Aspergillus sp., Fusarium sp., and Ramularia sp. were endophytic fungi isolated from Rumex gmelini Turcz (RGT), all of these three strains could produce some similar bioactive secondary metabolites of their host. However the ability to produce active components degraded significantly after cultured these fungi alone for a long time, and were difficult to recover. In order to obtain more bioactive secondary metabolites, the co-culture of tissue culture seedlings of RGT and its endophytic fungi were established respectively, and RGT seedling was selected as producer. Among these fungi, Aspergillus sp. showed the most significant enhancement on bioactive components accumulation in RGT seedlings. When inoculated Aspergillus sp. spores into media of RGT seedlings that had taken root for 20d, and made spore concentration in co-culture medium was 1×104mL–1, after co-cultured for 12d, the yield of chrysophaein, resveratrol, chrysophanol, emodin and physcion were 3.52-, 3.70-, 3.60-, 4.25-, 3.85-fold of the control group. The extreme value of musizin yield was 0.289mg, which was not detected in the control groups. The results indicated that co-culture with endophytic fungi could significantly enhance bioactive secondary metabolites production of RGT seedlings.
Endophytic fungi live in various tissues and organs of healthy plant, maintain an association with their hosts for at least a part of their life cycle without triggering host plant to show obvious symptoms of infection. Researches on endophytic fungi of medicinal plant showed that some of them could promote accumulation of bioactive secondary metabolites in their host plant,1,2 some endophytic fungi could synthesize the same or similar secondary metabolites of their hosts.3 These characteristics of endophytic fungi had provided a new approach for the production of active compounds through industrial fermentation, offered new ideas and methods for improving the accumulation of bioactive components in medicinal plants, and maintained the sustainable development of traditional Chinese medicine resources.
Rumex gmelini Turcz (RGT) belongs to Polygonaceae. In the folk its root and rhizome are used as medicine, which contain bioactive secondary metabolites such as resveratrol, polydatin, emodin, chrysophanol, chrysophaein, physcion and musizin. It has many pharmacological activities, such as anticancer, antifungal, antitussive, antiasthmatic, antihypertensive, antiviral and antioxidant effects.4 Our research group had done some researches on chemical composition, pharmacology and cultivation of RGT, and found it was a potential new drug. More than 300 strains of endophytic fungi had been isolated from roots, rhizomes, stems and leaves of RGT, belonging to 3 orders, 4 families, 37 genera and had obvious diversity. Detected by TLC and HPLC, strains that could produce the same or similar bioactive components of their host (such as resveratrol, polydatin, chrysophanol, emodin, musizin) by fermentation were screened out.5 Among these endophytic fungi, Aspergillus sp. could produce emodin, Fusarium sp. could produce polydatin, and Ramularia sp. could produce chrysophanol. All of these three strains had relatively strong abilities to produce bioactive components. However, these abilities would be degraded after cultured alone for a long time, and were difficult to recover.
