Gastric non-Hodgkin lymphoma is a rare tumour that represents approximately 7% of all stomach cancers and 2% of all lymphomas. The most frequent location of gastric MALT (mucosa associated lymphoid tissue) lymphomas is in the antrum in 41% of the cases, and 33% can be multifocal. The risk of spontaneous perforation of a gastric MALT lymphoma is 4–10%.
Clinical caseA 24-year-old male patient carrying the Human Immunodeficiency Virus, who began with signs and symptoms of acute abdomen and fever 72h before arriving in the emergency room. A computed tomography was performed that showed free fluid in the cavity, and gastric wall thickening. The patient underwent a laparotomy, finding absence of the anterior wall of the stomach, sealed with the left lobe of the liver, colon and omentum. Total gastrectomy, with oesophagosty and jejunostomy tube, was performed.
ConclusionsGastric perforation secondary to a MALT lymphoma is rare, with high mortality. There is limited information reported of this complication and should be highly suspected in order to provide appropriate treatment for a complication of this type.
El linfoma gástrico no Hodking es un tumor poco frecuente el cual representa aproximadamente el 7% de todas las neoplasias malignas del estómago y 2% de todos los linfomas. Siendo la localización gástrica la más frecuente de los linfomas MALT (linfoma de tejido linfoide asociado a mucosa), presentándose con mayor frecuencia en el antro gástrico en el 41% de los casos, y el 33% puede ser multifocal. El riesgo de perforación espontánea por un linfoma MALT gástrico es del 4-10%. El objetivo de este artículo es presentar el caso de una complicación rara en un paciente inmunosuprimido, y hacer una revisión del tema.
Caso clínicoPaciente masculino de 24 años portador de virus de la inmunodeficiencia humana, el cual inició con signos y síntomas de abdomen agudo de 72 horas de evolución y fiebre. Acude a Urgencias en donde se le realiza tomografía de abdomen la cual muestra líquido libre en cavidad, y engrosamiento de paredes gástricas. Se somete a laparotomía, encontrando ausencia de pared anterior de estómago sellada con lóbulo izquierdo del hígado, colon y epiplón. Se realiza gastrectomía total con esofagostoma y sonda de yeyunostomía.
ConclusionesLa perforación gástrica por un linfoma MALT es muy rara con una alta mortalidad, hay poca información reportada sobre esta complicación, se debe de tener una alta sospecha para poder ofrecer un tratamiento oportuno para una complicación de este tipo.
Gastric mucosa associated lymphoid tissue lymphoma (MALT) is a rare disease,1 described for the first time in 1983 by Isaacson and Wright2; it is classified as non-Hodgkin B-cells, extranodal lymphomas and within marginal zone lymphomas, which means it can disseminate to lymph nodes and other organs during its progression.1,2 This represents approximately 7% of all stomach malignant neoplasms, and 2% of all lymphomas.2 Gastric location is the most frequent location of MALT lymphomas, with a higher frequency in the gastric area in 41% of cases, and may be multifocal in 33% of cases.3
The most frequent age of presentation is after 50 years of age, more often in men than in women, 1.7:1. At the time of diagnosis it is a low grade tumour in 70–85% of cases.1,4
The risk of spontaneous perforation caused by gastric MALT is 4–10%, may be presented spontaneously as an emergency or as a complication of quimio or radiotherapy; but a complete absence of the anterior wall of the stomach, as in this case report, is an even rarer presentation.4,5
There is a close relation between the infection caused by Helicobacter pylori (H. pylori) and the development of gastric MALT lymphoma, as shown by various studies.
The risk of gastric perforation may be presented spontaneously as an emergency or as a complication of quimio or radiotherapy6; but the complete absence of the anterior wall of the stomach as in the case of our patient is rare, and therefore our goal is to perform a review of the few reports of this complication, and the correct management of this pathology.
Clinical caseA male patient, 24 years old, relevant history as HIV carrier from birth controlled by the Infectology service with last HIV viral load of 163copies/ml, CD4 19cel/AL under treatment with tenofovir/entricitabine and efavirenz, pulmonary tuberculosis for 3 years, denies other diseases or prior surgeries.
The current condition began 72h prior to admission into the Casualty department with sudden onset abdominal pain in the epigastrium with irradiation to the dorsum, burning type, constant and incapacitating, EVA 9/10, with nausea but no vomits, non-quantified fever, no self-medication, with 2 days of pain until attending Casualty because there was no improvement.
