A fracture repair involves complex cellular processes. However, despite optimal treatment, some fractures heal slowly or do not repair. These complications support the need for innovative therapies. Electromagnetic stimulation is a non-invasive technology that could have a direct impact on many cellular pathways.
ObjectiveTo demonstrate the effectiveness of electro-stimulation by alternating current applied during bone elongation to accelerate the consolidation process for 30 days in an animal model.
Materials and methodsA device with closed circuit and graduated voltage was designed and kept in contact with the external fixator. Group A was elongated without electro-stimulation and group B was electro-stimulated since the beginning of the distraction. Radiographs were taken at 15 and 30 days post-surgical. Haematoxylin and eosin staining and Masson's trichrome stain were performed.
ResultsNo significant difference were observed in bone density of group A (4.05±3.24, p=0.163). In group B there was a significant difference (61.06±20.17, p=0.03) in bone density. Group A maintained a fibrous tissue repair, with areas of cartilage and bone matrix. Group B had more organised tissue in the stages of bone repair.
ConclusionBecause there is a significant difference in the growth and callus formation at 15 and 30 days between groups, electro-stimulation could be considered as an adjuvant during bone elongation.
La reparación de una fractura implica procesos celulares complejos. Sin embargo, a pesar de un tratamiento óptimo, algunas fracturas curan lentamente o, simplemente, no se reparan. Estas complicaciones apoyan la necesidad de terapias innovadoras. La estimulación electromagnética es una tecnología no invasiva que pudiera tener un impacto directo sobre muchas vías celulares.
ObjetivoDemostrar la eficacia de la electroestimulación por corriente alterna, aplicada durante una elongación ósea para acelerar el proceso de consolidación, por 30 días en un modelo animal.
Materiales y métodosSe diseñó un dispositivo de circuito cerrado y voltaje graduado, que se mantuvo en contacto con el fijador externo. El grupo A fue elongado sin electroestimulación y el grupo B fue electroestimulado desde el inicio de la distracción. Se tomaron radiografías a los 15 y 30 días posquirúrgicos, se realizaron tinciones de hematoxilina y eosina, y de tricrómico de Masson.
ResultadosNo se observó una diferencia significativa en la densidad ósea del grupo A (4.05±3.24, p=0.163). En el grupo B existe una diferencia significativa (61.06±20.17, p=0.03), en la densidad ósea. El grupo A mantuvo un tejido de reparación fibroso, con zonas de cartílago y matriz ósea. El grupo B presentó un tejido más organizado en las fases de reparación ósea.
ConclusiónDebido a que existe una diferencia significativa en el crecimiento y formación del callo óseo a los 15 y 30 días entre ambos grupos, la electroestimulación podría considerarse como un adyuvante durante el proceso de elongación ósea.
A bone fracture is defined as the loss of normal continuity of bony tissues, resulting from a trauma or a pathological process which weakens its normal structure. In general, the primary cause is the result of applying force on the bone which surpasses its elastic resistence.1,2 A fracture repair involves complex processes of proliferation, cellular differentiation and several other factors such as growth, inflammatory cytokines, antioxidants, osteoclasts, osteoblasts, hormones, amino acids and several nutrients.3
It is difficult to establish a precise moment when a fracture should be rectified. Despite optimum treatment, however, several fractures repair slowly or simply do not repair.4 The majority of experts agree unless there is no clinical or radiological evidence of cure after at least 3 months the term “non union” should not be used to describe the fracture.5 Occasionally non unions occur with no apparent cause, but in many cases factors such as soft tissue damage, tissues associated with high energy open and closed fractures, infection, segmentary fractures, pathological fractures, fractures with soft tissue interposition, poor local blood supply, systemic diseases, malnutrition, vitamin D deficiency, use of corticosteroids and a poor mechanical fixation, and iatrogenic interferences are involved.6
To understand the processes leading to bone lengthening, the terms osteodistraction osteogenesis should be analysed, as these refer to the production of new bone between vascular bony surfaces, generated by an osteotomy and separated by a gradual distraction7 and distraction epiphysiolysis, which refers to the mechanical compression and distraction forces in the growth platelet without an osteotomy but with the presence of an extra-articular fracture.8,9
