TJP1 gene encodes a ZO-1 protein that is required for the recruitment of occludins and claudins in tight junction, and is involved in cell polarisation. It has different variations, the frequency of which has been studied in different populations. In Mexico there are no studies of this gene. These are required because their polymorphisms can be used in studies associated with medicine and surgery. Therefore, the aim of this study was to estimate the frequency of alleles and genotypes of rs2291166 gene polymorphism TJP1 in Mexico Mestizos population, and to estimate the conformational effect of an amino acid change.
Material and methodsA total of 473 individuals were included. The rs2291166 polymorphism was identified PASA PCR-7% PAGE, and stained with silver nitrate. The conformational effect of amino acid change was performed in silico, and was carried out with servers ProtPraram Tool and Search Database with Fasta.
ResultsThe most frequent allele in the two populations is the ancestral allele (T). A genotype distribution similar to other populations was found. The polymorphism is in Hardy–Weinberg, p>0.05. Changing aspartate to alanine produced a conformational change.
ConclusionsThe study reveals a high frequency of the ancestral allele at rs2291166 polymorphism in the Mexican population.
El gen TJP1 codifica para una proteína ZO-1, necesaria para el reclutamiento de las ocludinas y claudinas en las uniones estrechas y participa en la polarización celular. Tiene diferentes variaciones cuya frecuencia ha sido estudiada en numerosas poblaciones; sin embargo en México no hay estudios de este gen, siendo necesarios ya que sus polimorfismos pueden ser usados en estudios de asociación en medicina y en cirugía. Por tal motivo el objetivo de este estudio fue estimar la frecuencia de alelos y genotipos del polimorfismo rs2291166 del gen TJP1 en población mestiza de México; así como estimar el efecto conformacional del cambio de un aminoácido.
Material y métodosSe incluyeron 473 individuos. El polimorfismo rs2291166 se identificó por PCR-PASA y PAGE al 7% teñida con nitrato de plata. El efecto conformacional de cambio de aminoácido se realizó in silico con los servidores ProtPraram Tool y Search Database with Fasta.
ResultadosEl alelo más frecuente en las dos poblaciones es el alelo ancestral (T). Se encontró una distribución similar a otras poblaciones respecto a los genotipos. El polimorfismo está en equilibrio de Hardy-Weinberg, p>0.05. El cambio de aspartato por alanina produce un cambio conformacional.
ConclusionesEl estudio revela alta frecuencia del alelo ancestral en población mexicana del polimorfismo rs2291166 y produce un cambio en la estructura de ZO-1.
In the postgenomic era there are three paradigms of interest in general surgery: the carrying out of customised surgical procedures, prediction and the prevention of pre-inter- and post-surgical complications.1–3 Few studies on genes have been carried out on the Mexican population, and the ones that have focused on the process of fibrogenesis, on polymorphisms in genes TGF-β, PAI-1, AT, which are associated with post-mammoplasty capsular contracture or response to treatment with pirfenidone.4–6 In clinical practice the polymorphism rs1345365 of ELMO17 has recently been proposed as a marker.
Apart from these genes, new markers must be sought to be used in everyday diseases of the oncology, urology, gynaecological, internal medicine and gastroenterological surgeon. These would be, for example: acute pancreatitis, chronic pancreatitis, hydatiform mole; several types of cancer (thyroid, pancreas, biliary ducts, liver and colon). Variants of the TJP1 (Tight Junction Protein-1) gene are proposed as markers in customised practice. This is for two reasons: first, that the TJP1 gene encodes ZO-1 (Zona Occludens-1) protein, which forms part of the narrow or contact cell-to-cell bonds because it participates in cellular differentiation, cytokinesis and chemotaxis.8–12Second, because alteration in the architecture of ZO-1 was reported in animal models, in in vivo studies and tissue cultures, in two groups: either through reduction of the protein expression, or its increase; for the first case this corresponds to partial molar pregnancies, complete hydatiform moles, intrahepatic cholangiocarcinomas and extra hepatics such as extraheptatic tumours, tumours of the gallbladder, and stage 4TLM breast tumours.12–16 The second group includes pancreatic ductal adenocarcinoma, stage I-II de cancer of the colon and cancer of the colon with liver metástasis.17–22 The increase of TJP1 has been recently reported in 71% of gastrointestinal neoplasia cases, where expression is proportional to tumour diameter, histological staging and the patients 22 survival; for example, in non-small cell lung cancer, the expression of this gene is an indicator of good prognosis, in accordance with the TNM scale.23
Evidence suggests that in surgery, the ideal genetic markers of TJP1 are those which drive changes in amino acid, because they modify the structure of the proteins with the reduction or gain in function, and also for their accumulation. In both cases disease occurs due to structural alteration.24 This also applies to those loci in intronic cryptic locations, in promoter or enhancer regions, because they increase or diminish the generic expression and therefore that of the protein.
Gene TJP1 studies worldwide are limited, there are only four polymorphisms with loci in intronic regions studied in the phenotypes of the autistic spectrum, response to treatment of antipsychotics and keratoconus.25–27 Furthermore, of the 155 SNP (Single Nucleotide Polymorphism) type polymorphisms and three of the DIV (deletion, insertion, variation) type polymorphisms recorded, which lead to changes in amino acid, only six have been analysed in the Mexican American population and of these the variant rs2291166 is associated with albuminuria. This polymorphism consists of a T >G transversion in exon 23, codon 1334, which drives the change from aspartate to alanine in a not yet functionally characterised ZO-1 domain. In the Mexican population there are no previous reports of these. To study them is challenging since the structure of the genetic variation of the ethnic groups would be a significant contribution. A genome association study is required as a previous step to determine whether a polymorphism is present and can be validated, as a clinical and surgical marker.
AimsTo analyse the frequency of alleles and genotypes, and establish the Hardy Weinberg equilibrium in the mestizo Mexican population, with regards to rs2291166 (p.D1334A) with locus g.29716773T>G gene polymorphism TJP1. We also wished to compare the frequencies obtained with other populations and establish through in silico analysis, whether polymorphism rs2291166 would lead to a conformational change in protein ZO-1.
