Although photocoagulation reduces the incidence of moderate visual loss in eyes with focal diabetic macular oedema, some eyes may lose some vision after treatment. The proportion of eyes with poor functional response after photocoagulation, and whether any retinal variable is associated with this, is unknown.
ObjectiveTo determine the proportion of eyes with diabetic macular oedema that have a poor functional response after focal photocoagulation, and their associated features.
Material and methodsA non-experimental, longitudinal, comparative and retrospective study was conducted. The proportion and 95% confidence intervals (CI) of diabetics with macular oedema that had a poor functional response after focal photocoagulation (any visual loss after 6 weeks) were identified. The means of retinal variables before treatment were compared between eyes with and without a poor functional response using the Student t test for independent means.
ResultsThe study included 115 eyes of patients aged 59.3 (SD 9.24) years. Visual acuity was greater than or equal to 0.5 in 63 eyes (54.8%). A total of 33 eyes had a poor functional response after photocoagulation (28.7%, 95% CI: 13.3–44.1). The comparison between retinal variables and visual acuity before treatment did not show any differences between eyes with or without a poor functional response and eyes (p>0.05).
ConclusionRetinal thickening and visual acuity improved or did not change in 71.3% of eyes with diabetic macular oedema with a single photocoagulation procedure. Retinal variables that are usually evaluated were unable to identify the remaining 28.7%, which could lose vision after that treatment, and would require additional interventions.
La fotocoagulación reduce la incidencia de pérdida visual moderada en ojos con edema macular diabético focal, aunque en algunos disminuye la visión. Se desconoce la proporción de ojos que presenta mala respuesta funcional después de la fotocoagulación y si esta se asocia con alguna característica retiniana previa.
ObjetivoDeterminar la proporción de ojos con edema macular diabético que presenta mala respuesta funcional a la fotocoagulación y, comparar las características retinianas entre ojos con y sin ella.
Material y métodosEstudio observacional, longitudinal, comparativo, retrospectivo. Se identificó la proporción e intervalos de confianza (I.C.) del 95% de ojos con edema macular diabético focal, que tuvo mala respuesta funcional después de la fotocoagulación (disminución de agudeza visual 6 semanas después). Se compararon las variables retinianas previas al tratamiento, entre ojos con y sin mala respuesta funcional (t de Student).
ResultadosSe trabajó sobre 115 ojos de pacientes con edad promedio 59.3±9.24 años. La agudeza visual era ≥ 0.5 en 63 (54.8%); 33 ojos tuvieron mala respuesta funcional después de la fotocoagulación (28.7%, I.C. 95%: 13.3 a 44.1). Las variables retinianas no difirieron entre los ojos con mala respuesta funcional y los ojos sin ella (p>0.05).
ConclusiónEn 71.3% de los ojos con edema macular diabético focal, el engrosamiento retiniano remitió y la agudeza visual se conservó o mejoró con un solo procedimiento de fotocoagulación; las variables retinianas evaluadas habitualmente no permitieron identificar al 28.7% restante, cuya visión puede disminuir después de este tratamiento, y que requeriría intervenciones adicionales.
Diabetic retinopathy is the main cause of blindness in people of working age in the world; it threatens vision in two ways; proliferative retinopathy and macular oedema.1 The latter is the most common cause of sight loss in diabetics with retinopathy, and consists of the enlargement of the retina in the area of best sight, due to abnormal capillary permeability.2
Six percent of diabetics develop clinically significant macular oedema,1 which can cause moderate sight loss: a three-line reduction on the sight chart, which affects 33% of untreated cases.3 The Early Treatment Diabetic Retinopathy Study [ETDRS]) demonstrated the efficacy of photocoagulation in reducing this outcome to 13%.4
Although photocoagulation reduces the incidence of moderate sight loss, in a few cases it improves vision; visual gain is less frequent if the capillary filtration on a retinal angiography is diffuse.5 When this condition exists, endothelial vascular growth factor (EVGF) blockers via the intra-vitreous route are more effective than photocoagulation in reducing retinal thickness and improving vision6; this treatment requires from 3 to 5 injections at intervals of one month.7,8
EVGF blockers have been evaluated as limited in eyes with clinically significant macular oedema and visual acuity>20/30, and for these cases photocoagulation is the therapy of choice9; most of these eyes have focal filtration, and their oedema regresses after one single photocoagulation session; this is an advantage of this treatment over others. Even when macular oedema subsides, eyes treated with photocoagulation can lose one or 2 lines of vision; this sight loss is not “moderate”, but would represent a poor functional response to photocoagulation, if one considers that vision improves in most eyes treated with EVGF blockers.