Physical examination: blood pressure 110/70mmHg, heart rate 117 per minute, respiratory rate 18 per minute, temperature 37.5°C. Cachectic patient, pale teguments, dehydrated, no neurological impairment with appearance of pain, oriented in time and space, rhythmic heart area, tachycardia with no murmurs or adventitious sounds, well ventilated lung fields with no rales or wheezes. Abdomen at auscultation with decreased peristaltic noises, tympanic on percussion, with involuntary rigidity, pain on superficial palpation in all quadrants, mass palpated in epigastrium and mesogastrium, positive rebound, with clear data of peritoneal irritation.
Laboratory results upon admission: leukocytes 4.0 thousand/Ul, haemoglobin 6.2g/dl, haematocrit 21.7%, platelets 254 thousand/Ul, neutrophils 84.9%, lymphocytes 8.8%, TP 12.9, glucose 81mg/dl, creatinine 0.9mg/dl, K 4.5mmol/l, Na 122mmol/l, lactic dehydrogenase 493U/l. Arterial blood gas: pH 7.35, PCO2 29, PO2 76, HCO3 17, CO2 18, EB-6.1, SatO2 95.5% with no supplementary oxygen support.
The assessment using the APACHE II scale proved to be 15 points with 25% mortality.
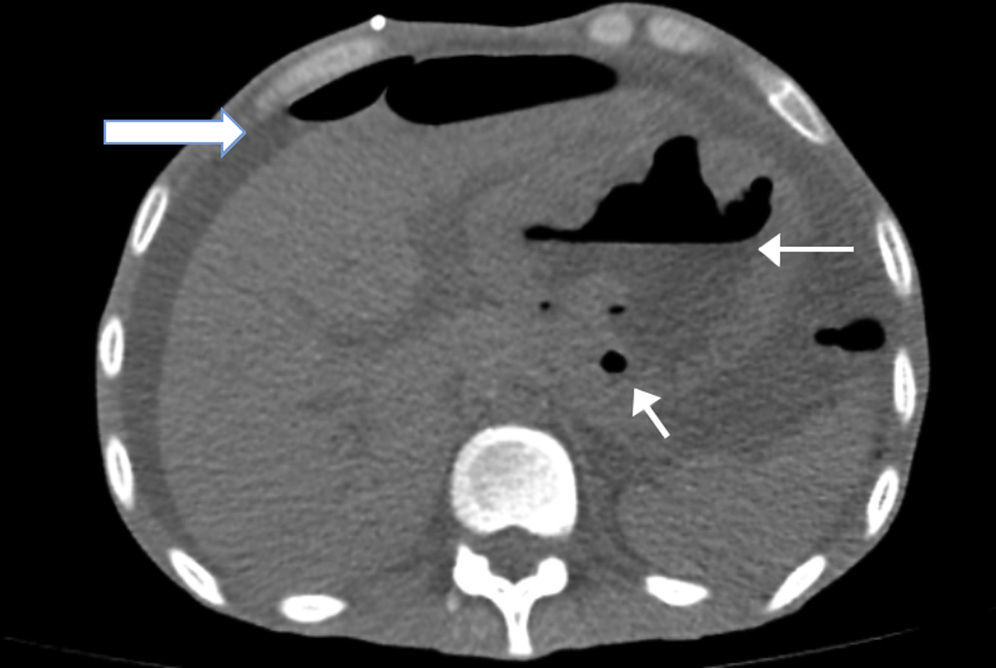
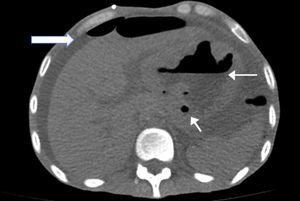
A simple abdomen tomography was taken, showing left pleura thickening with a small effusion, abdomen with free fluid in all the abdominal cavity, and stomach with thickened walls where the left lobe of the liver adhered to the stomach (Fig. 1).
