Angiotensin converting enzyme inhibitors are effective in delaying the progression of diabetic retinopathy. It is unknown if their use is associated with a better visual outcome in patients with diabetic macular oedema.
Material and methodsA non-experimental, comparative, longitudinal and retrospective study was performed on patients with diabetic macular oedema treated by focal photocoagulation, and with systemic arterial hypertension treated with angiotensin converting enzyme inhibitors (Group 1), and without hypertension (Group 2). The dependent variable was the proportion with visual improvement, operatively defined as the gain of one or more lines of vision three weeks after photocoagulation. The independent variable was the use of angiotensin converting enzyme inhibitors. The proportion of eyes with visual improvement after treatment was compared between groups using the Chi squared (χ2) test.
ResultsA total of 33 eyes (51.6%) were assigned to group 1, and 31 (48.2%), to group 2. The mean of visual acuity improved after three weeks, compared with baseline (p=0.002). The proportion of eyes with visual improvement did not differ between patients treated with angiotensin converting enzyme inhibitors (45.5%) and those that did not use them (51.6%, p=0.4).
ConclusionsThere was no statistical difference in the proportion of eyes with visual improvement between patients treated with angiotensin converting enzyme inhibitors and in those where they were not used. There is no support for the inhibition of angiotensin II in addition to photocoagulation for improving the outcome in patients with diabetic macular oedema.
El uso de inhibidores de la enzima convertidora de angiotensina es eficaz para retardar la progresión de retinopatía diabética; se desconoce si su empleo se asocia con un mejor desenlace visual en pacientes con oedema macular diabético.
Material y métodosEstudio observacional, comparativo, longitudinal y retrospectivo. Se evaluaron pacientes con oedema macular diabético tratados mediante fotocoagulación, y con hipertensión arterial sistémica tratados con inhibidores de la enzima convertidora de angiotensina (grupo 1), y no hipertensos (grupo 2). La variable dependiente fue la proporción de mejoría visual, definida operativamente como la ganancia de una o más líneas de visión, 3 semanas después de la fotocoagulación; la variable independiente fue la presencia o ausencia del tratamiento con inhibidores de la enzima convertidora de angiotensina. Se comparó la proporción de ojos con mejoría visual entre grupos mediante χ2.
ResultadosTreinta y tres ojos (51.6%) se asignaron al grupo 1 y 31 (48.4%) al 2; el promedio de agudeza visual mejoró a las 3 semanas, con respecto al basal (p=0.002). La proporción de ojos con mejoría visual no difirió entre los pacientes tratados con inhibidores de la enzima convertidora de angiotensina (45.5%) y aquellos que no los recibían (51.6%, p=0.4).
ConclusionesLa proporción de ojos con mejoría visual entre los pacientes tratados con inhibidores de la enzima convertidora de angiotensina y quienes no lo usaban no tuvo diferencia significativa. No se sustenta inhibir la angiotensina II como terapia adjunta a la fotocoagulación focal, para mejorar el desenlace en pacientes con oedema macular diabético.
Macular oedema is the main cause of sight loss in patients with any degree of diabetic retinopathy. Macular oedema is characterised by thickening of the macula due to the accumulation of fluid in the retinal tissue, which separates the photoreceptors, and therefore reduces vision.1
The loss of support cells in the capillaries of the retina (pericytes) forms microaneurysms and enables the filtration of liquid and inflammatory elements which cause macular oedema.2–4 Capillary leakage can come from a localised lesion (focal oedema), or from an extensively damaged network of capillaries (diffuse oedema).
The standard treatment for macular oedema with localised filtration is focal photocoagulation, which closes the sites of capillary leakage and encourages the resorption of the extravasated fluid. This treatment reduces the incidence of moderate sight loss (3 lines of vision) from 33% to 13%.5
Although photocoagulation achieves involution of oedema, it seldom improves vision.6,7 This lack of improvement might be associated with other elements which contribute towards the formation of thickening, such as inflammation, vascular permeability factors and angiotensin II (Ang II).8
Vascular endothelial growth factor, also known as the vascular permeability factor, enables the transcytotic transport of fluid towards the retina via structures of the endothelial wall, known as caveolae. The fluid that leaks through the caveolae can be removed using the ATPase enzyme of the pigment epithelium. This mechanism is insufficient in eyes with macular oedema, due to the increased retinal concentration of vascular endothelial growth factor, which encourages thickening of the macula to persist.
