification | 0.004 | |||
| I | 23 (3.9) | 3 (2.7) | 20 (4.2) | |
| II | 58 (9.8) | 1 (0.9) | 57 (12) | |
| III | 217 (37.2) | 41 (36.6) | 178 (37.4) | |
| IV | 215 (36.6) | 49 (43.7) | 166 (34.8) | |
| NR | 73 (12.5) | 18 (16.1) | 55 (11.6) | |
| Lauren classification | 0.002 | |||
| Intestinal | 251 (42.7) | 39 (34.8) | 212 (44.5) | |
| Diffuse | 247 (42.0) | 63 (56.3) | 184 (38.7) | |
| Indeterminate | 90 (15.3) | 10 (89.3) | 80 (16.8) | |
| Tumour differentiation | 0.000 | |||
| Well differentiated | 16 (2.7) | 0 | 16 (3.4) | |
| Moderately differentiated | 152 (25.9) | 13 (11.6) | 139 (29.2) | |
| Poorly differentiated | 260 (44.2) | 60 (53.6) | 200 (42.0) | |
| Not differentiated | 45 (7.6) | 21 (18.7) | 24 (5.0) | |
| NR | 115 (19.6) | 18 (16.1) | 97 (20.4) | |
| Morpological classification | 0.184 | |||
| Papillary | 5 (0.9) | 2 (1.8) | 3 (0.6) | |
| Colloid or mucinous | 17 (2.9) | 0 | 17 (3.6) | |
| Medullary | 10 (1.7) | 1 (0.9) | 9 (1.9) | |
| Seal ring cell CA | 421 (71.5) | 81 (72.3) | 340 (71.4) | |
| NR | 135 (23.0) | 28 (25.0) | 107 (22.5) | |
NR: not representative; CA: cancer.
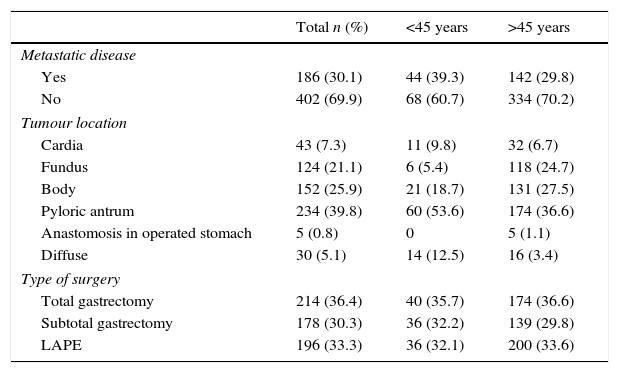
The surgical findings and results are shown in Table 3; a higher percentage of metastatic disease was found (39.3 vs 29.8%), as well as diffuse location (12.5 vs 3.4%), but a lower percentage of resectable disease (52.7 vs 61.3%) in patients aged under 45 years compared with the older age group.
Outcomes of surgery.
| Total n (%) | <45 years | >45 years | |
|---|---|---|---|
| Metastatic disease | |||
| Yes | 186 (30.1) | 44 (39.3) | 142 (29.8) |
| No | 402 (69.9) | 68 (60.7) | 334 (70.2) |
| Tumour location | |||
| Cardia | 43 (7.3) | 11 (9.8) | 32 (6.7) |
| Fundus | 124 (21.1) | 6 (5.4) | 118 (24.7) |
| Body | 152 (25.9) | 21 (18.7) | 131 (27.5) |
| Pyloric antrum | 234 (39.8) | 60 (53.6) | 174 (36.6) |
| Anastomosis in operated stomach | 5 (0.8) | 0 | 5 (1.1) |
| Diffuse | 30 (5.1) | 14 (12.5) | 16 (3.4) |
| Type of surgery | |||
| Total gastrectomy | 214 (36.4) | 40 (35.7) | 174 (36.6) |
| Subtotal gastrectomy | 178 (30.3) | 36 (32.2) | 139 (29.8) |
| LAPE | 196 (33.3) | 36 (32.1) | 200 (33.6) |
LAPE: laparoscopy.