The proportion of eyes with focal oedema with a poor functional response, and its associated characteristics after a single photocoagulation session, is unknown. Objective measurement of the retina which is provided by optical coherence tomography might identify associations between poor functional response and photocoagulation, and alterations in variables measured by the rapid macular mapping test, such as central point thickness (thickness at the intersection of 6 radial scans of 6mm which cross the centre of the macula), the thickness of the central field (average retinal thickness in a circle with a 1mm diameter, concentric to the centre of the macula) or the macular volume.10
A study was performed to determine the proportion of eyes with clinically significant macular oedema which presented a poor functional response, 6 weeks after focal photocoagulation, and the association of this outcome with the characteristics prior to the procedure.
Materials and methodsAn observational, longitudinal, comparative and retrospective study was performed. The target population was type 2 diabetics of the Distrito Federal and its metropolitan area; the accessible population was diabetics attended in a general hospital between 1 January 2012 and 30 June 2014. The study took place from 1 September to 30 November of 2014, in compliance with the principles of the Helsinki Declaration and was approved by the Research and Research Ethics Committees of the hospital where it was undertaken.
Type 2 diabetic patients aged between 35 and 80 were included in the study, with any grade of diabetic retinopathy, and clinically significant macular oedema with focal filtration on retinal fluoroangiography, and spongiform pattern on optical coherence tomography, who had been treated with focal photocoagulation recorded with best-corrected visual acuity, and with optical coherence tomography macular mapping of 6mm, on the day of treatment and 6 weeks afterwards, in whom the retinal swelling had resolved. Eyes which had developed any other eye disease which reduced best-corrected visual acuity, those presenting an increased foveal vascular area on retinal fluoroangiography, and those whose rapid macular mapping had measurement errors were excluded from the study.
Visual acuity under subjective refraction in decimal equivalent was measured in all the patients. The proportion of eyes was identified whose best-corrected visual acuity was <0.5 before treatment, as this is the cut-off point used by the ETDRS to qualify poor visual acuity, and the degree of diabetic retinopathy was determine in each treated eye, according to the classification of the American Academy of Ophthalmology.
Retinal thickness was measured by means of the rapid macular mapping test of 6mm, of Stratus (Carl Zeiss Meditec, Inc, Dublin, CA, U.S.A., software version 4.01) optical coherence tomography equipment, under the following standard procedure: mydriasis≥6mm, scan for dark eyes, identification of the plane of the retina by acoustic alarm, and optimisation of the Z-axis and polarisation. All the images were obtained with flash between 09.00 and 11.00 am.
The central point thickness was measured in all the eyes and the central field thickness in μm, and macular volume in mm3. The measurements were taken automatically by the optical coherence tomography equipment. Any deviation of the optical coherence tomography line with respect to the real limit of the retina was considered a measurement error, at a standard deviation ratio of central point thickness/central point thickness>0.111 and a signal intensity of<4.
The variable under study was the poor functional response after photocoagulation, defined operationally as any reduction in best-corrected visual acuity 6 weeks after the procedure, compared to that recorded before it. Predictive variables were considered at best-corrected visual acuity<0.5, at central field thickness, and at the macular volume prior to treatment; the proportion of eyes with central thickening was identified, operationally identified as thickness of the central field>212.5μm, which represents a value 2 standard deviations above the average reported in diabetic patients without oedema in our population (188.7μm).12
The proportion and 95% confidence intervals (CI) were identified of eyes with a poor functional response after photocoagulation; the central point thickness averages, central field thickness, macular volume and best-corrected visual acuity in decimal equivalent, were compared between the eyes with poor functional response after photocoagulation and the remainder, by means of Student's t-test for independent measurements. The central point thickness averages, central point thickness, and macular volume before and after the treatment were compared in each group by means of a Student's t-test for paired samples.