Material and methodsRecruitment of subjectsThis was a descriptive study, which included 473 healthy subjects (n=946 chromosomes), between 18 and 82 years of age. All subjects had mestizo ancestry (born in Mexico, with a surname of Spanish origin) and ancestors of Mexican origin, from at least three generations). They were recruited from the municipal centre Desarrollo Integral de la Familia (DIF) of Chapala Jalisco, from the Laboratorios Tolsa, from the Hospital Regional Valentín Gómez Farías, from the Delegación Sur in the State of Oaxaca, from the Sociedad de Gerontogeriatría in the State of Jalisco A.C. (SOGEJAL), from the Instituto de Investigaciones sobre la Salud Pública of the Universidad de la Sierra Sur, from the Instituto de Genética Humana, Centro Universitario de Ciencias de la Salud (CUCS) from the Benemérita Universidad de Guadalajara.
All participants signed the letter of informed consent.
This study forms part of the project entitled: “Population screening study for the identification of environment and genetic risk factors, associated with the development of complex diseases related to nutrition in the West and South of Mexico”, registered with numbers IBM/DIF/2010-2012 and IISSP/BAMM/03, approved by the Research, Ethics and Biosecurity Committees. This was carried out in compliance with the Declaration of Helsinki and the Belmont treaty principles.
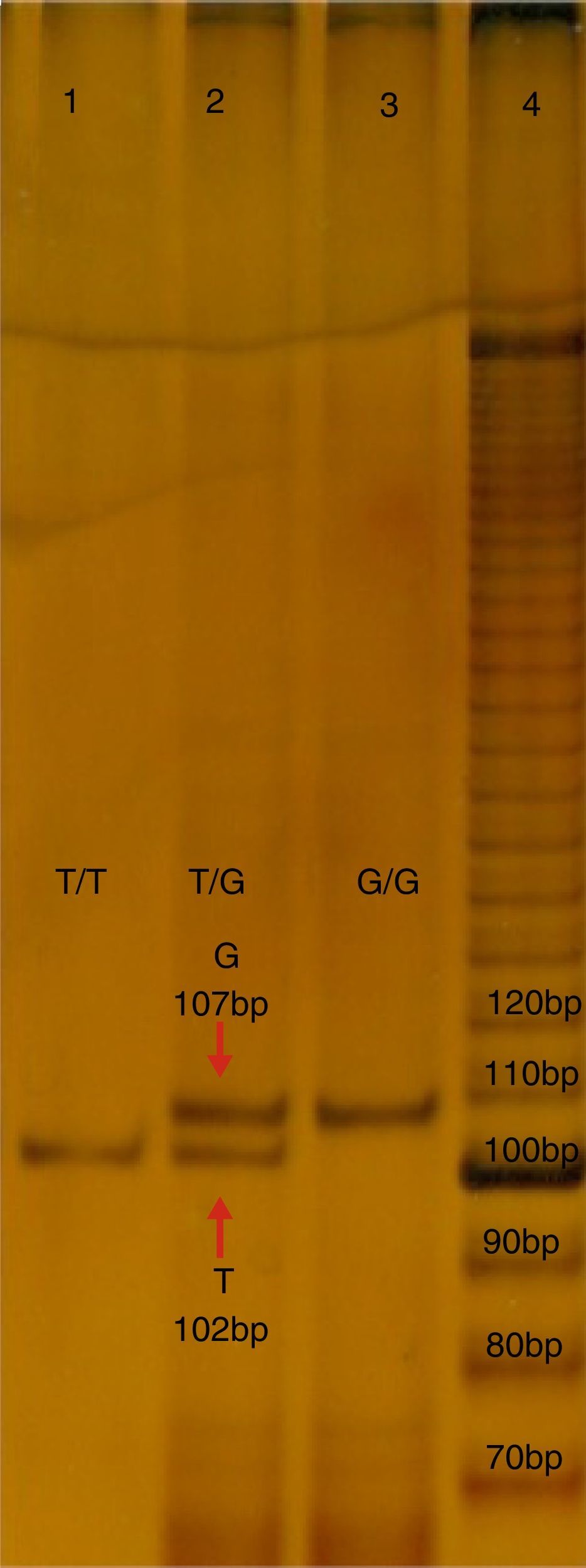
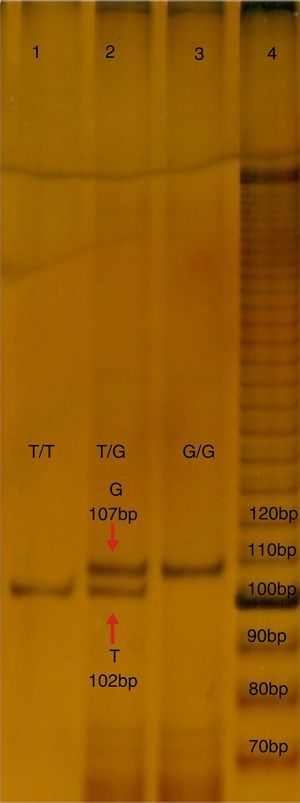
Molecular studyFive millilitres of peripheral blood was obtained from each subject, in a tube with ethylenediaminetetraacetic acid (EDTA), which was used to isolate the DNA with a commercial kit (GeneCatcher, Invitrogen). The detection of polymorphism rs2291166 was performed using the specific allele polymerase chain reaction (PCR-PASA). For this the following primers were designed: FW1G 5′-CTTCATCTTCTTCAGGTT-3′, FW2A 5′-ATATTCTTCATCTTCTTC AGG TG¿-3, RW3 5′-GTCATTCATTATCTGTTAGG-3′ (Genosys Sigma-Aldrich). The amplification programme made in the TECHNE TC-412 model thermal cycles, consisted of 30 cycles: 95°C, for 5min (initial denaturalisation), at 95°C, for 30s (denaturalisation), 48°C, 45s (hybridisation), at 72°C, 30s (polymerisation), with a final extension of 72°C, 5min. The mixture of reaction; buffer KCl 2.5μl (1X), MgCl2 1.5μl (25mM), 0.5μl de dNTP's (0.2mM), 0.5μl of each initiator (25pmoles), 2μl of hardened DNA (200ng), DNA Pol Taq 0.3μl (3U/μl) (Invitrogen), and finally 17.20μl of water, final reaction 25ml. The PCR products were analysed using electrophoresis in poliacrylamide in the proportion of: 19:1 at 7%, with ulterior electrophoresis running with buffer TBE 1X for 1.5h at 200V, 80–84mA. The products were distinguished by sizes; that of 102 pb corresponds to the T allele, whilst that of 107 pb corresponds to the G allele, as may be observed in Fig. 1.