Another component in the formation of diabetic macular oedema is the rennin–angiotensin–aldosterone system, which also exists in the retina.9 Ang II is a hormone which regulates the balance of liquid and sodium, and cellular growth,10 and exerts its effect via 2 principal receptors: AT1and AT2. The AT1 receptors mediate vasoconstriction, the release of vasopressin and aldosterone, fibrosis, cellular growth and migration. The AT2 receptors involve contra-regulatory reactions such as vasodilation, the release of nitric oxide and inhibition of proliferation and growth.11
Synthesis of Ang II in the retina takes place through the action of the angiotensin converting enzyme, which is produced in the capillary endothelium and in the retinal pigment epithelium. Ang II receptor type 1 is located in the same site as the vascular endothelial growth factor.12
It has been reported that inhibitors of the angiotensin converting enzyme are more effective in reducing the incidence and progression of diabetic retinopathy, and in promoting its regression in type 2 diabetics.13 The effect of blocking Ang II in a short-term event has not been described, such as resolving thickening after focal photocoagulation, in eyes with diabetic macular oedema.
Ang II increases the expression of vascular endothelial growth factor, and therefore blocking it pharmacologically would theoretically accelerate the resolution of macular thickening, by reducing the capillary filtration dependent on the caveolae. This mechanism might facilitate resolution of the oedema and help to improve visual function after focal photocoagulation.
A study was undertaken to compare the proportion of eyes with visual improvement after photocoagulation, between patients already treated with angiotensin-converting-enzyme inhibitors and patients who had not been treated with them, in order to establish whether blocking Ang II might be effective in improving the functional outcome in diabetics with macular oedema.
Material and methodsAn observational, comparative, longitudinal and retrospective study was performed on patients with focal diabetic macular oedema.
The target population was diabetic patients with macular oedema, who attended the Opthalmology Department of the Hospital Juárez de México, of the Secretaría de Salud in Mexico City and its metropolitan area. The accessible population comprised patients treated in a general hospital between 26 March 2008 and 3 December 2013. The study took place between 20 April and 20 May 2015 and was authorised by the Research and Ethics Committee and Research Committee of the institution where it was carried out.
Adult patients of either gender were included, diagnosed with clinically significant macular oedema, who had been treated using focal photocoagulation by the same retinal consultant, and who also had systemic arterial hypertension treated with angiotensin-converting-enzyme inhibitors or who did not have arterial hypertension, with a record of best-corrected visual acuity under subjective refraction, measured in decimal equivalent, with a rapid macular map from optical coherence tomography on the date of the photocoagulation and 3 weeks afterwards.
Patients under any other anti-hypertensive treatment, or with crystalline opacity, or any other eye diseases which reduced vision were excluded from the study.
Patients who did not attend follow-up visits and those with incomplete data were removed from the study.
The dependent variable was improvement of visual acuity, defined operationally as the gain of one or more lines of vision after photocoagulation. The independent variable was the use of angiotensin-converting-enzyme inhibitors. Both nominal qualitative variables were qualified as present or absent.
Central point thickness, central field thickness and macular volume were defined as secondary variables, and measured by optical coherence tomography using Stratus OCT equipment (Carl Zeiss, Meditec, Dublin, CA, USA). The averages of change were measured 3 weeks after photocoagulation using the Student's t-test for paired samples.
The proportion of eyes with visual improvement was compared between the patients treated with angiotensin-converting enzyme inhibitors and those who were not, using the χ2 test.
A p<0.05 was considered significant; the information was stored and analysed using SPSS IBM version 22, for Windows.
ResultsSixty four eyes of 53 patients were evaluated, aged between 25 and 80 (mean 61, SD±9.7); 35 were male (54.7%). The course of their diabetes was from one to 30 years (mean 14.4, SDE±6.9), 17 eyes were those of patients treated with insulin (26.6%) and 48 of patients treated with oral hypoglycaemics or diet. Thirty-three eyes belonged to patients with systemic arterial hypertension (44.6%).
The degree of diabetic retinopathy mild non-proliferative in 10 eyes (15.6%), moderate non-proliferative in 35 (54.7%), severe non-proliferative in 3 (4.7%), and proliferative in 16 (25%); in 45 eyes the macular oedema was monofocal (70.3%).