The proportion of cases with poor functional response after photocoagulation was compared with the eyes with a previous visual acuity of<0.5, and the eyes with a previous visual acuity of≥0.5, and between the eyes with and without central thickening, by means of the χ2 test. The proportion and 95% confidence intervals (CI) of eyes which presented moderate sight loss 6 months after treatment were also identified; the information was stored and analysed using SPSS for Windows version 22; a p value of<0.05 was considered statistically significant.
Results115 eyes were assessed, of 58 patients, aged from 35 to 77 (average age 59.3, standard deviation [S.D.]±9.24); 49 of the eyes were those of females (42.6%); the patients had a history of diabetes from 1 to 30 years (average 15.15, S.D.±7.14), 37 eyes were those of patients treated with insulin (32.2%). The average of random capillary glucose was 167.25mg/dl (S.D.±70.93) and that of glycated haemoglobin was 9.15% (S.D.±2.56), 62 eyes were those of patients with systemic arterial hypertension (53.9%).
Best-corrected visual acuity before photocoagulation had a distance of 0.06 to 1 (average 0.56, S.D.±0.29); it was ≥0.5 in 63 eyes (54.8%) and<0.5 in 52 (45.2%); 13 patients presented mild non-proliferating retinopathy (11.3%), 63 moderate non-proliferating retinopathy (54.8%), 6 severe non-proliferating retinopathy (5.2%), and 33 proliferating retinopathy (28.7%).
The average central point thickness before photocoagulation was 182.26μm (S.D.±47.53μm), the average central field thickness 215.16μm (S.D.±42.07μm) and average macular volume 7.76mm3 (S.D.±0.87mm3). Six weeks after photocoagulation the average central point thickness was 182.05±43.33μm (p=0.9), the average central field thickness 214.59±38.83μm (p=0.8) and the average macular volume 7.63±0.77mm3 (p<0.001).
After photocoagulation, visual acuity had a distance of 0.03–1.00 (average 0.60±0.29, p=0.16); it improved in 51 eyes (44.3%), did not change in 31 (26.9%) and reduced in 33 (28.7%). Three eyes presented moderate sight loss (2.6%, C.I. 95%: 0–5.5%) and 33 had poor functional response after photocoagulation (28.7%, 95% CI: 20.4–37.0).
In the eyes with poor functional response after photocoagulation, the average central point thickness changed from 192.45±67.7μm before the treatment to 187.64±57.9μm after it (p=0.5); the average central field thickness changed from 224.8±58.5μm to 219.6±50.8μm (p=0.4) and the average macular volume changed from 7.84±1.06 to 7.61±0.81mm3 (p=0.01). In the remaining eyes the central point thickness average changed from 178.2±36.2μm to 179.8±36.04μm (p=0.6); the average central field thickness changed from 211.3±32.9μm to 212.6±32.9μm (p=0.6) and the average macular volume changed from 7.73±0.78 to 7.64±0.76mm3 (p=0.01).
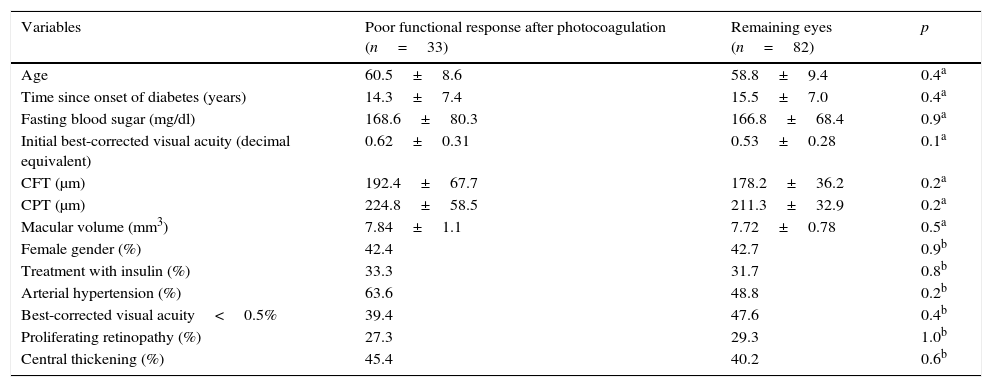
The comparison of the ocular and systemic variables before photocoagulation, between the eyes that presented poor functional response and the remainder, is shown in Table 1; no significant differences were found which enabled any variable prior to the treatment to be associated with poor functional response after photocoagulation.