Ilizarov10 introduced the concept of callostasis, and was highly successful in achieving bone lengthening. His concepts were subsequently extended in accordance with Peña Martínez et al.11
The Ilizarov method enables the surgeon to undertake complex and prolonged lengthening of both short extremities, either congenital or acquired, but the technique may be difficult and time is required to master this procedure, compared with methods which involve the use of a monolateral fixator.12,13 The De Bastiani et al.14 method has gained prestige among paediatric orthopaedists because it is technically less demanding for the surgeon and the monolateral fixator tends to be more comfortable for the patient than a circumferential fixator. The method is based on a more conventional osteotomy, consisting of the opening of the periosium, perforation of both cortexes and in several directions, the interconnection of visible perforations with an osteotomy and the finalisation of the osteotomy with manual osteoclasis.14
With regard to the stimuli based on electric discharges or electro-stimulation, as stated by with Boyer,15 background information on the treatment of non unions made by Birch in 1812 exists. After this Yasuda (1953) conducted studies which showed that there is an electric effect in the bone when it is submitted to lineal or angular charges, and a small current is applied to the bone, which is able to stimulate osteogenesis.16 Given that these electrical endogenous fields may change cellular activities in the bone, several systems for the therapeutic use of electro-stimulation were developed.17,18 Electro-stimulation equipment may be inductive in effect (like an electromagnetic pulsatile therapy), of training effect and direct current, currently accepted by the FDA for the treatment of non unions and vertebral fusions.19,20 However, efficacy in the use of callostasis tissue has not been demonstrated, and our work therefore aims at demonstrating the efficacy of electro-stimulation for reducing bone lengthening time, corresponding with remodelling and corticalisation.
Materials and methodsOnce previous authorisation from the Ethics Committee of the Faculty of Medicine and the Universidad Autónoma de Nuevo León had been obtained, 14 dogs of mixed breed were used and with weight and age averaging 10kg and 3 years, respectively. 2 study groups were designed: Group A comprised 7 dogs treated with bone elongation without electro-stimulation and Group B comprised 7 dogs treated for bone elongation with electro-stimulation.
Electro-stimulator. A bone electro-stimulator was designed which was connected to an external fixator through a cathode receptor and an anode receptor. Kischner type 0.62mm nails were designed for the drive with a polypropylene cover with a 5mm drive area at both ends, to prevent an interface with soft tissues. The alternating current was 6V to 20μA, with positive feed of dual 9V source.
Surgical technique. The MiniRail System de Orthofix® was used for external fixation which consists of a fixator with 2 articulated bodies for fixing the screws and a central body with attachment for elongation of 0–8cm. The De Bastiani screw insertion technique involved dissection of the flat bones up to the periosteum of the femoral diaphyseal bone. After this, a series of holes were made with the 2mm drill bit throughout the whole diameter of the bone. These holes were joined together with a flat osteotome 0.5–1cm wide until the coricotomy was completed.21 The periosteum was finally closed, as were the adjacent tissues and the skin with 3-0 (ETHICON® Johnson & Johnson S.A.) nylon sutures.
X-rays. The animals were sedated with xylazine so that x-rays in anteroposterior and lateral positions could be taken 15 and 30 days after surgery.
Euthanasia. This was performed in accordance with Official Mexican Regulation NOM-062-ZOO-1999, on technical specifications for the production, care and use of laboratory animals, with the administration of sodium pentobarbital at a dose of 120mg/kg intravenously and xylazine at a dose of 2mg/kg.
Histological staining. The samples were fixed with the Bouin solution and were decalcified with the Calci-Clear Rapid (Fisher Scientific, United Kingdom) solution. The tissue was then dehydrated and soaked in paraffin, to obtain the histological slices. Masson's trichrome stain and haematoxylin and eosin staining were used to observe cellular morphology, the amorphous matrix and the collagenous content.
Densitometry analysis. For analysis of radiographic images, the Image Pro Plus version 6 (Media Cybernetics, Inc. U.S.A.) software was used, to measure the amount of matter present in a material, measuring the amount of light passing through it.
Statistics. Statistical analysis was performed using the Student's t-test for both groups, comparing the bone density at 15 and 30 days, with the version 20 IBM SPSS (SPSS, Inc., Armon, NY, U.S.A.) software.