Electrophoresis in polyacrylamide at 7%, TBE 0.5X, of the PCR-PASA for the SNP rs2291166 of TJP1. Lane number 4 corresponds to the running of the marker of 10 bases (life-technologies). Lane 1 and 3 correspond to the sample of homozygote subjects T and G, lane 2 corresponds to a herterozygote. Bp: pairs of bases.
To determine whether polymorphism rs2291166 would lead to a conformational change, the primary and secondary structure of protein ZO-1 was analysed by scanning the following software; ProtPraram Tool available on http://web.expasy.org/protparam, and Search Database with Fasta with access on http://Fasta.bioch.virginia.edu/fasta_www2/, considering the following parameters: secondary structure (Hydropathy/Secondary-Structure/Garnier plot for 4 stages); α-helices, sheets-β, loops and coils). In both cases the FASTA sequences were inserted into the respective portal with the reference number NCBI>gi|666335569|ref|NP_001287954.1[Homo sapiens], in two versions, one with the aspartate residue at 1334 and the other with the alanine residue at 1334.
Statistical analysisThe lower allele rate (MAF, MinorAllele Frequency) was established by direct observation. To validate the differences in allele y genotype distribution based on frequencies observed with those expected, we used χ2. Hardy–Weinberg equilibrium was considered if the sum of the values of χ2 were lower than 9.21 and P<0.001 with a level of freedom.
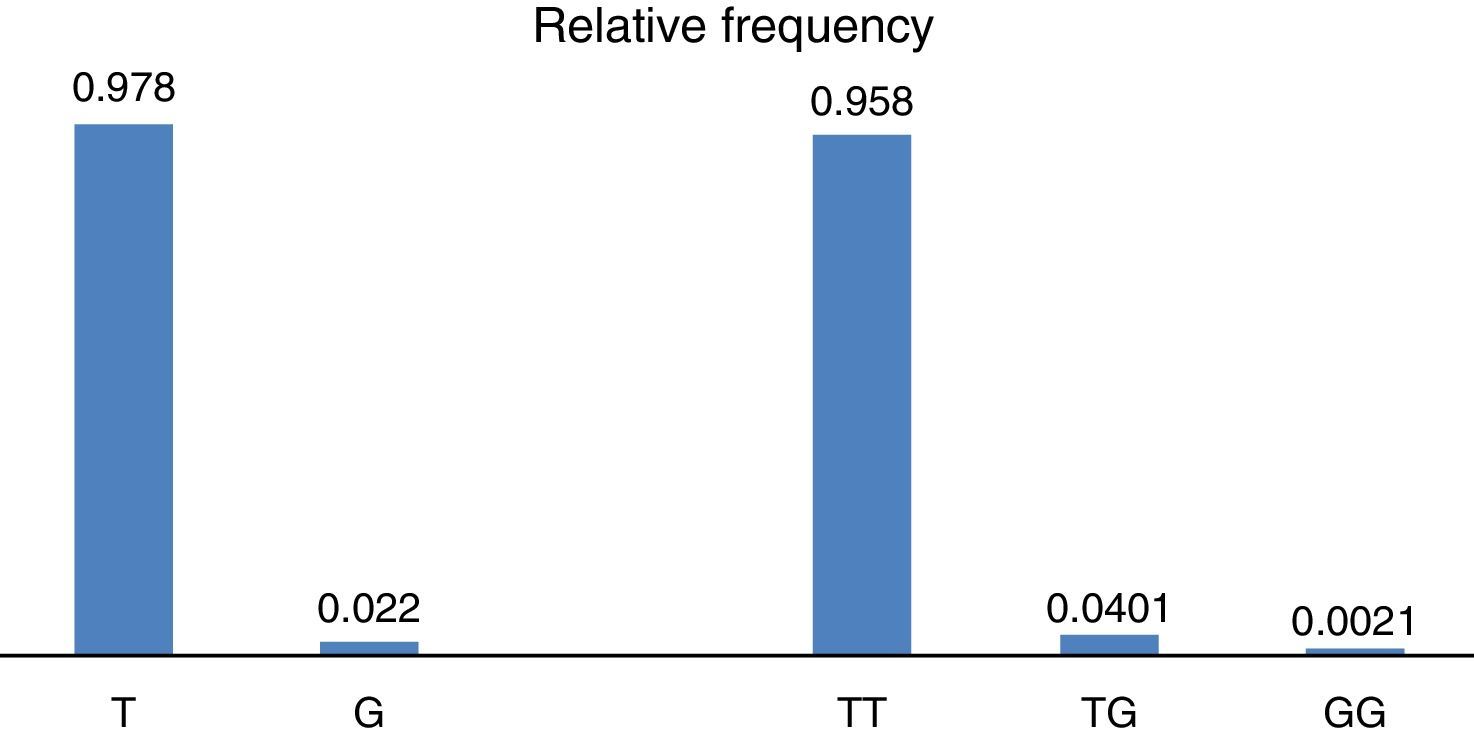
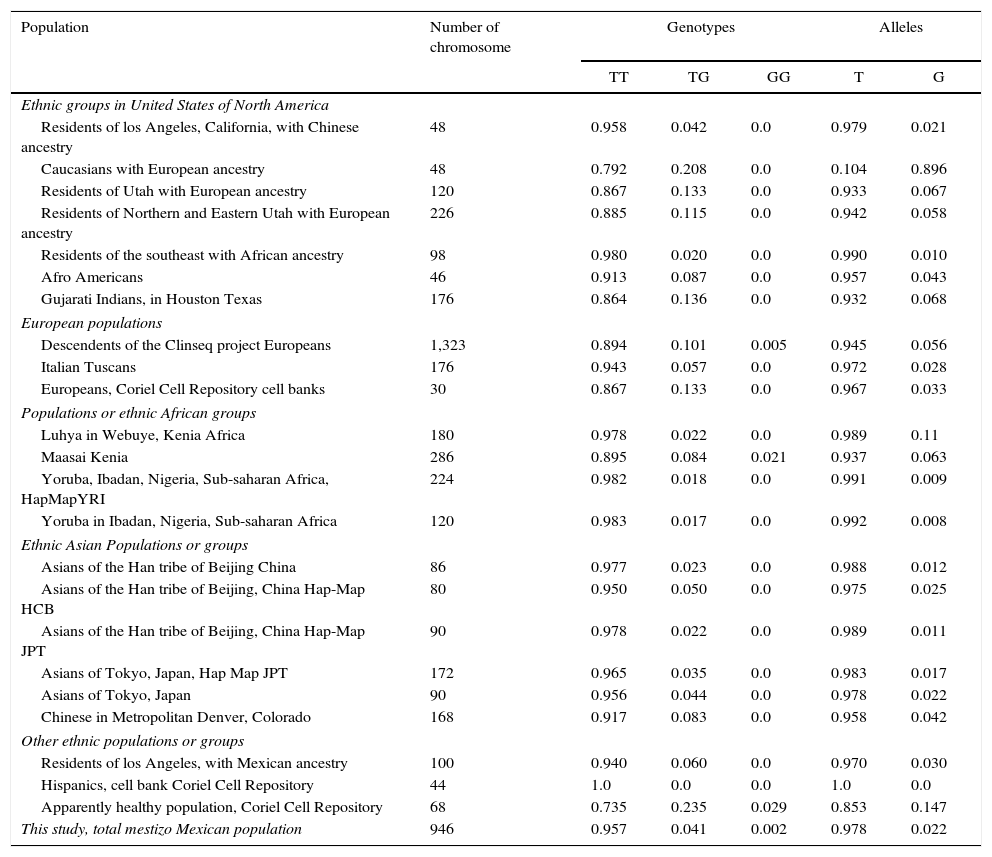
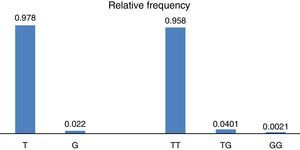
ResultsWith regard to allele distribution of the TJP1 gene polymorphism rs2291166 relative frequency (rf) in the total mestizo Mexican population analysed was as follows: 0.978 (n=925) for the ancestral or wild-type T and 0.022 (n=21) for the G allele (Fig. 2). MAF rate was G=0.022/21. Genotype distribution was as follows: 0.958 for T homozygotes (n=453), 0.0401 for heterozygotes (n=19) and 0.0021 for G homozygotes (n=1), as may be observed in Fig. 2. The average heterozosity index in the total population analysed was 0.05 and homozygosity was 0.95. The G homozygotes were low in the population analysed. On comparing the relative frequencies of alleles and genotypes rs2291166 with the other populations reported in the SNP bank, we found a similar distribution for the different ethnic groups in which the G homozygote genotype was infrequent, it is only described in the Maasai population in Kenia (fr=0.021) and in the apparently healthy population of the AGI-ASP collection (Coriel Apparently Healthy Collection) (fr=0.029) (Table 1).
Frequency of alleles and genotypes of the polymorphism rs2291166 of the TJP1 gene.
| Population | Number of chromosome | Genotypes | Alleles | |||
|---|---|---|---|---|---|---|
| TT | TG | GG | T | G | ||