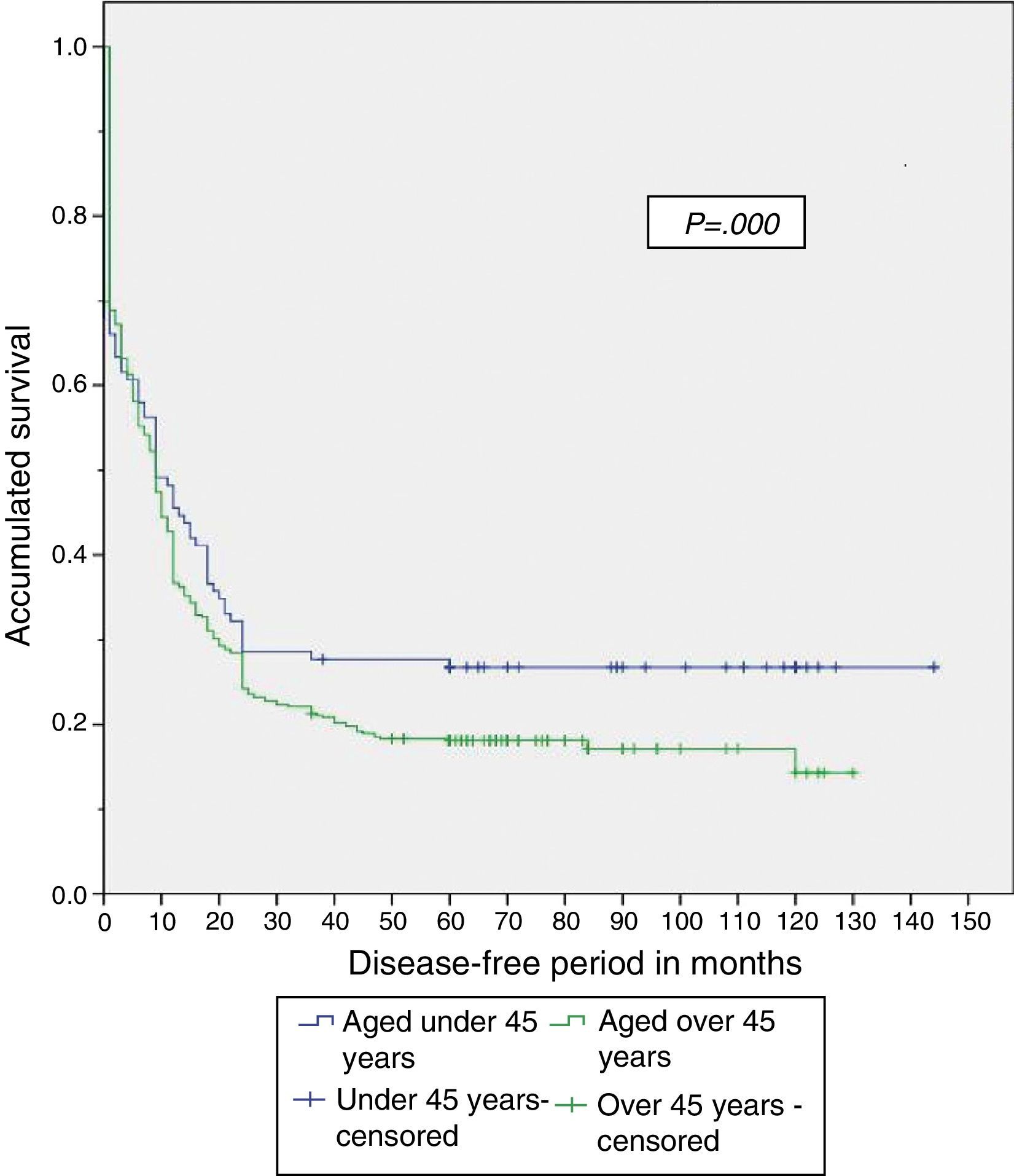
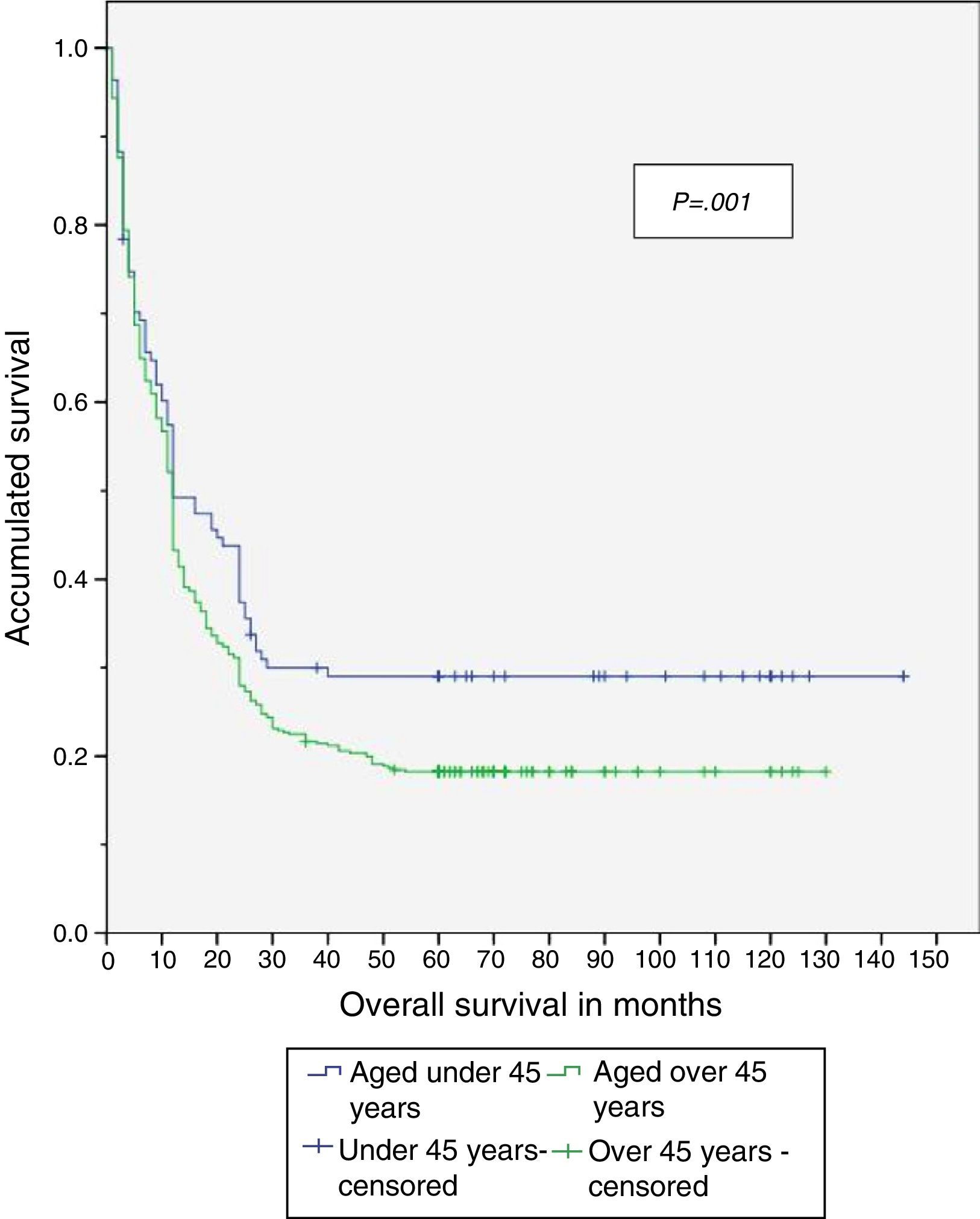
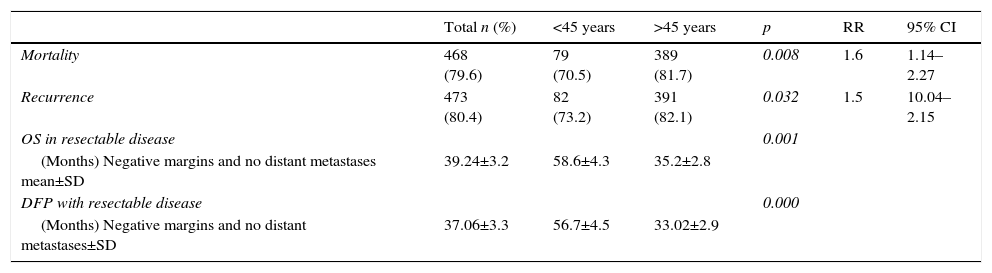
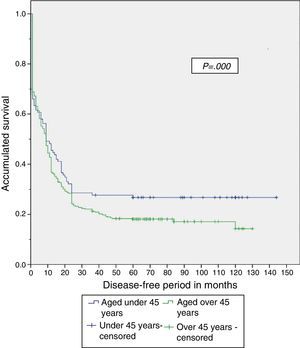
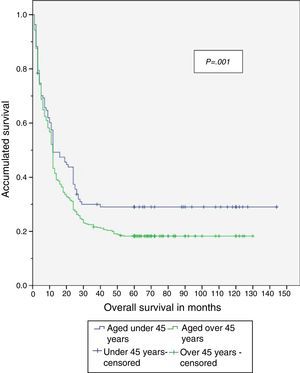
When the study was completed, 29.5% of patients aged under 45 years were alive, of which 26.8% had no recurrence of the disease (Table 4). The disease-free period and overall survival of resectable disease is shown in Figs. 1 and 2. Fifty percent of the patients from both groups died after 12 months. The difference in overall survival is presented from 24 months, and corresponds to fewer than 30% of the patients. Overall survival at 5 years was 28% in the group aged under 45 years and 18% in those aged over 45.