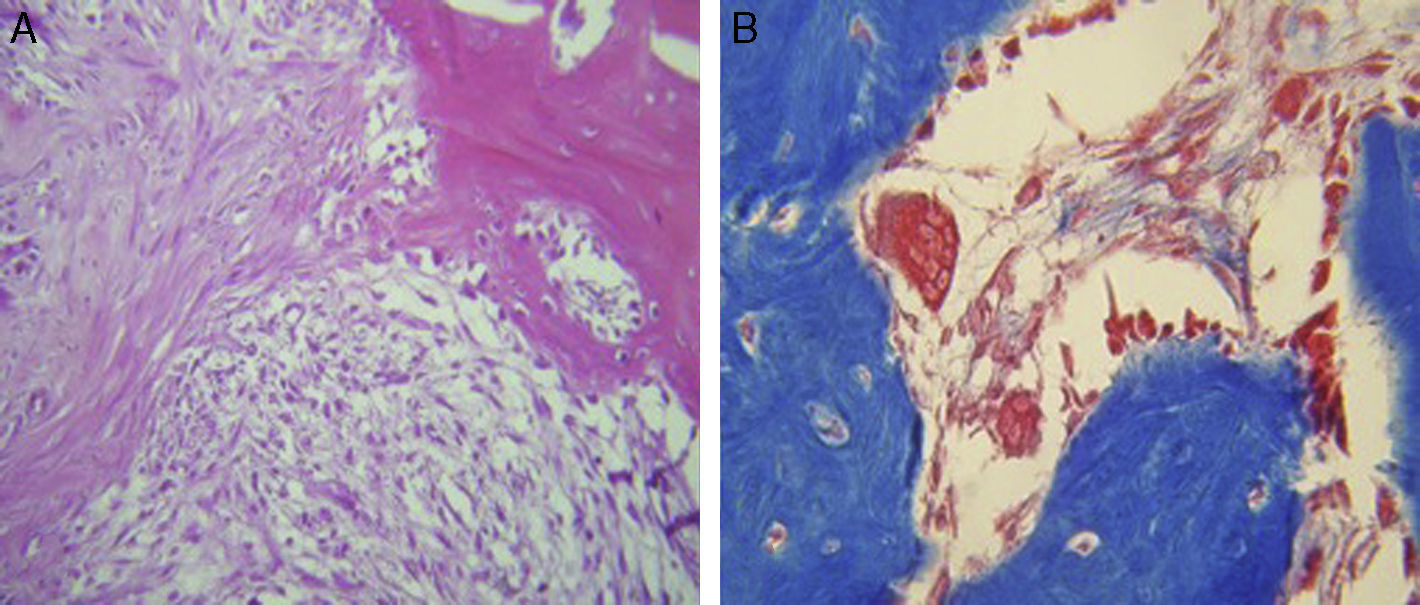
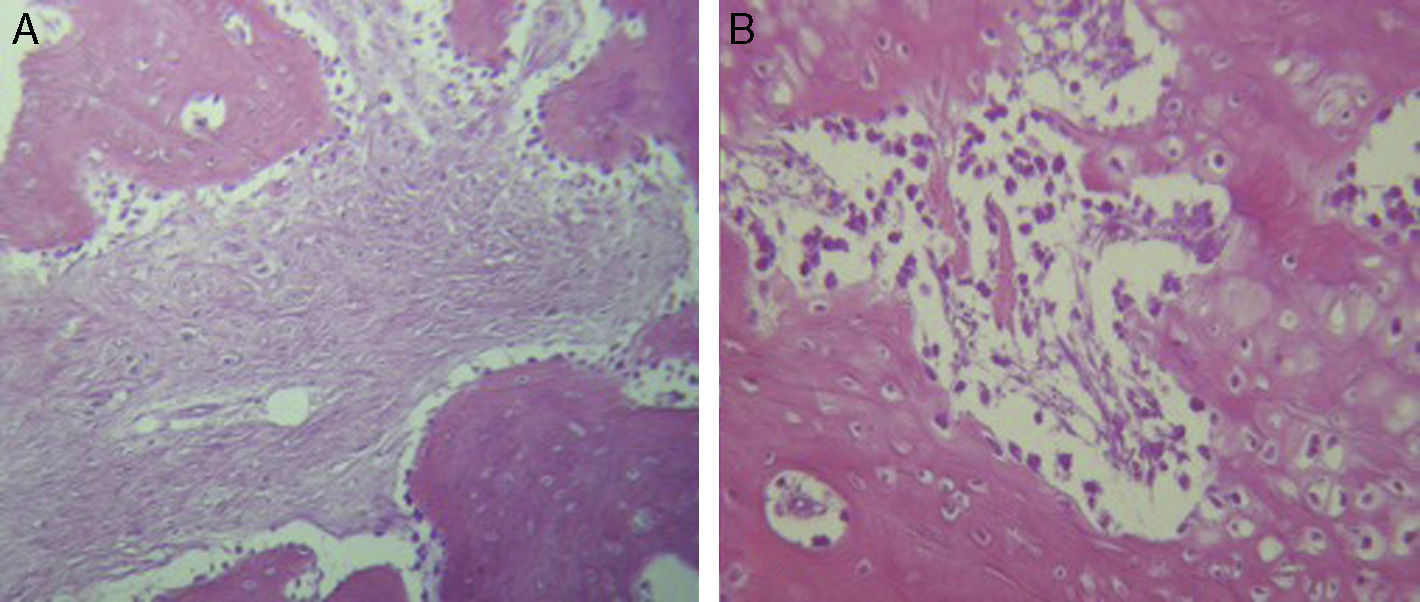
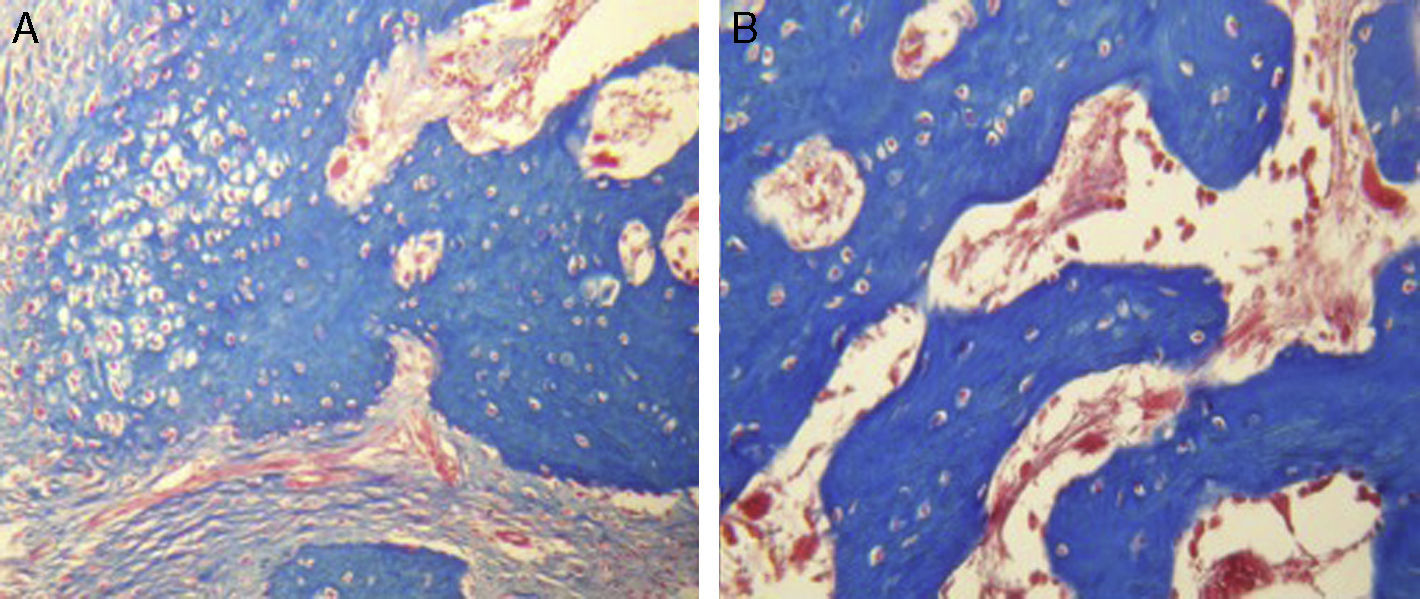
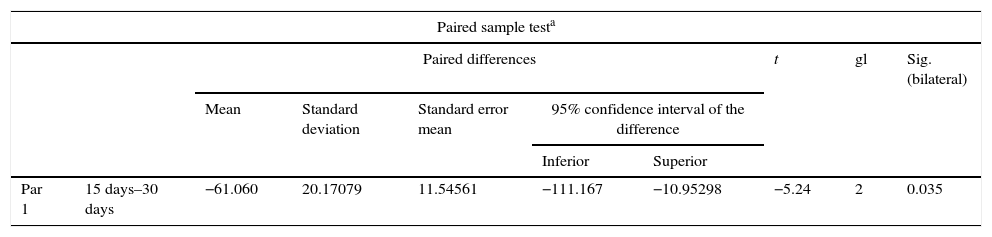
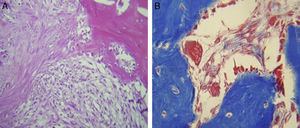
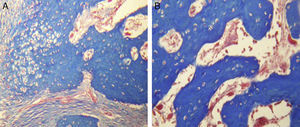
ResultsFor Group A (Fig. 1), in the cases where there had been no electrical stimulus, the slices stained with haematoxylin and eosin were analysed: in general we observed that the repair tissue had fibrous features. At other cut-off levels a transition from cartilaginous tissue to thick fibrous tissue was observed, up to the formation of the primary bone. Periosteum components were appreciated which gave way to fibroblasts and to thick-collagenous tissue, forming parallel bundles which interconnected with the primary bone, with the latter replacing the hyaline cartilaginous tissue. There was a presence of blood vessels in the fibrous, hyaline regions, in addition to a very thick fibrous scar. In the healthy bone, we observed bone trabeculae with flat osetoblasts, and the formation of new trabeculae with large, cuboid osteoblasts in free proliferation. The formation of loose connective tissue was