| Ethnic groups in United States of North America | ||||||
| Residents of los Angeles, California, with Chinese ancestry | 48 | 0.958 | 0.042 | 0.0 | 0.979 | 0.021 |
| Caucasians with European ancestry | 48 | 0.792 | 0.208 | 0.0 | 0.104 | 0.896 |
| Residents of Utah with European ancestry | 120 | 0.867 | 0.133 | 0.0 | 0.933 | 0.067 |
| Residents of Northern and Eastern Utah with European ancestry | 226 | 0.885 | 0.115 | 0.0 | 0.942 | 0.058 |
| Residents of the southeast with African ancestry | 98 | 0.980 | 0.020 | 0.0 | 0.990 | 0.010 |
| Afro Americans | 46 | 0.913 | 0.087 | 0.0 | 0.957 | 0.043 |
| Gujarati Indians, in Houston Texas | 176 | 0.864 | 0.136 | 0.0 | 0.932 | 0.068 |
| European populations | ||||||
| Descendents of the Clinseq project Europeans | 1,323 | 0.894 | 0.101 | 0.005 | 0.945 | 0.056 |
| Italian Tuscans | 176 | 0.943 | 0.057 | 0.0 | 0.972 | 0.028 |
| Europeans, Coriel Cell Repository cell banks | 30 | 0.867 | 0.133 | 0.0 | 0.967 | 0.033 |
| Populations or ethnic African groups | ||||||
| Luhya in Webuye, Kenia Africa | 180 | 0.978 | 0.022 | 0.0 | 0.989 | 0.11 |
| Maasai Kenia | 286 | 0.895 | 0.084 | 0.021 | 0.937 | 0.063 |
| Yoruba, Ibadan, Nigeria, Sub-saharan Africa, HapMapYRI | 224 | 0.982 | 0.018 | 0.0 | 0.991 | 0.009 |
| Yoruba in Ibadan, Nigeria, Sub-saharan Africa | 120 | 0.983 | 0.017 | 0.0 | 0.992 | 0.008 |
| Ethnic Asian Populations or groups | ||||||
| Asians of the Han tribe of Beijing China | 86 | 0.977 | 0.023 | 0.0 | 0.988 | 0.012 |
| Asians of the Han tribe of Beijing, China Hap-Map HCB | 80 | 0.950 | 0.050 | 0.0 | 0.975 | 0.025 |
| Asians of the Han tribe of Beijing, China Hap-Map JPT | 90 | 0.978 | 0.022 | 0.0 | 0.989 | 0.011 |
| Asians of Tokyo, Japan, Hap Map JPT | 172 | 0.965 | 0.035 | 0.0 | 0.983 | 0.017 |
| Asians of Tokyo, Japan | 90 | 0.956 | 0.044 | 0.0 | 0.978 | 0.022 |
| Chinese in Metropolitan Denver, Colorado | 168 | 0.917 | 0.083 | 0.0 | 0.958 | 0.042 |
| Other ethnic populations or groups | ||||||
| Residents of los Angeles, with Mexican ancestry | 100 | 0.940 | 0.060 | 0.0 | 0.970 | 0.030 |
| Hispanics, cell bank Coriel Cell Repository | 44 | 1.0 | 0.0 | 0.0 | 1.0 | 0.0 |
| Apparently healthy population, Coriel Cell Repository | 68 | 0.735 | 0.235 | 0.029 | 0.853 | 0.147 |
| This study, total mestizo Mexican population | 946 | 0.957 | 0.041 | 0.002 | 0.978 | 0.022 |
Alleles: T or G; ClinSeq:a Large-Scale Medical Sequencing Clinical Research Pilot Study; Genotypes: T (TT) or G (GG) homozygotes, (TG) heterozygotes; HapMap: map of haplotypes, catalogues of common genetic variants in humans; HCB: samples of phase 3 of the ethnic Han group of Beijing, China; JPT: sample of phase 5 of Japanese population of Tokyo; YRI: sample of phase 3 of population with Yoruba ancestry from Ibadan Nigeria, Africa.