Mortality and survival.
| Total n (%) | <45 years | >45 years | p | RR | 95% CI | |
|---|---|---|---|---|---|---|
| Mortality | 468 (79.6) | 79 (70.5) | 389 (81.7) | 0.008 | 1.6 | 1.14–2.27 |
| Recurrence | 473 (80.4) | 82 (73.2) | 391 (82.1) | 0.032 | 1.5 | 10.04–2.15 |
| OS in resectable disease | 0.001 | |||||
| (Months) Negative margins and no distant metastases mean±SD | 39.24±3.2 | 58.6±4.3 | 35.2±2.8 | |||
| DFP with resectable disease | 0.000 | |||||
| (Months) Negative margins and no distant metastases±SD | 37.06±3.3 | 56.7±4.5 | 33.02±2.9 |
OS: overall survival; DFP: disease-free period; SD: standard deviation.
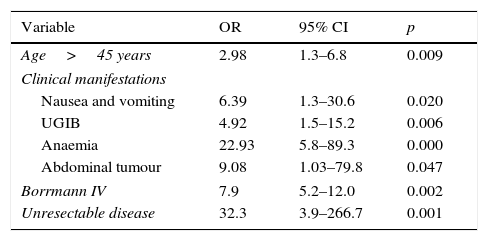
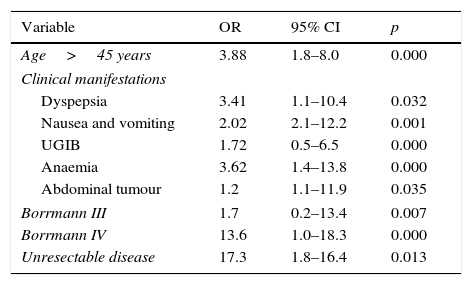
Multiple logistic regression is shown in Table 5 on comparing the 2 study groups. The adverse prognostic factors for survival were: aged over 45 years (OR 2.98), nausea and vomiting (OR 6.39), upper gastrointestinal bleeding (OR 4.92), anaemia (OR 22.93), abdominal tumour (OR 9.08), Borrmann (OR 7.9) and unresectable disease (OR 32.3). The adverse prognostic factors for recurrence with respect to those aged over 45 years (OR 3.88) were dyspepsia (OR 3.41), anaemia (OR 3.62), abdominal tumour (OR 1.2), Borrmann IV (OR 13.6) and unresectable disease (OR 17.3) (Table 6).