At the present stage, under controlled conditions, endophytic fungi could directly produce bioactive secondary metabolites by fermentation,6 but separation from their hosts always led to degeneration of this capacity,7 so many studies use different kinds of elicitors to maintain or promote fungal production of bioactive components. Water-extracted polysaccharide of its host was found to be the most effective elicitor to enhance diepoxin zeta production of endophytic fungus Berkleasmium sp.,8 methyl jasmonate were proven to be an optimum elicitor in view of that camptothecin yield was increased 3.4-fold after its use in culture medium of endophytic fungus ly357.9
At the same time, it was also found that adding endophytic fungi elicitor into plant cell suspension culture medium could improve the ability of plant cells to produce secondary metabolites, when beauvericin10 and oligosaccharide11 extracted from endophytic fungi of Dioscorea zingiberensis C. H. Wright were added into media of D. zingiberensis C. H. Wright cell cultures, the production of diosgenin would be enhanced. Dried mycelia of endophytic fungi were homogenated and collected the entire contents as elicitors, such as after endophytic fungi F4-3 elicitors were added into the cell suspension culture system of Tripterygium wilfordii Hook. f., the highest wilforgine and total alkaloids production were obtained.12
Both plant cell elicitors and endophytic fungi elicitors had promotion effect on accumulation of bioactive secondary metabolites, but they could not reflect the natural interaction of host and its endophytic fungi, so the promotion effect in co-cultured of plant tissue or cell and endophytic fungi were studied. The co-culture of the suspension cells of Taxus chinensis var. mairei and its endophytic fungi Fusarium mairei was successfully established for paclitaxel production, and got a productivity 38-fold higher than that by uncoupled culture.13 Cell of Catharanthus roseus (L.) G. Don co-cultured with its endophytic fungus could get a 48% higher alkaloid yield than the control group.14 Some studies also showed that sometimes the promotion effect of the fungus on the growth of host seedlings was much better than that of its elicitor, while the fungus could more effectively enhance the quality of herbal medicines.15
Through above review, it was found that in the research on interaction of hosts and endophytic fungi, both elicitors and living body were used, among which co-culture of tissue culture seedlings and endophytic fungi were most similar to their daily growth state and it was also a potential form to enhance accumulation of bioactive components, hence co-cultured host tissue culture seedlings and endophytic fungi were chosen to study. In consideration of reaction conditions affected the yield of metabolites, the co-culture system of excellent strains could be optimized to improve the production efficiency. RGT is a potential new drug, however, wild resources are limited, and the growth period is long. If the induced effects of endophytic fungi could promote the production of active substances of RGT tissue culture seedlings, it would provide a strong protection of plant resources, as well as a theoretical basis for the low cost production of these bioactive secondary metabolites.
The objective of this study was to establish co-culture system of RGT tissue culture seedlings with its endophytic fungi, detect influence of this system on the yield of bioactive secondary metabolites in plant tissue, selected strain with strongest promotion effect, optimize their co-culture system and provide theoretical basis for industrial production.
Materials and methodsSeedling culture of RGTRhizomes of RGT aseptic seedlings were used as explants, then inoculated on the medium of MS+6-BA 3.0mg/L, 2,4-D 0.1mg/L and cultured to obtain adventitious buds. These induced buds were cut, placed in liquid MS medium for rooting culture in order to acquire seedlings of RGT.
Culture of endophytic fungiAspergillus sp., Fusarium sp. and Ramularia sp. were preserved in Traditional Chinese Medicine Resources Laboratory of Heilongjiang University of Chinese Medicine. These endophytic fungi were inoculated on PDA culture medium, cultured at 37°C for 7–10d in a digital biochemical incubator.
Establishment of co-culture system of RGT seedlings and its endophytic fungiHealthy, vigorous RGT tissue culture seedlings which had taken root for 15d were chosen, and then randomly divided into 4 groups, 18 bottles in each group. Punch was used to get small circular colonies (d=5mm) in the edge of the colony. Aspergillus sp. were inoculated in MS liquid culture medium of RGT seedlings as group A, Fusarium sp. were inoculated in culture medium of RGT seedlings as group F, Ramularia sp. were inoculated in culture medium of RGT seedlings as group R, one small circular colonies (d=5mm) in each sample. The fourth group were cultured as control, without inoculating any fungi. All of these seedlings were cultured in light incubator at 25±2°C, light of 14h/d, light intensity of 1500–2000Lx.
Culture media were replaced every 5d to ensure essential nutrients for the growth of seedlings (In order to avoid loss of fungi as less as possible, eight layers of sterilized gauze were used to filter out the endophytic fungi, and then fungi were added to the new liquid culture medium). Symbiotic situation were observed every day, and symbiotic period was determined according to the specific experimental results.
After cultured for a certain period of time, RGT seedlings were removed, washed with distilled water, then the stems and leaves were cut off with a dissecting knife. Because roots and rhizomes of RGT seedlings in each bottle were limited, in order to reduce error of dry weight weighing, the roots and rhizomes of each group was randomly divided into three parts, each part contained seedling roots and rhizomes from six bottles. The roots and rhizomes were put in oven drying to constant weight at 45°C, weighed and stored at 4°C.