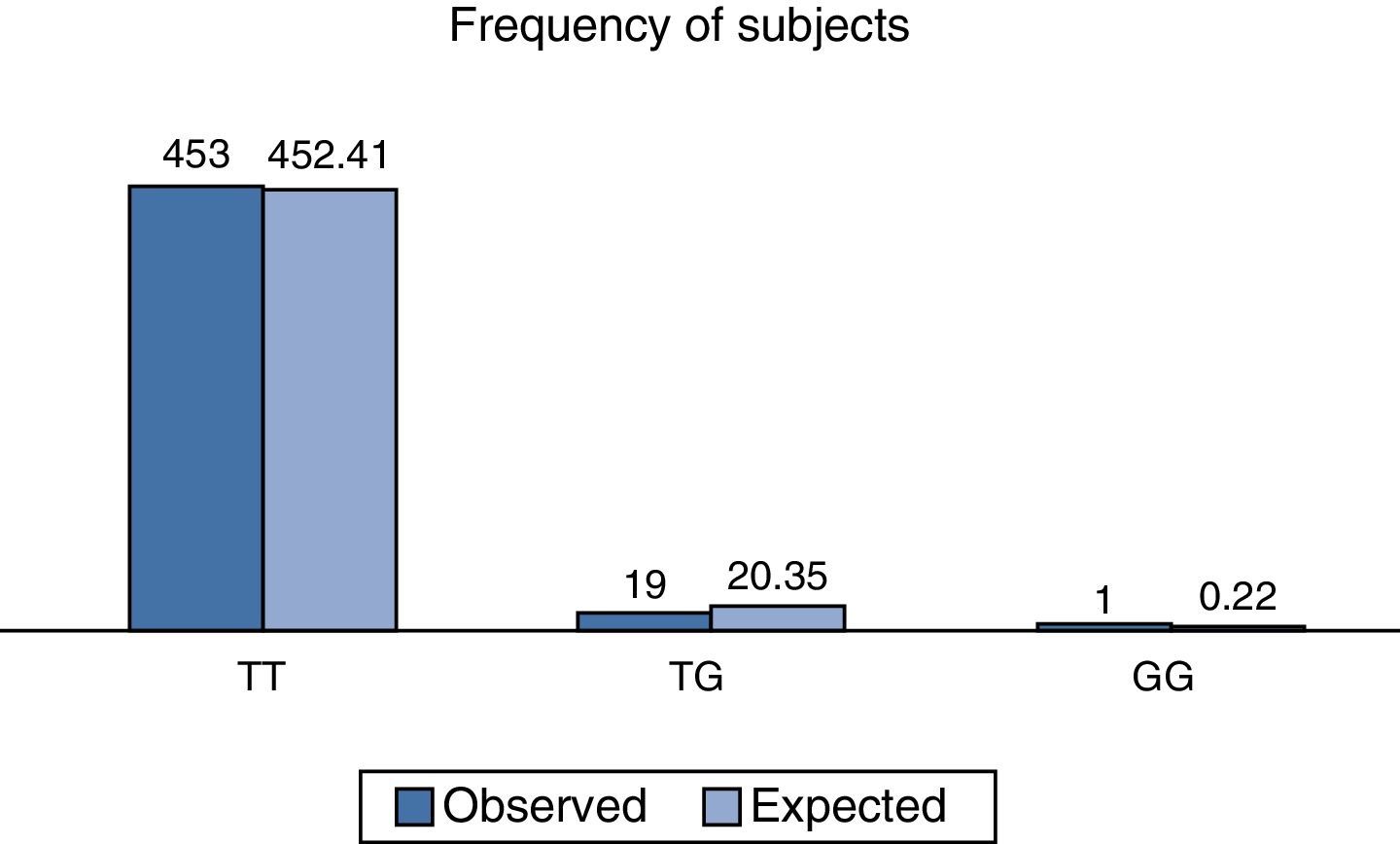
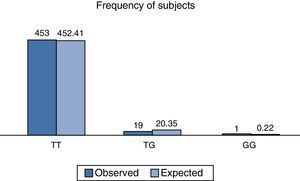
On analysing the distribution of relative observed frequencies, and comparing them with those which were expected, a value of χ2 of 2.3, respectively for the total population analysed was found, and with a value of P>0.05; the polymorphism rs2291166 of the TJP1, gene was therefore within the Hardy Weinberg equilibrium, as observed in Fig. 3, which is very similar to that reported in other populations (Table 1).