detected in different areas, with fusiform, star-shaped cells, and abundant blood vessels of different calibres. Towards the lateral area bone trabeculae of varying thicknesses were observed and towards the inside of the bone, the connective tissue was thicker with collagen bundles. This part of the bone also contained mesenchymal cells, islet cells of hyaline cartilage with morphological changes caused by the condensing of the matrix towards a more compact fibrous tissue. In Masson's trichrome stains (Fig. 1) for the same Group we observed the presence of a large, fibrous scar between the newly forming bone trabeculae which was a continuation of pre-existing trabeculae. Collagen fibres were forming and integrating into the trabeculae. Loose fibrous cartilaginous tissue was detected, hypertrophying towards a thick tissue. There were numerous osteoclasts in the periphery of the new formation trabeculae which suggested restoration of the centre towards the periphery. In the endosteum region, macrophages and osteoclasts were observed which had migrated towards the newly formed bone trabeculae. At the scar site, bone breakdown was actively enabling bone tissue to develop.
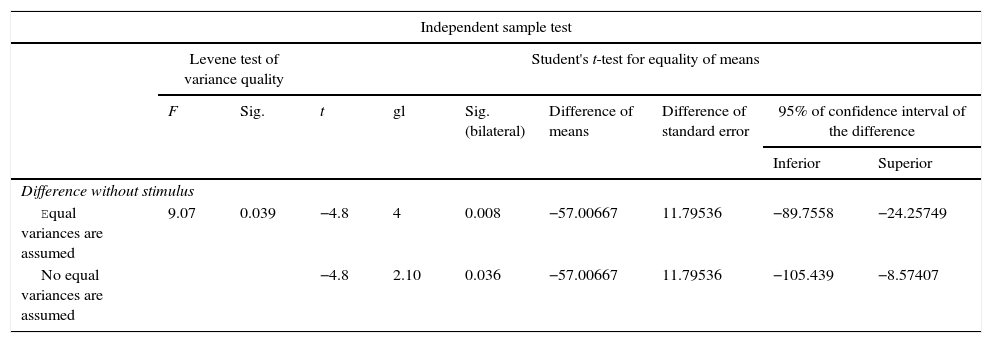
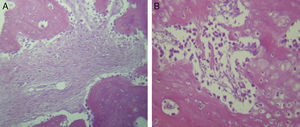
For Group B, in the haematoxylin and eosin (Fig. 2) staining the general observation was larger areas of hyaline cartilage, and transitions of fibrous tissue to cartilaginous tissue until the tissue became osteo collagenous. A more organised tissue was clearly distinguished with regard to the bone repair phases in comparison with the Group A slices. The fibrous tissue was observed to be more consistent with regards to how the collagen fibres were arranged in their integration with the trabeculae. In all the Masson trichrome stained slices (Fig. 3), it was observed that there was profuse fibrous tissue which was very thick and compact. In general, presence of osteoclasts in the fibrin areas was low, and the fibrin was thinner. Few macrophages and osteoclasts were observed in the repair bone trabeculae ends. Towards the bone periphery, a more organised and fibrous periosteum was observed. In the central area of the bone there was a concentration of osteoclasts and thick, compact bundles, running perpendicularly to the collagen fibres.