Optimization of co-culture system of Aspergillus sp. and RGT seedlingsCertain amounts of spore suspension were respectively added to MS liquid culture media to make the final concentrations of Aspergillus sp. spores were 1×103mL–1, 1×104mL–1 and 1×105mL–1. RGT seedlings that had taken root for 10d, 15d, 20d and 25d were respectively cultured in the above MS culture media with certain spore concentration, in light incubator at 25±2°C, light of 14h/d, light intensity of 1500–2000Lx. A total of 12 groups, 18 bottles of RGT seedlings each group.
Culture media were replaced every 5d to ensure essential nutrients for the growth of seedlings. Symbiotic situation were observed every day, and symbiotic period was recorded when most of the leaves have withered and turned yellow. After cultured for a certain period of time, RGT seedlings were removed, washed with distilled water, then the stems and leaves were cut off with a dissecting knife, the roots and rhizomes of each group were randomly divided into three parts, each part contained seedling roots and rhizomes from six bottles. The roots and rhizomes were put in oven drying to constant weight at 45°C, weighed and stored at 4°C.
HPLC analysis of bioactive secondary metabolitesSample preparation for HPLC analysisDry roots and rhizomes of RGT seedlings from every group were ground into powder and then passed through 80 mesh sieve before used. 0.5g dry powder were precisely weighed, put in Soxhlet extractor, mixed with 70mL 50% ethanol and kept at room temperature for 4h, and then reflux extracted at 85°C for 4h, after that, the solution was filtrated, the filtrate was concentrated to dryness on a rotary evaporator, diluted with methanol to volume 5mL (5000μL), this solution filtrated by 0.45μm microporous membrane to use as sample for HPLC analysis.
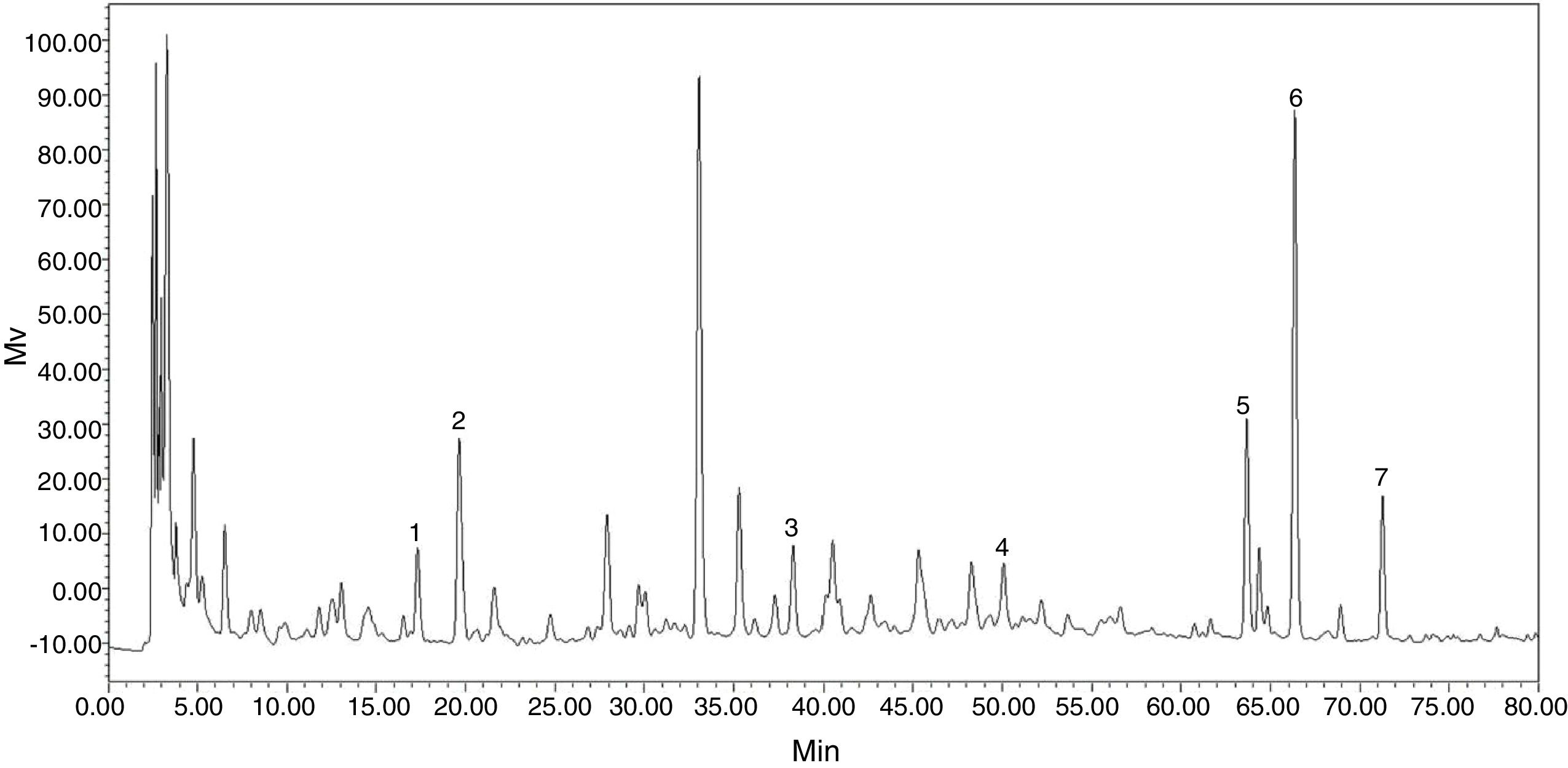
The conditions of HPLCA Dimma, Diamonsil C18 column (150mm×4.6mm ID, 5μm), Phenomenex ODS-C18 (4.0mm×3.0mm) pre-column, 2998 PDA Detector and waters2695 pump were used. The column temperature was set at 25°C. The mobile phase was methanol, 0.1% phosphoric acid water and at a flow rate of 1.0mL/min. The gradient elution condition: the mobile phase was from 30% methanol to 100% methanol. The analysis time was 70min. Tested wavelength was 254nm and 303nm. The sample injection volume was 10μL. The content of polydation, resveratrol, musizin, emodin, physcion, chrysophanol and chrysoretin could be detected and quantified by standard curves.