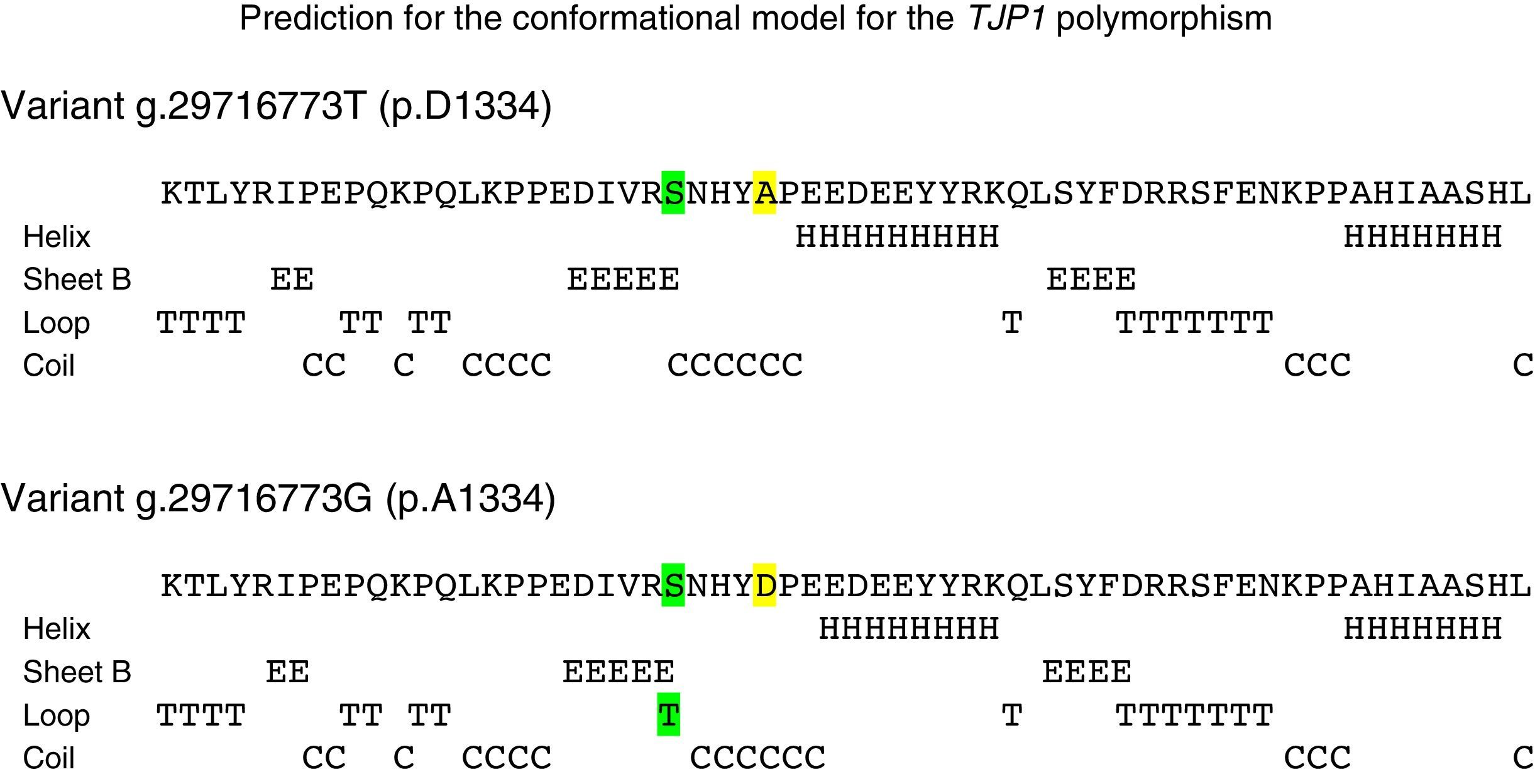
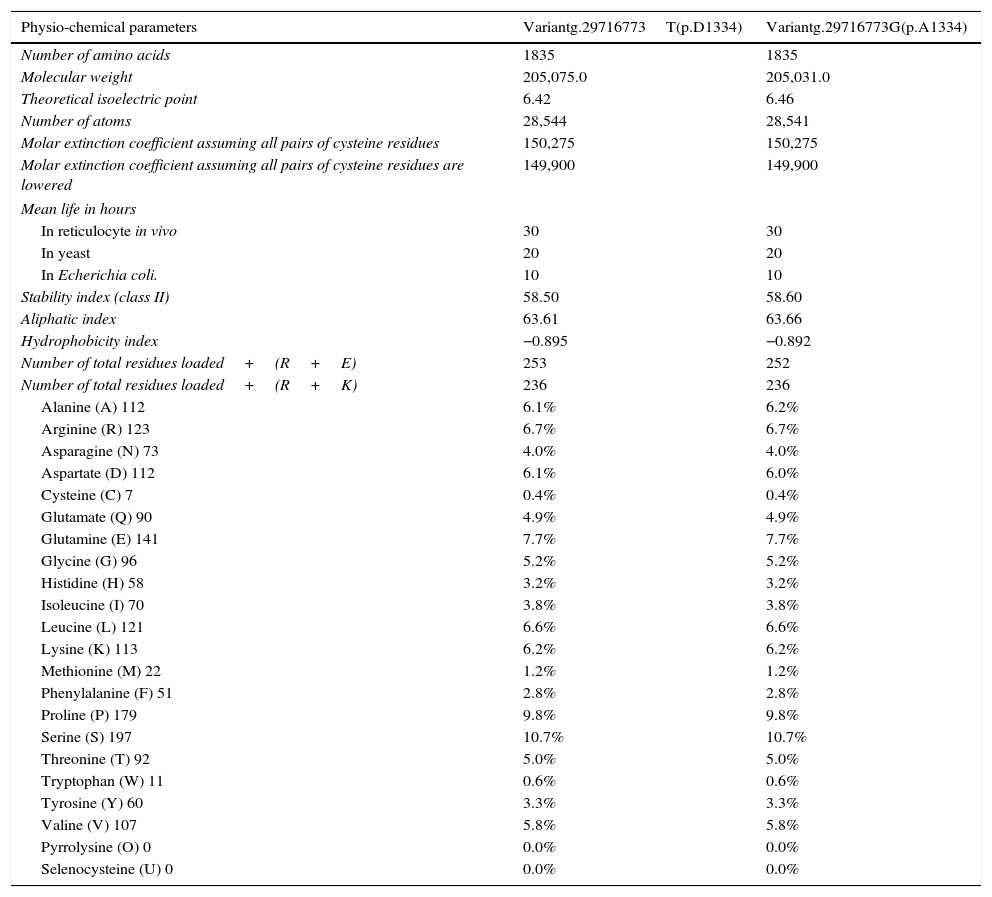
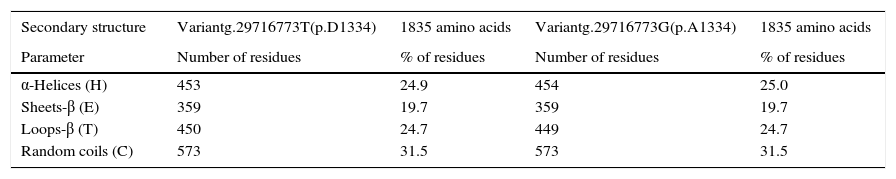
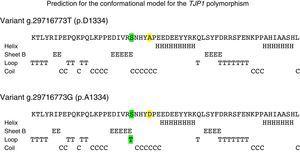
The in silico study of the primary structure shows differences between the variants g.29716773T (p.D1334) and g.29716773G (p.A1334) for the molecular weight, isoelectric point, stability index, aliphatic index, hydrophobicity index, number of positive load residues, aspartate percentage; and in the alanine percentage (Table 2). The ancestral variant g.29716773T leads to a lower isoelectric point, a more stable protein (although it is class II) and leads to ZO-1 with a higher index of hydrophobicity. Study of the secondary structure shows that the ancestral variant g.29716773T de TJP1 produces a ZO-1 protein with a higher content of loops-β and lower content of α-helices (Table 3 and Fig. 4).