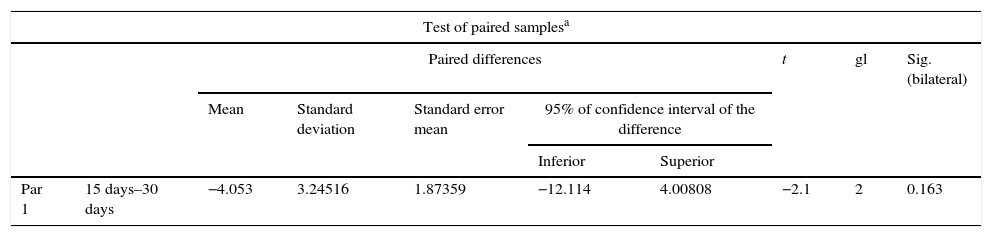
The x-ray images of both groups were digitalised. The first x-rays were made 15 days after surgery and the second ones 30 days after surgery. For both groups, in the new growth area in general a predominant radio lucid area was observed with little presence in radio-opaque areas, which were selected for analysis and subsequently compared between the different days. The mean of the values from the densimetric analysis was estimated at 15 days as 113.66 and at 30 as 117.71. When the paired samples were tested, we found a difference of 4.05 with a p value of 0.163, and there was therefore no statistical difference in the bone density from a radiographical viewpoint (Table 1). For the group which received stimulus (Group B), 15 days after treatment a mean of 65.60 was found and at 30 days a density mean of 126.66 was found. When data were paired, a difference of 61.06 was found with a p value of p=0.03, which indicates a significant difference (Table 2). On comparing both Groups with their respective p values, we found a statistically significant difference as equal variances had not been assumed (Table 3).
Difference of paired samples for group A.
| Test of paired samplesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Paired differences | t | gl | Sig. (bilateral) | ||||||
| Mean | Standard deviation | Standard error mean | 95% of confidence interval of the difference | ||||||
| Inferior | Superior | ||||||||
| Par 1 | 15 days–30 days | −4.053 | 3.24516 | 1.87359 | −12.114 | 4.00808 | −2.1 | 2 | 0.163 |
Difference of paired samples for group B.
| Paired sample testa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Paired differences | t | gl | Sig. (bilateral) | ||||||
| Mean | Standard deviation | Standard error mean | 95% confidence interval of the difference | ||||||
| Inferior | Superior | ||||||||
| Par 1 | 15 days–30 days | −61.060 | 20.17079 | 11.54561 | −111.167 | −10.95298 | −5.24 | 2 | 0.035 |
Student's t-test for equality of means.
| Independent sample test | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Levene test of variance quality | Student's t-test for equality of means | ||||||||
| F | Sig. | t | gl | Sig. (bilateral) | Difference of means | Difference of standard error | 95% of confidence interval of the difference | ||
| Inferior | Superior | ||||||||
| Difference without stimulus | |||||||||
| Equal variances are assumed | 9.07 | 0.039 | −4.8 | 4 | 0.008 | −57.00667 | 11.79536 | −89.7558 | −24.25749 |
| No equal variances are assumed | −4.8 | 2.10 | 0.036 | −57.00667 | 11.79536 | −105.439 | −8.57407 | ||
Non unions continue to be the primary complication in fracture treatment.22 It is known that they affect from 5% to 10% of the 7.9 million fractures which occur annually in the United States.23 The socio economic burden associated with fracture repair, including hospital costs and loss of salaries is considerable.24,25 These complications support the need for innovative therapies to optimise fracture healing. Electromagnetic stimulation is a non-invasive technology that improves fracture healing.26–28 The use of electromagnetic stimulation in the treatment of fractures without sufficient union began in the mid 1800s.29,30 In 1957, Fukada and Yasuda31 demonstrated that there was a relationship between electricity and callus formation. Recent studies have shown that electromagnetic stimulation could have a direct impact on many cellular pathways, including the synthesis of growth factors,32–34 in proteoglycans, in the regulation of collagen35,36 and the production of cytokines.37 These pathways allow the bone to respond to changes in the environment, and respond to the stimulation of the cacium-calmoduline pathways and thus promote bone consoldation.24,38 Several random trials have assessed the effect of electromagnetic stimulation in bone consolidation, but clinical findings vary.39,40
The aim of our study was to demonstrate the efficacy of electro-stimulation, through the use of a direct alternating current applied with a closed circuit device with graduated voltage, in the maturing of a tissue obtained by distraction in bone lengthening, and thus accelerate the consolidation process (on average 30 days), specifically focused on elongation. Eyres et al.41 explored the use of electro-magnetic stimulation in lengthening procedures in the lower extremity and reported a statistically significant improvement in bone density, in the group who underwent surgery 12 months after treatment, in segments proximal to the osteotomy. Borsalino et al.42 reported an increase in the callus and in the trabecular bridge with electro-magnetic stimulation in the middle and lateral cortex at 40 and 90 days, and an improvement in osseous callus density 90 days in patients who underwent a femoral intertrochanteric osteotomy.