Adverse prognostic factors for survival.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age>45 years | 2.98 | 1.3–6.8 | 0.009 |
| Clinical manifestations | |||
| Nausea and vomiting | 6.39 | 1.3–30.6 | 0.020 |
| UGIB | 4.92 | 1.5–15.2 | 0.006 |
| Anaemia | 22.93 | 5.8–89.3 | 0.000 |
| Abdominal tumour | 9.08 | 1.03–79.8 | 0.047 |
| Borrmann IV | 7.9 | 5.2–12.0 | 0.002 |
| Unresectable disease | 32.3 | 3.9–266.7 | 0.001 |
UGIB: upper gastrointestinal bleeding.
Adverse prognostic factors for recurrence.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age>45 years | 3.88 | 1.8–8.0 | 0.000 |
| Clinical manifestations | |||
| Dyspepsia | 3.41 | 1.1–10.4 | 0.032 |
| Nausea and vomiting | 2.02 | 2.1–12.2 | 0.001 |
| UGIB | 1.72 | 0.5–6.5 | 0.000 |
| Anaemia | 3.62 | 1.4–13.8 | 0.000 |
| Abdominal tumour | 1.2 | 1.1–11.9 | 0.035 |
| Borrmann III | 1.7 | 0.2–13.4 | 0.007 |
| Borrmann IV | 13.6 | 1.0–18.3 | 0.000 |
| Unresectable disease | 17.3 | 1.8–16.4 | 0.013 |
UGIB: upper gastrointestinal bleeding.
Gastric cancer in Mexico according to the Histological Report on Neoplasms, comprise 3% of the cancers diagnosed in the country, with 3 cases recorded per 100,000 inhabitants.1–4
Although the percentages of resectability have increased (60–80%) and postoperative mortality is from 6 to 14%, the rates of survival at 5 years remain at 8–26% in some series.5–7 Despite the fact that some patients with gastric cancer are reported as resectable and free from lymph node disease in the definitive histopathological study, which would imply a better prognosis, they eventually die due to recurrent malignant disease.8–10
Being aged under 40 has been associated with the increased frequency of poorly differentiated tumours, with seal ring cell histology and diffuse tumours.10 However, recent studies contradict this and report that despite the adverse histological features in young people, and the predominance in females, survival at 5 years is better in people under 50 years (54 vs 46%, p=0.035).11
The study included 2 groups divided into patients aged under 45 years, 19% (n=112) of the cases, and those aged over 45 years, 81% (n=476).
It has been reported that gastric cancer affects males by 4% more in all age groups.12–15 In this study we found that there were more male than female cases (55.5 vs 40.5%), and that males predominated in the group of patients aged over 45 years (67%). However, this trend was reversed n the group of patients under 45, with a predominance of females (77%).
The average course of the disease was 8.1 months (SD: 4.6), this was not significant in either group (p=0.829).
When the groups were compared, there was a difference in the initial symptoms. For those aged under 45 years, the main symptom was nausea (41.1%) followed by dyspepsia (29.4%). In the older group, weight loss was more frequent (23.1%) followed by abdominal pain (22.3%), with no statistically significant difference.