Effect of polymorphism rs2291166 in the primary structure of ZO-1.
| Physio-chemical parameters | Variantg.29716773T(p.D1334) | Variantg.29716773G(p.A1334) |
|---|---|---|
| Number of amino acids | 1835 | 1835 |
| Molecular weight | 205,075.0 | 205,031.0 |
| Theoretical isoelectric point | 6.42 | 6.46 |
| Number of atoms | 28,544 | 28,541 |
| Molar extinction coefficient assuming all pairs of cysteine residues | 150,275 | 150,275 |
| Molar extinction coefficient assuming all pairs of cysteine residues are lowered | 149,900 | 149,900 |
| Mean life in hours | ||
| In reticulocyte in vivo | 30 | 30 |
| In yeast | 20 | 20 |
| In Echerichia coli. | 10 | 10 |
| Stability index (class II) | 58.50 | 58.60 |
| Aliphatic index | 63.61 | 63.66 |
| Hydrophobicity index | −0.895 | −0.892 |
| Number of total residues loaded+(R+E) | 253 | 252 |
| Number of total residues loaded+(R+K) | 236 | 236 |
| Alanine (A) 112 | 6.1% | 6.2% |
| Arginine (R) 123 | 6.7% | 6.7% |
| Asparagine (N) 73 | 4.0% | 4.0% |
| Aspartate (D) 112 | 6.1% | 6.0% |
| Cysteine (C) 7 | 0.4% | 0.4% |
| Glutamate (Q) 90 | 4.9% | 4.9% |
| Glutamine (E) 141 | 7.7% | 7.7% |
| Glycine (G) 96 | 5.2% | 5.2% |
| Histidine (H) 58 | 3.2% | 3.2% |
| Isoleucine (I) 70 | 3.8% | 3.8% |
| Leucine (L) 121 | 6.6% | 6.6% |
| Lysine (K) 113 | 6.2% | 6.2% |
| Methionine (M) 22 | 1.2% | 1.2% |
| Phenylalanine (F) 51 | 2.8% | 2.8% |
| Proline (P) 179 | 9.8% | 9.8% |
| Serine (S) 197 | 10.7% | 10.7% |
| Threonine (T) 92 | 5.0% | 5.0% |
| Tryptophan (W) 11 | 0.6% | 0.6% |
| Tyrosine (Y) 60 | 3.3% | 3.3% |
| Valine (V) 107 | 5.8% | 5.8% |
| Pyrrolysine (O) 0 | 0.0% | 0.0% |
| Selenocysteine (U) 0 | 0.0% | 0.0% |
Effect of the polymorphism rs2291166 in the secondary ZO-1 structure.
| Secondary structure | Variantg.29716773T(p.D1334) | 1835 amino acids | Variantg.29716773G(p.A1334) | 1835 amino acids |
|---|---|---|---|---|
| Parameter | Number of residues | % of residues | Number of residues | % of residues |
| α-Helices (H) | 453 | 24.9 | 454 | 25.0 |
| Sheets-β (E) | 359 | 19.7 | 359 | 19.7 |
| Loops-β (T) | 450 | 24.7 | 449 | 24.7 |
| Random coils (C) | 573 | 31.5 | 573 | 31.5 |
Conformational effect of TJP1 polymorphism rs2291166 in ZO-1. The yellow highlighting shows the change of amino acid and the green the conformational change which is characterised by an introduction of a loop-β associated with the alanine in residue 1334. The colour of this figure may only be appreciated in the electronic version of the article.