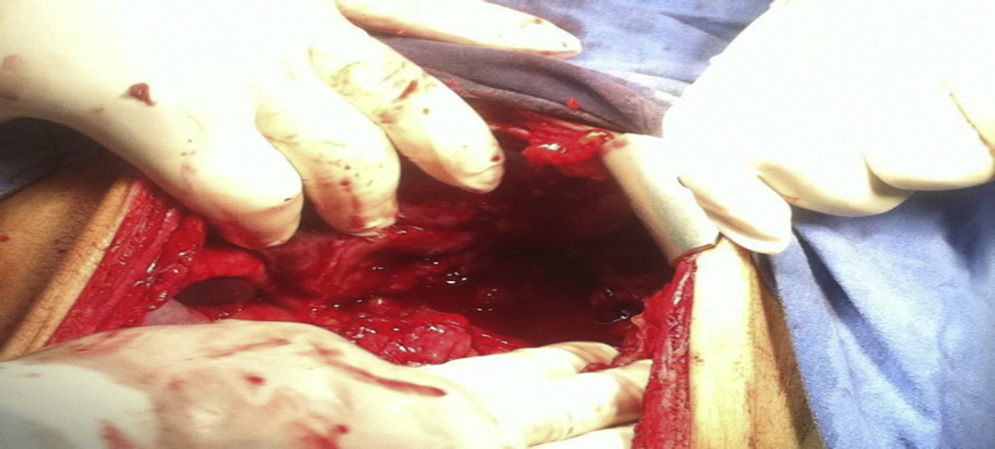
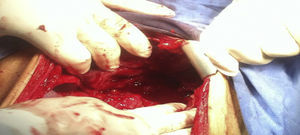
An emergency laparotomy was performed, where 600cc of inflammatory free fluid was found in the cavity, with no data of active bleeding; a mass involving the liver, stomach and colon; absence of anterior gastric wall found upon dissection; from 3cm below the oesophagogastric junction up to the pylorus, the folds of the gastric mucosa were thickened and indurated; inflammatory adenopathies in the greater and lesser curvature of approximately 0.5cm; and transverse colon dilated approximately 5cm of diameter (Fig. 2). With these findings, it is decided to perform a full gastrectomy with oesophagostoma plus placing of jejunostomy tube.
The patient was transferred to the Intensive Care Unit with pressor amines support, with multiple organ failure secondary to abdominal sepsis, and died 24hours later.
Later, a peritoneal fluid culture was received where yeast Gram is reported, low levels of polymorphonuclear cells and Candida albicans. Cytochemical examination of unclear peritoneal fluid, leukocytes 10.710mm,3 polymorphonuclear cells 90% with crenocytes, piocytes, bacteria and mononuclear cells 10%.
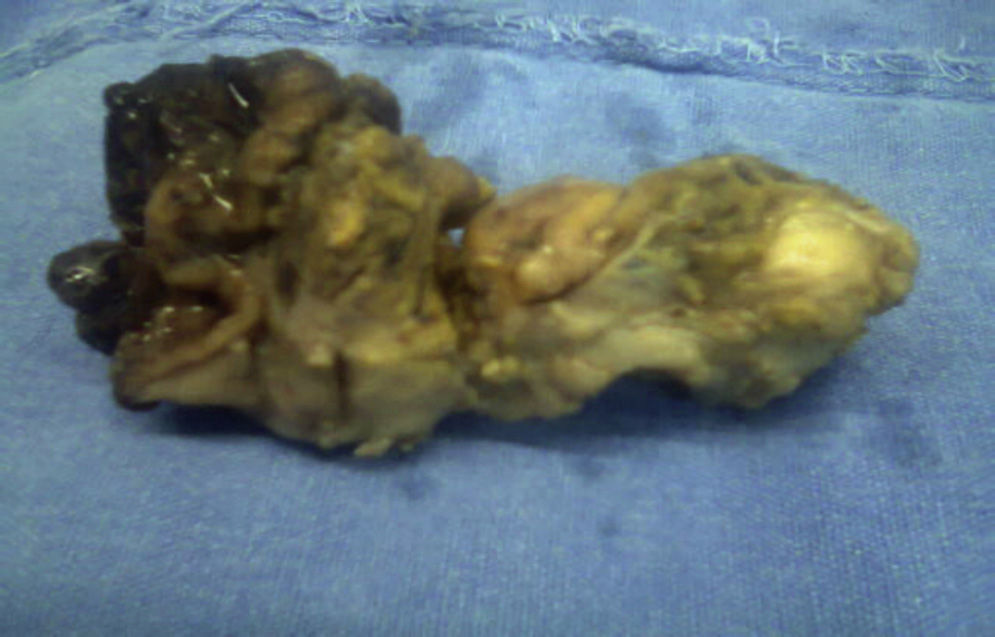
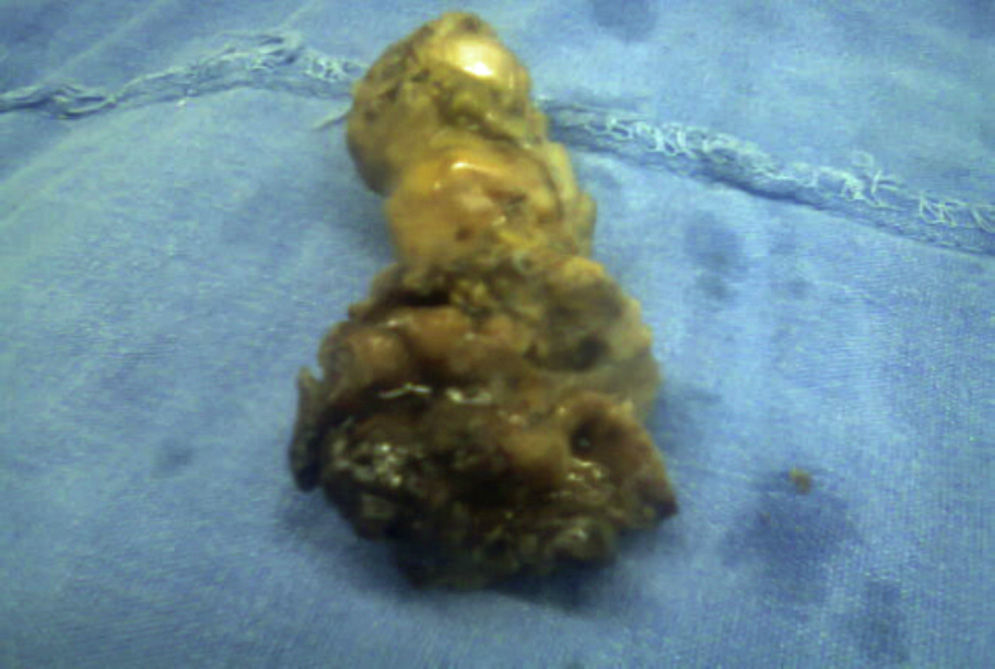
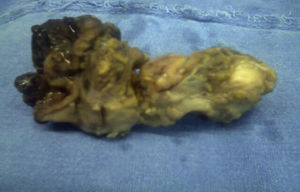
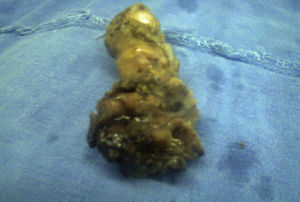
The histopathological result of the piece reported a histological image with high grade malignant lymphoproliferative lesion in relation to MALT lymphoma, positive immunohistochemistry for CD20++, CD21+++, and CD35++, negative for CD10, and CD5 (Figs. 3 and 4).
Spontaneous gastric perforation secondary to lymphoma is a rare complication, and we found only one case report of this type of complication, reported by Ayako and Kitsukawa,7 where the initial presentation was through gastric ulcers as a consequence of the tumour; in our case the patient never presented gastric symptomatology, and never had an endoscopy during his lifetime, which made it very complicated to make an earlier diagnosis.
Gastric MALT lymphoma is a neoplasia producing scarce clinical manifestations in its initial states, and may even be asymptomatic; many times confused with gastric cancer; usually causes a dyspeptic condition, with prevalence of epigastric pain, with or without ulcerous rhythm, feeling of fullness, nausea and vomiting. Advanced tumours produce a condition similar to gastric carcinoma, with weight loss, fatigue, anorexia, digestive bleeding, and in 10–20% of cases it is possible to feel an abdominal tumour.1,2,4,7
The diagnosis of MALT lymphoma was based on the gastroscopy, taking biopsies, and a characteristic but non-pathognomonic finding of MALT lymphomas with thickened gastric walls, which were clearly visible in the tomography taken on admission. Also, we observed the presence of ulcerations, with thickened folds and/or irregular or polypoid tumours. The finding of these lesions next to multiple, star-like configuration ulcers and sometimes confluent ulcers (which may even pass the pylorus and affect the duodenum) suggests lymphomatose lesion. Even when there is just a suspicion of the existence of MALT lymphoma, numerous samples should be taken from the antrum to the fornix, mapping all the gastric mucosa.1,2,8