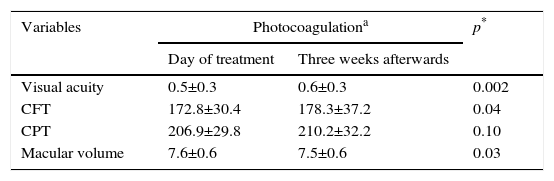
Before photocoagulation, the visual acuity course was from 0.13 to 1.00, central field thickness from 146 to 287μm, central point thickness of 122–260μm, and the macular volume from 6.24 to 8.76mm3. Three weeks after photocoagulation, the visual acuity course was from 0.10 to 1.00, central field thickness from 131 to 313μm, central point thickness from 112 to 307μm and macular volume from 6.35 to 8.87mm.3 The comparison of the variables measured on the day that the selective macular laser was applied and 3 weeks afterwards is shown in Table 1.
Comparison of visual acuity and anatomical variables on the day of treatment and 3 weeks afterwards.
| Variables | Photocoagulationa | p* | |
|---|---|---|---|
| Day of treatment | Three weeks afterwards | ||
| Visual acuity | 0.5±0.3 | 0.6±0.3 | 0.002 |
| CFT | 172.8±30.4 | 178.3±37.2 | 0.04 |
| CPT | 206.9±29.8 | 210.2±32.2 | 0.10 |
| Macular volume | 7.6±0.6 | 7.5±0.6 | 0.03 |
CFT: central field thickness; CPT: central point thickness.
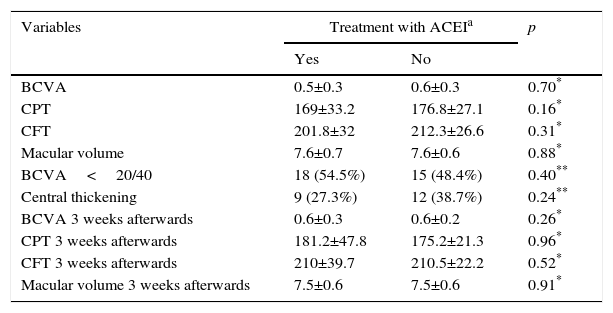
From the total sample, 33 eyes of patients treated with angiotensin-converting-enzyme inhibitors (51.6%) were assigned to group 1, and the remaining 31 (48.4%) to group 2. The comparison of variables between groups is shown in Table 2; there were no differences in the variables between the two groups prior to treatment.
Comparison of visual acuity and anatomical variables between groups.
| Variables | Treatment with ACEIa | p | |
|---|---|---|---|
| Yes | No | ||
| BCVA | 0.5±0.3 | 0.6±0.3 | 0.70* |
| CPT | 169±33.2 | 176.8±27.1 | 0.16* |
| CFT | 201.8±32 | 212.3±26.6 | 0.31* |
| Macular volume | 7.6±0.7 | 7.6±0.6 | 0.88* |
| BCVA<20/40 | 18 (54.5%) | 15 (48.4%) | 0.40** |
| Central thickening | 9 (27.3%) | 12 (38.7%) | 0.24** |
| BCVA 3 weeks afterwards | 0.6±0.3 | 0.6±0.2 | 0.26* |
| CPT 3 weeks afterwards | 181.2±47.8 | 175.2±21.3 | 0.96* |
| CFT 3 weeks afterwards | 210±39.7 | 210.5±22.2 | 0.52* |
| Macular volume 3 weeks afterwards | 7.5±0.6 | 7.5±0.6 | 0.91* |
BCVA: best corrected visual acuity; CFT: central field thickness; CPT: central point thickness.
The proportion of eyes with visual improvement of patients treated with angiotensin-converting-enzyme inhibitors (45.5%) did not differ significantly from that found in patients who were not receiving this treatment (51.6%, p=0.40) (Table 3).
Comparison of visual improvement between groups.
| Treatment with ACEI | Visual improvement | No visual improvement | p* |
|---|---|---|---|
| Yes | 15 (45.5%) | 18 (54.5%) | 0.40 |
| No | 16 (51.6%) | 15 (48.4%) |
ACEI: angiotensin-converting-enzyme inhibitors.