Statistical analysisThe results were represented by their mean values and standard deviations. The data were submitted to single factor variance analysis to detect significant differences by spss18.0.
ResultsEstablishment of co-culture system of RGT and its endophytic fungiRGT seedlings that had taken root for 15d already had developed root system and in good growth state, respectively inoculated Aspergillus sp., Fusarium sp. and Ramularia sp. in media of these seedlings. In the first 3d after inoculation, there was no change either in seedlings nor in media, which showed that endophytic fungi needed a certain adaptation period.
After co-cultured Fusarium sp. and RGT seedlings for 5d, there were mycelia blocks in the medium and black spots on root hair. Symbiosis for 7d, culture medium became turbid and thick, but no mycelia on the surface of the medium, meanwhile new leaves were growing. After 10d, more new leaves appeared, some old leaves became yellow, the roots became stronger, the whole seedlings were in good growth condition (Fig. 1-F). This status could last for another 5–10d.
Ramularia sp. and RGT seedlings were in poor symbiotic state, 7d after inoculation, mycelia of Ramularia sp. had covered the surface of culture medium, color of medium turned dark and became thick and sticky, root tips of seedlings were black. Co-cultured for 12d, the edge of new leaves turned yellow, and were wilted (Fig. 1-R), although new leaves appeared, they were grew slow, most seedlings were in morbid state, the others were died. Through observation, it was found that in the beginning of this symbiosis, Ramularia sp. grew too fast and excessively consumed nutrients of the culture medium, resulting in the malnutrition of seedlings. In later stage, seedlings were sick and died, which might because seedlings could not tolerate serious infection of Ramularia sp. During this process, Ramularia sp. might produced some metabolites that turned the culture medium yellow and sticky, moreover, some of these metabolites were not conducive to the growth of seedlings.