The histological diagnosis is shown through immunohistochemistry techniques, Southern Blot and PCR to detect monoclonality of B-lymphocytes, specifically in differential diagnosis with pseudolymphomas. Tumour cells are small-medium sized B-lymphocytes, with non-abundant cytoplasm and irregular-shaped nuclei, called centrocytoid lymphocytes, and small or monocytoid-type lymphocytes are less frequent; in one lesion there may be a clear prevalence of one cell shape, or several shapes may coexist. The presence of lymphoepithelial lesion is considered as the most characteristic morphological feature of MALT lymphoma, which consists of the invasion of the crypt by aggregate centrocitoid lymphocytes. Other histological findings are the moderate atypical cell of tumour lymphocites and the presence of lymphocites with Dutcher bodies, although their absence does not rule out the diagnosis.1,3,4,8
Immunohistological studies show the following as positive: CD20, CD21, CD35 and IgM, and the following as negative: CD5, CD10, CD23 and cyclin D1(-). The presence of monoclonality does not equal malignancy, and there may be monoclonality without lymphoma or persistence thereof some time after the tumour has disappeared.1,2,4,8
The division into low and high grade lymphomas is based on the proportion of blast cells in the lesion. The classification of the tumour into high or low grade is important, since the high grade represents a more aggressive clinical condition and a worse prognosis. The histological diagnosis of the grade can be difficult in certain patients, since both grades can coexist in the same lesion or different multifocal lesions, having gone through the evolutional transformation of low and high grade in MALT lymphomas. It is considered that the presence of islets of more than 20 transformed cells, or a proportion above 15–20% of high grade cells, is clinically relevant. In certain high grade lymphomas there are no signs of low grade lesion, and therefore these tumours may be considered high grade “de novo”. However, this information lacks prognosis value because there are no clinical differences in lymphomas progressing from low to high grade.1,2,8
There are several therapeutic possibilities for gastric MALT lymphomas, including: treatment to eradicate the infection by H. pylori; surgical treatment; non-surgical oncological treatment with quimio and/or radiotherapy. These modalities of treatment can supplement each other. In the case of low grade lymphomas, no significant differences have been found in survival after treatment for eradication, whether surgery, chemotherapy, or surgery plus chemotherapy or radiotherapy.1,2,9 The survival of patients operated for healing purposes up to 5 years is 93.7% in stage I, stage II 55% and stage III 25%.1,6,9 When the surgery is palliative, the survival is 37.1% up to 5 years, while survival of operated patients with negative lymph nodes is 92.3% up to 5 years, and with positive lymph nodes it is 41.3% and in both up to 5 years.1,6,9