Comparison of the variables prior to treatment, between eyes with poor functional response to photocoagulation and eyes with good functional response.
| Variables | Poor functional response after photocoagulation (n=33) | Remaining eyes (n=82) | p |
|---|---|---|---|
| Age | 60.5±8.6 | 58.8±9.4 | 0.4a |
| Time since onset of diabetes (years) | 14.3±7.4 | 15.5±7.0 | 0.4a |
| Fasting blood sugar (mg/dl) | 168.6±80.3 | 166.8±68.4 | 0.9a |
| Initial best-corrected visual acuity (decimal equivalent) | 0.62±0.31 | 0.53±0.28 | 0.1a |
| CFT (μm) | 192.4±67.7 | 178.2±36.2 | 0.2a |
| CPT (μm) | 224.8±58.5 | 211.3±32.9 | 0.2a |
| Macular volume (mm3) | 7.84±1.1 | 7.72±0.78 | 0.5a |
| Female gender (%) | 42.4 | 42.7 | 0.9b |
| Treatment with insulin (%) | 33.3 | 31.7 | 0.8b |
| Arterial hypertension (%) | 63.6 | 48.8 | 0.2b |
| Best-corrected visual acuity<0.5% | 39.4 | 47.6 | 0.4b |
| Proliferating retinopathy (%) | 27.3 | 29.3 | 1.0b |
| Central thickening (%) | 45.4 | 40.2 | 0.6b |
CFT, central field thickness; CPT, central point thickness.
There was no significant difference between the eyes with a poor response after photocoagulation, and the eyes without it, compared to the absolute averages of change in central point thickness (−4.8±44.1μm vs 1.6±30.1μm), in central field thickness (−5.1±35μm vs 1.3±24.0μm) and in macular volume (−0.23±0.47mm3vs −0.08±0.29mm3).
The proportion of cases with poor functional response to photocoagulation did not differ between the eyes with visual capacity of<0.5, and the eyes with visual acuity≥0.5 (Table 2); neither did it differ between the eyes with and without thickening in the centre of the macula (Table 3).
Comparison of the proportion of cases with poor functional response after photocoagulation, between eyes with visual acuity < 0.5 and eyes with visual acuity≥0.5 before treatment.
| Visual acuity | Poor functional response to photocoagulation (n=33), n (%) | Remaining eyes (n=82), n (%) | pa |
|---|---|---|---|
| <0.5 | 13 (25) | 39 (75) | 0.4 |
| ≥0.5 | 20 (31.7) | 43 (68.3) | |
| Total | 33 (28.7) | 82 (71.3) | |
Comparison of the proportion of cases with poor functional response after photocoagulation, between eyes with and without thickening of the centre of the fovea before treatment.
| Thickening of the centre of the macula | Poor functional response to photocoagulation (n=33), n (%) | Remaining eyes (n=82), n (%) | pa |
|---|---|---|---|
| Present | 18 (26.9) | 49 (73.1) | 0.4 |
| Absent | 15 (31.3) | 33 (28.7) | |
| Total | 33 (28.7) | 82 (71.3) | |
In 28.7% of the eyes with macular oedema treated with focal photocoagulation visual acuity decreased, even when the retinal thickening resolved; in the remaining 71.3%, the treatment was efficient in preventing sight loss, with one single procedure.