The proportion of eyes with macular oedema showing visual improvement 3 weeks after focal photocoagulation, was similar between the patients treated with angiotensin-converting-enzyme inhibitors and those who were not.
It was expected that the proportion of eyes with visual improvement after photocoagulation would be greater in patients treated with angiotensin-converting-enzyme inhibitors, due to the beneficial effect of these drugs on the incidence and progression of diabetic retinopathy.14
In 2007, Cha and Kim15 reported that small doses of angiotensin-converting-enzyme inhibitors showed no beneficial effects in delaying the progression of severe non-proliferative diabetic retinopathy.
In 2009, Mauer et al.16 described a reduction of 2 or more stages in the incidence of diabetic retinopathy progression using an angiotensin-converting-enzyme inhibitor and an Ang II receptor 1 blocker, irrespective of changes in blood pressure. These results coincide with those found by Harindhanavudhi et al.17 who reported in 2011 that 62 of the patients treated with enalapril and losartan (27.8%) had a significant reduction of 65% and 70% in the incidence of diabetic retinopathy progression, respectively.
Hogeboom van Buggenum et al.18 found in 2002 that the eyes of patients treated with angiotensin-converting-enzyme inhibitors had lower concentrations of vascular endothelial growth factor in the vitreous, compared with those of patients who did not receive this treatment. Reducing the ocular concentration of vascular endothelial growth factor with its antagonist drugs is the principle of intravitreous treatment in patients with macular oedema. The effect of Ang II was theoretically an element that might have encouraged involution of the thickening and visual improvement.
The proportion of visual improvement did not change between the patients treated with angiotensin-converting-enzyme inhibitors and those who were not, 3 weeks after photocoagulation. This might be because the intervention on Ang II might have had less of an effect short term. Studies evaluating diabetic retinopathy progression involve long-term measurements, since a change in the degree of retinopathy is not expected short term.
Another factor which might have intervened is that the Ang II blocker might have inhibited protective functions, such as those regulated by the Ang II type 2 receptor. Photocoagulation has been demonstrated to increase the gene expression of Ang II type 2 receptors,19 which promote the removal of intraretinal fluid. Completely blocking the production of Ang II would prevent the beneficial effects of activating the type 2 receptors, and those associated with the production of Ang 1–7.14
Angiotensin-converting-enzyme inhibitor 2 turns Ang I into Ang 1–9 and also converts Ang II into angiotensin 1–7; the latter, which can also be generated from Ang 1–9, has an anti-proliferative and vasodilatory action, capable of counteracting the effects mediated by Ang II receptor type 1 (Burrell et al.20 2004). The expression of 1–7 reduces as diabetes progresses; Verma et al. demonstrated in 201214 that injecting recombinant Ang 1–7 provides a protective effect against the incidence of diabetic retinopathy in rodents.
It has been described that angiotensin-converting-enzyme inhibitors and angiotensin receptor antagonists (ARA II) do not cross the haemato-retinal barrier, due to their molecular weight.21 However, in clinically significant macular oedema, damage to this structure enables larger molecules (lipoproteins) to exit,22 which would allow these drugs to reach the retinal tissue; furthermore, the expected effect would be completed in the endothelial cells,23 and therefore it would not be essential for them to cross the haemato-retinal barrier.
The participation of the renin–angiotensin system in the pathophysiology of diabetic macular oedema has already been documented, but the site has not yet been established on which to intervene to obtain functional improvement with adjunct therapy. Moreover, from the available biochemical information, the results of this study do not support the use of angiotensin-converting-enzyme inhibitors to increase the incidence of visual improvement after photocoagulation in diabetic macular oedema. Therefore the use of Ang II receptor 1 antagonists should be evaluated prospectively in order to determine whether its use as adjunct therapy after photocoagulation increases the incidence of visual improvement in diabetic patients with macular oedema.
ConclusionsThe proportion of eyes with visual improvement did not differ significantly between the patients treated with angiotensin-converting-enzyme inhibitors and the patients who were not. There is no evidence to support blocking the production of Ang II as adjunct therapy after focal photocoagulation, in diabetic macular oedema.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Somilleda-Ventura SA, García-Rubio YZ, Razo Blanco-Hernández DM, Lima-Gómez V. Asociación entre la mejoría visual y el uso de inhibidores de la enzima convertidora de angiotensina, en edema macular diabético. Cir Cir. 2016;84:269–274.