After co-cultured Aspergillus sp. and RGT seedlings for 8d, only some white suspended mycelia of Aspergillus sp. appeared in medium without large mycelia blocks, the color of medium did not change significantly, seedlings could continue to grow with a few white fluffy mycelia covered the surface of their roots. As co-culture continued, new leaves appeared, surface area of old leaves increased, the whole bright green seedlings were in good growth condition (Fig. 1-A). After co-cultured for 20d, the tissue culture seedlings were strong without any pathological changes.
Effect of co-culture on the yield of bioactive secondary metabolitesChromatogram of roots and rhizomes of RGT seedlings could be obtained by HPLC (Fig. 2). The contents of seven chemical compositions were calculated according to their regression equations.
As RGT seedlings could only co-exist with Ramularia sp. for 12d, in order to compare the yield of bioactive secondary metabolites in these co-cultured seedlings with different endophytic fungi, we chose symbiotic period of 12d to do further research.
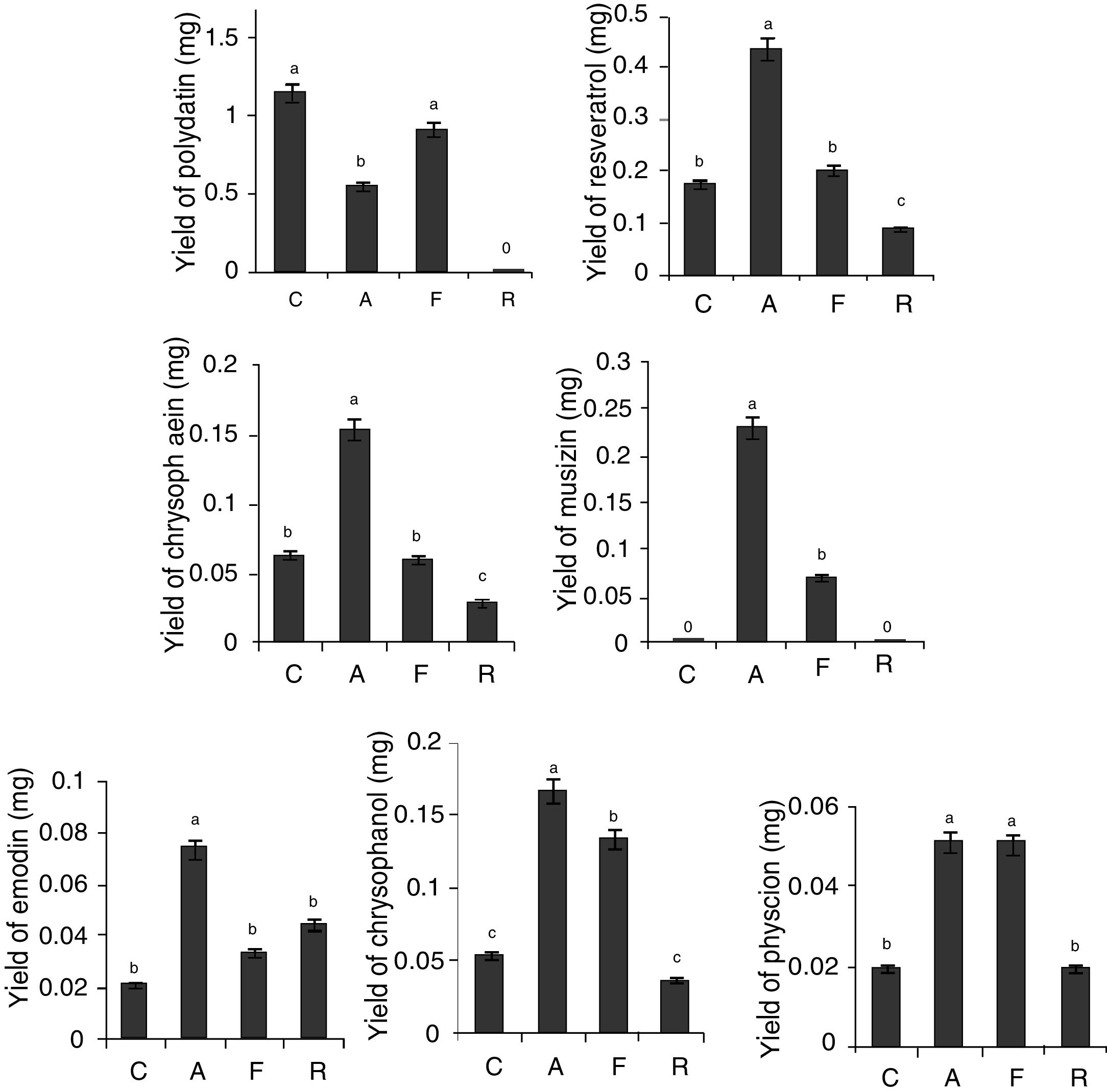
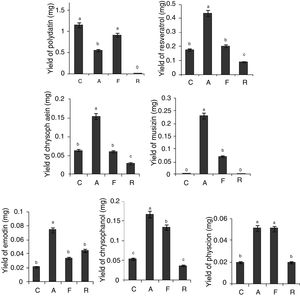
As Presented in Fig. 3, the yield of effective components of the roots and rhizomes of RGT seedlings that co-cultured with Aspergillus sp. were higher than that of the control except polydatin, in addition, musizin was detected in co-cultured seedlings, but not in the control.