The warning signs at the time of diagnosis are major independent negative prognostic factors associated with survival at 5 years. The most relevant are: dysphagia 7%, palpable mass 11%, anaemia 12% and vomiting 14% (p=0.001).16,17 We found when we performed the statistical analysis in our study that the symptoms with adverse recurrence and survival prognosis were principally anaemia (OR=22.93), palpable abdominal tumour (OR 9.08), nausea and vomiting (OR 6.39) and upper gastrointestinal bleeding (OR 4.92) (Table 6).
With regard to performance status, in our analysis 32.3% of the patients were classified as ECOG 2 and 6.5% as ECOG 3 treated surgically. They had an associated mean survival rate of 27.02 and 5.48 months respectively, with similar results in both age groups (Table 1). Cunningham identified that patients with ECOG 2 and 3 have poorer rates of survival (HR 1.712, p>0.0001) compared to patients with performance status 0 and 1 (HR 1.0).18–21
With regard to the features of the tumour, a difference in endoscopic classification was found for both age groups, since more cases classified as Borrmann IV (43%) presented in those aged under 45, followed by Borrmann III (36%), whereas in those aged over 45 the reverse was observed: a greater frequency of Borrmann III tumours (37%), followed by Borrmann IV (34%) (p=0.004). Mean survival was 59 months for Borrmann III and 13 months for Borrmann IV.
A survival of 62.7% was observed for tumours invading the muscularis propria (pT2), of 42.2% with infiltration of the subserosa (pT3) and of 30.1% with involvement of the serosa (pT4) (p≤0.0001).22–25 The depth of invasion in the pathological report had no statistical difference and depths pT4 (31.1%) and pT4b (34.4%) presented, with similar percentages in each group.
Gastric cancer is classified as early-stage, limited to the mucosa and submucosa, which in the United states represents 6–8%, and in countries like Japan, thanks to their screening methods, represents from 30% to 50%.26–29 We know that survival is poor in Mexico; in the year 2000 it was reported that almost 80% of patients were diagnosed at advanced stages III or IV, with resectability rates of 33% and a survival rate at 5 years <15%.30–33
In our analysis, 5.2% were classified as early-stage (EC IA and IB) with a difference in each age group, at 1.8% for those aged under 45 and 6.3% for those aged over 45 years (p=0.002). 82.2% of our patients had stage III and IV of the disease, a similar percentage in both groups. With a mean survival of 110 months with EC IA, 44.3 months with EC IIB, 20.8 months with IIIA, 11.1 months with IIIB and 3.41 months at EC IIIC.
The pyloric antrum was the most common anatomic site for the tumour in both groups (39.8%). It is worth noting that in the group of patients under 45 years, the percentage of diffuse tumour location was 12.5 vs 3.4% in the older patients. However, the type of surgical resection was similar in both groups, with total gastrectomy in 35.4% of the patients, with no statistical significance.
Metastatic disease was found in 30.1% of the cases during surgical exploration, with a higher percentage in the group of patients aged under 45 compared to the older group (39.3 vs 29.8%, respectively) (p=0.05) (RR: 0.09).
Similarly, there was a greater predominance of resection of tumours with negative surgical margins in patients aged over 45, with the same pattern observed when the disease was classified as resectable or unresectable. In other words, more patients with negative surgical margins and no metastatic disease were also found in the older age group (61.3%) when compared with the group of patients aged under 45 (52.7%). However, this was not statistically significant (p=0.96).
Therefore, even though a lower percentage of resectable disease presented in the group aged under 45, it was found that both the disease-free period and overall survival were significantly greater in that group when compared with the group aged over 45, with a disease-free period of 56 vs 33 months and overall survival of 58 vs 35 months, respectively.
ConclusionYoung patients aged under 45 years who have undergone complete resection of their cancer have a better survival rate after two disease-free years, despite poor prognostic factors, such as advanced presentation of the disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Medrano-Guzmán R, Valencia-Mercado D, Luna-Castillo M, García-Ríos LE, González-Rodríguez D. Factores pronóstico de sobrevida en adenocarcinoma gástrico avanzado resecable. Cir Cir. 2016;84:469–476.