The operational definition of poor functional response after photocoagulation in this study did not correspond to the outcome that one wishes to avoid in patients with clinically significant macular oedema: moderate sight loss. Only 2.65% of the eyes in the sample presented this outcome, compared to the 13% reported by the ETDRS. Although the results at 6 weeks did not represent the outcome at 3 years, it should be considered that the proportion of moderate sight loss at 6 weeks in the ETDRS was greater in the treated eyes than in the untreated eyes.4
The poor functional response after photocoagulation did not correspond to a therapeutic failure, a definition that refers to the persistence of retinal thickening 4 months after the procedure. In the sample, thickening reduced significantly both in the eyes with poor functional response after photocoagulation, and in the remaining eyes.
In this study, any decrease in visual acuity was defined as a poor functional response after photocoagulation, because there are efficient therapies to improve vision for eyes with diffuse oedema.13–15 In eyes with focal macular oedema not only should we try to prevent the loss of 3 lines of vision, but any reduction in visual acuity.
Unlike photocoagulation, therapies with glucocorticoids or EVGF antagonists have a transitory effect, and therefore various procedures are required to achieve the therapeutic goal. However, they are currently indicated for treating diffuse oedema (in combination or otherwise with photocoagulation)16 because their effect on vision is better than that of isolated photocoagulation.
In focal macular oedema general sight loss and retinal thickening are of greater magnitude than in diffuse macular oedema; therefore, focal photocoagulation remains effective in preventing moderate sight loss. Photocoagulation treats isolated sites of capillary leakage, and causes less inflammation than that caused by 60–100 spots of grid laser treatment for diffuse oedema. However, eyes with focal oedema whose vision has reduced by at least one line after photocoagulation might require other treatment.
The variables that have traditionally been evaluated as predictive of a response to photocoagulation are visual acuity<0.5, and the central location of macular oedema; these variables did not modify the proportion of eyes with poor functional response after photocoagulation, according to the operational definition. Neither did the other anatomical variables measured by optical coherence tomography, one of the principal tools for guiding treatment of macular oedema, the correlation of which with visual acuity is slight.17
Strict control of glycaemia reduces the incidence of diabetic retinopathy, but the extent to which it contributes towards the visual result 6 weeks after photocoagulation is not known in eyes with focal macular oedema. Recent studies evaluating its participation in the response to therapy with VEGF blockers have encountered differences in anatomical outcome, but not in functional outcome,18 and have identified that insulin therapy compared to oral glucose-lowering drugs does not change the efficacy of the treatment.19
Other functional variables might modify the response to treatment, such as retinal sensitivity, a form of estimating the overall function of the macula which can be measured with conventional perimetry or micro-perimetry. In patients with macular oedema a reduction in sensitivity has been reported even in eyes with slightly increased retinal thickness,20,21 Which might explain the variability of response to photocoagulation and require specific evaluation.
It would also be useful to specifically evaluate the condition of the temporal perifoveal sector which, if it does not present thickening, has been associated with a greater incidence of visual improvement after focal photocoagulation.22 Another variable which might participate would be the condition of the perifoveal capillary network. Although none of the eyes in the sample had characteristics of macular ischaemia, the condition of the perifoveal capillaries might have differences associated with poor functional response after photocoagulation.
At this time there is no available data which enables patients to be identified with clinically significant focal macular oedema who might present a poor functional response after photocoagulation. In order to propose an adjunct therapy with the procedure, variables other than the traditional need to be identified, which are significantly associated with poor functional response, and which can be changed; otherwise, there is the risk of giving unnecessary treatments to people whose response is adequate.
Transitory sight loss associated with photocoagulation is caused by inflammation, and adjunt therapy with non-steroidal anti-inflammatory drugs results in more eyes with visual improvement.23 It is not known whether this treatment is also effective in reducing the amount of cases with poor functional response, and therapy should be assessed prospectively, as should other variables which might be associated with a reduction in visual acuity, although not falling under the definition of moderate sight loss.
ConclusionRetinal variables prior to treatment, evaluated traditionally in eyes with diabetic macular oedema, did not show differences between the eyes with poor functional response after focal photocoagulation and the eyes without this outcome.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Ávila-Alcaraz Y, Razo Blanco-Hernández DM, García-Rubio YZ, Lima-Gómez V. Falta de asociación entre las características retinianas previas al tratamiento y la mala respuesta funcional a la fotocoagulación focal, en edema macular diabético. Cir Cir. 2016;84:3–8.