This is the first study conducted in the Mexican population showing the frequencies of alleles and genotypes of the TJP1 gene polymorphism rs2291166. The T allele, and the TT and TG genotypes were the most frequent, similarly to Mexican Americans and other populations (Table 1).8 The homozygote G (GG) genotype of the SNP rs2291166, has only been reported in a broad sample of over 1,000 Europeans (ClinSeq project; A Large-Scale Medical Sequencing Clinical Research Pilot Study) (fr=0.005), in the Maasai of Kenia (fr=0.021), in the apparently healthy population of the AGI-ASP collection (fr=0.029) and now in Mexico (fr=0.002). These findings may be explained by three hypotheses: the first, which postulates that the frequencies presented in this study are the product of crossbreeding, supported by several previous reports with Y-STR's (Short Tandem Repeats), which show up to 3%-5% of African ancestry and 65% of European ancestry.28 The second, which argues in favour of the frequency of alleles was preserved during the crossbreeding of Amerinian natives, as observed for alleles *A, *B and *C of the polymorphism (GC) of the gene for erythrocyte acid phosphatase.29 The third, which refers to the low frequency of the G homozygoteis related to an anti-selection, having a severe phenotypic effect associated with death by kidney damage, which is supported by studies carried out in Mexican Americans Lehman et al.8 Variant rs2291166 was also validaded as a polymorphism which is present in Mexico, since its distribution of genotypes is in the Hardy Weinberg equilibrium and to establishing this parameter it was necessary to carry out a prior association study, to reduce the possibility of false positives or Information weightings related to the lack of credibility of the marker, as reported by Topete-González et al.7
In this study a higher number of subjects was included than those reported in SNP's bank, very similar to the sample of European descendents from the ClinSeq project, which significantly reduces the possibility of these weightings, providing certainly to the presented findings,30 which is reflected in the detection of G homozygote carriers, the frequency of which is very low; on the other hand, the in silico study reveals a pathogenic effect of the TJP1 SNP rs2291166, since the T>G transversion becomes the replacement of aspartate by valine, which leads to a conformational change in ZO-1. The p.1334D variant produces a more stable isoform (Table 2 and Fig. 4), this change is correlated to modifications in the primary structures; hydrophobicity index, which is higher in the ancestral isoform (Table 2). These results plus the association of variant p.1334D>A, with the albumin/creatin urinary rate support the theory of the conformational pathology in the pathogenesis of diseases, such as that put forward by Hayden et al.24 The effect of the structure change would have to be demonstrated by isolelectro focusing studies, cristalography and diffraction with X rays which may be carried out because the two isoforms of ZO-1 present a different isoelectric point; however, these concerns do not form part of the aims of this study.
If we consider that acute pancreatitis, hydatiform mola and several types of cancer share the alteration of cellular architecture, whether this be either due to the loss of cell-to-cell contact molecules or the increase in the expression of these molecules, and the mobility through the extracellular matrix, TJP1 polymorphism rs2291166 must be analysed to determine the effect in the risk of development, taking into consideration that these alternations are caused also by structural changes of cytoeskeletal proteins and the extracellular matrix. These markers may also be susceptible to complex or emerging diseases, where the architecture of narrow bonds may be changed (ZO-1), as shown by the cultures of acute leukaemia cells and myelodysplasic syndromes, through hypermatilation muting.31–33 In the case of infections by cosackievirus, adenovirus, hepatitis C virus, human type 1 immunodeficiency viruns (HIV) and papilomavirus (VPH): in the first two infection is contained so long as the narrow epithelial unions maintain their structures; in the case of hepatitis C virus it bonds with tight junction leading to retention of occludins in the endoplasmatic reticulum; and for the third and fourth cases, the expression of HIV protein in the cervical epithelial muscosa correlates with disruption of ZO-1, and increases the expression of pseudovirion of the papillomavirus for the penetration of basal cells and parabasal cells, in which the life cycle of HIV begins. ZO-1 alteration therefore increases the carcinogenesis of the cervix associated with papillomavirus, relevant for colposcopy gynaecologists.34–36 The genetic predisposition of the SNP rs2291166 for gastritis by Helicobacter pylori, will be the subject of future studies, since it produces disruption of ZO-1, in gastric epithelial cells.37
Lastly, the changes in amino acids modifies the structure and function of proteins (for ZO-1, cellular permeability) and therefore leads to analysing the effect of ZO-1 polymorphisms with cardiovascular problems, since production of hydrolysis of lipoprotein lipase takes place in endothelial cells and in triglycerides, which increases permeability of the ZO-1, and serum hypercholesterolemy leads to alteration of the distribution and pathway of PI3K (phosphatidyl-inositol-3-kinase). In cardiac arrest from ischaemic or dilated cardiomyopathy there is also a marked reduction of ZO-1 and connexin 43.38–40
In dermatology the TJP1 polymorphisms such as SNP rs2291166 may be a modifying factor of severity in dermatosis characterised by the loss of skin architecture, such as psoriasis and epidermolysis bullosa. This is supported in that the psoriasis is related to insulin resistance and TJP1 expression is positively regulated by IGF-1(insulin-like growth factor type 1, a growth factor similar to insulin type 1), as show in the epidermoid carcinoma cell cultures.41,42
ConclusionsThe frequency of alleles and genotypes was established, as was the Hardy Weinberg equilibrium in the Mexican population, with respect to polymorphism rs2291166 of the TJP1 gene, and further studies of association in the clinical-surgical field in Mexico are therefore validated. The similarity of the relative distribution of alleles and genotypes with other populations was also established, resembling European and African populations. And finally, it was proven in silico that this polymorphism led to a structural putative change of protein ZO-1, generating two isoforms, the ancestral isoform or p.1334D, which provides a protein with greater conformational stability.
FinancingFinancing by the Laboratorio de Variación Genética y Enfermedad, of the Benemérita Universidad de Guadalajara and by the Grupo Multidisciplinario para el Estudio Integral de las Enfermedades Metabólicas e Infecciosas in the Mexican population.
Conflict of interestsThe authors have no conflict of interests to declare.
Our thanks to Sergio A. Ramirez Garcia and Luis Javier Flores Alvarado, as both authors equally contributed to the development of the study. For their technical and collaborative support. Thanks also to the trainees of the Programa de Incorporación a la Investigación Temprana en Ciencias Biomédicas y Sociales, Delegación Sur de SOGEJAL 2010–2012: Jareth Marco Cruz Bastida and Jafet Said Cruz Bastida, who participated in sample collection, collection of informed consent forms, statistical analysis, table and figure design. Also to Mrs. Dora Cervantes, Chairwoman and to Magdalena Cruz, Director from 2010 to 2012 of the Sistema para el Desarrollo Integral de la Familia de la Delegación Chapala Jalisco, for assistance in external consultation of the Biomolecular Research Unit, for recruitment of subjects. Our thanks also to the General Director of Laboratorios Tolsa, Mr. Eduardo Ascuita Ramos for his support in conducting this study.
Please cite this article as: Ramirez-Garcia SA, Flores-Alvarado LJ, Topete-González LR, Charles-Niño C, Mazariegos-Rubi M, Dávalos-Rodríguez NO, et al. Alta frecuencia del alelo ancestral del polimorfismo rs2291166 de TJP1 en población mexicana, efecto conformacional así como las aplicaciones en cirugía y medicina. Cirugía y Cirujanos. 2016;84:28–36.