In low grade tumours, it has been shown that eradication of H. pylori leads to tumour remission, by interrupting the immunological stimulus which maintains its growth. It is apparently permanent, but confirmed up to 5 years with no data of tumour activity.
The recommended first line treatment for clinical stages I and II is to treat the infection by H. pylori, which implies the combination of any inhibitor of the proton bombs together with amoxicillin and clarithromycin, replacing the first one with metronidazole and, in case of allergy to the former, it can be replaced with metronidazole. Control of the infection for H. pylori is made via endoscopy; if it is confirmed that there is still H. pylori infection or the patient continues with symptoms and is negative for H. pylori, radiotherapy should be considered, or rituximab if radiotherapy is contraindicated.
For clinical stages III and IV where the patient presents bleeding, symptomatology, advanced disease, affection of an organ and shows data of progression of the disease, if there is any of the above, management with immunological therapy, chemotherapy or locoregional radiotherapy is indicated.6,9 The most used chemotherapy schemes are bendamustine plus rituximab, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone), and RCVP (rituximab, cyclophosphamide, vincristine and prednisone).1,6,9
Until recently, surgical resection was the treatment used for maltoma. Currently the indication of the surgery would be confirmed failure of the treatment for eradication or high grade lymphoma, limited to the stomach walls. Although the results of gastric surgery are still good, with mortality lower than 3%, the ideal is to use non-surgical treatments.
Indications of emergency surgery for a MALT lymphoma are perforation with an incidence of 4–10%, pyloric obstruction, and in some cases haemorrhage with a prevalence of 19%,7,9 the latter being the most frequent. Spontaneous gastric perforation represents a very serious complication with high mortality.1,8,9
The clinical stage is the main prognosis factor. It is also said that tumours smaller than 5cm permit 100% survival up to 5 years, while if they are more than 10cm, the survival rate is only 20%.9–11
ConclusionsMALT lymphoma represents 50% of gastric lymphomas and is developed secondary to an infection by H. pylori as a result of chronic gastritis. However, in our case the patient denied history of acid peptic disease. Finally, the patient began with an important gastric perforation, which is one of the rarest and most lethal complications, which is why we decided to report this case.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: López-Zamudio J, Ramírez-González LR, Núñez-Márquez J, Fuentes Orozco C, González Ojeda A, Leonher-Ruezga KL. Perforación gástrica por linfoma MALT. Reporte de caso. Cir Cir. 2015;83:217–21.