In our experimental study with an animal model in dogs, the x-ray images corresponding to 15 and 30 days after surgery were analysed. For the group without any type of eclectic stimulus (Group A) it was found that there was no significant difference in bone density between the established analysis periods, with 4.05±3.24 (p=0.163). For the group where electro-stimulation had been applied (Group B), a significant difference was found between the 15 and 30 days of electric stimulus, with a value of 61.06±20.17 (p=0.03). On comparing the data from both groups (with and without electo-stimulation), statistical difference may be assumed due to the differences found between the variances (0.036).
Since the majority of studies based on electro-stimulation report clinical and radiographical findings, it was difficult to compare our study with others where histological slices of an animal model were used. Chen et al.43 examined the evolution of an osteotomy histologically to determine the effect of postsurgical electric stimulation, in bone repair of a facial model in rats. They applied a 20μA current at the osteotomy site, but subcutaneously. They analysed the histological slices to determine the healing process of the osteotomy. Their results did not show any statistically significant differences between the 3 groups of animals (p>0.005).
In our study for the group which did not received electro-stimulation, when the histological slices were analysed, we observed that the repair tissues had fibrous features, areas of cartilage with transition towards the bone matrix, i.e. the primary bone structure. We also observed periosteum components which gave way to fibroblasts and to thick-collagenous tissue, forming parallel bundles which interconnected with the primary bone, with the latter replacing the hyaline cartilaginous tissue. In the Masson's trichrome staining we observed the presence of a fibrous scar between the new growth bone trabeculae which extended to the pre-existing trabeculae. There was also a large amount of osteocloasts in the periphery of the newly growing trabeculae, which indicated repair from the centre outwards. In the group where a direct electric stimulation of 20μA had been applied, better organised tissue was observed, for the bone repair stages, compared with the group A specimens, together with larger areas of hyaline cartilage, transitions of fibrous tissue which extended to an ostecollageneous one. There was little presence of osteoclasts in the fibrin areas.
Petersson and Johnell44 described an experimental model in rabbit tibia, where a delayed union was established by using a silicon spacer on the site of the osteotomy for 48 days. When the spacer was removed, an electric transistor of continuous current regulated to 20μA was applied though stainless steel electrodes, to stimulate the osteogenesis on the right-hand side for 62 days. A simulated operation was performed on the left-hand side, inserting stainless steel electrodes with no current: a well formed callus was formed on the stimulated side and on the control side there was synostosis between the ends of the fibula and tibia. Their end report was that there had been no significant difference in the formation of synostosis between the right and left side, and no adverse histological effect was provoked by the electric current.
ConclusionsUnder the conditions of our study using the animal model, we observed a significant radiographic difference in the growth and formation of callus at 15 and 30 days. Histologically we observed more organised tissue when electro-stimulation was applied in the animal model of osteosynthesis at 15 and 30 days. A significance in consolidation time in the group with electro-stimulation (Group B) was demonstrated by x-ray and laboratory studies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the staff of the Vivarium of the Department of Physiology of the Faculty of Medicine of the Autonomous University of Nuevo León, for their good care and management of the animals used for this study.
Please cite this article as: Peña-Martínez V, Lara-Arias J, Vilchez-Cavazos F, Álvarez-Lozano E, Montes de Oca-Luna R, Mendoza-Lemus O. Electroestimulación interósea en un modelo de elongación con fijación externa. Cir Cir. 2017;85:127–134.