Effect of co-culture on the yield of bioactive secondary metabolites. Different letters indicated significant differences among the treatments at p=0.05 level. C: control; A: RGT seedlings co-culture with Aspergillus sp.; F: RGT seedlings co-culture with Fusarium sp.; R: RGT seedlings co-culture with Ramularia sp.
During co-culture process of RGT seedlings and Ramularia sp., seedlings showed wilt and sick earlier than the other co-cultured groups, polydatin and musizin were not determined in co-cultured seedlings, and the yield of other effective components were low (Fig. 3), but the yield of emodin was 2.5-fold of the control.
Fusarium sp. could significantly improve the yield of musizin, chrysophanol and physcion (Fig. 3), however its promotive effect was weaker than Aspergillus sp. In addition, dry weight of RGT seedlings co-cultured with Fusarium sp. was dramatically less than that of the control, but there was no significant difference between dry weight of RGT seedlings co-cultured with Aspergillus sp. and that of the control. Hence Aspergillus sp. was considered as the most effective fungus that could promote bioactive components accumulation in RGT seedlings.
Optimization of co-culture conditions for RGT seedlings and Aspergillus sp.As Aspergillus sp. exhibited an excellent promoting effect on bioactive components accumulation in RGT seedlings, the inoculation amount and time were further studied. Because the colony's growth state often changes, in order to better quantify the inoculation amount of fungus, fungal spores were used to inoculate into media of RGT seedlings.
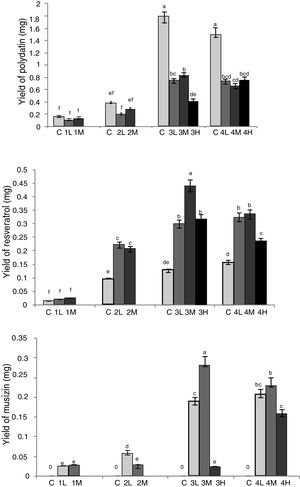
Aspergillus sp. spores were respectively inoculated into media of RGT seedlings that had taken root for 10d (1), 15d (2), 20d (3), 25d (4), and made final concentrations of spores in co-culture media were 1×103mL–1 (L), 1×104mL–1 (M), 1×105mL–1 (H). RGT seedlings in group 1H (10d, 1×105mL–1), 2H and 3H all showed phenomenon of withered leaves and whole plant death after co-cultured for 5–12d, this phenomenon appeared in group 4H slightly later, but the symbiotic periods were not more than 17d. Symbiotic periods of other groups were all more than 20d, group 3L, 3M, 4L and 4M showed the best state, whose symbiotic periods were over 25d. In these groups, vitality of RGT seedlings were strong with new leaves growing. As group 1H and 2H only could be co-cultured for 5d, RGT seedlings in these groups were too small to do further research. The symbiotic period of group 3H was 12d, in addition, the best symbiotic state of the other groups appeared 10 to 15d after inoculation of Aspergillus sp., in later stage seedlings grew very slow, so we chose symbiotic period of 12d to do further research.
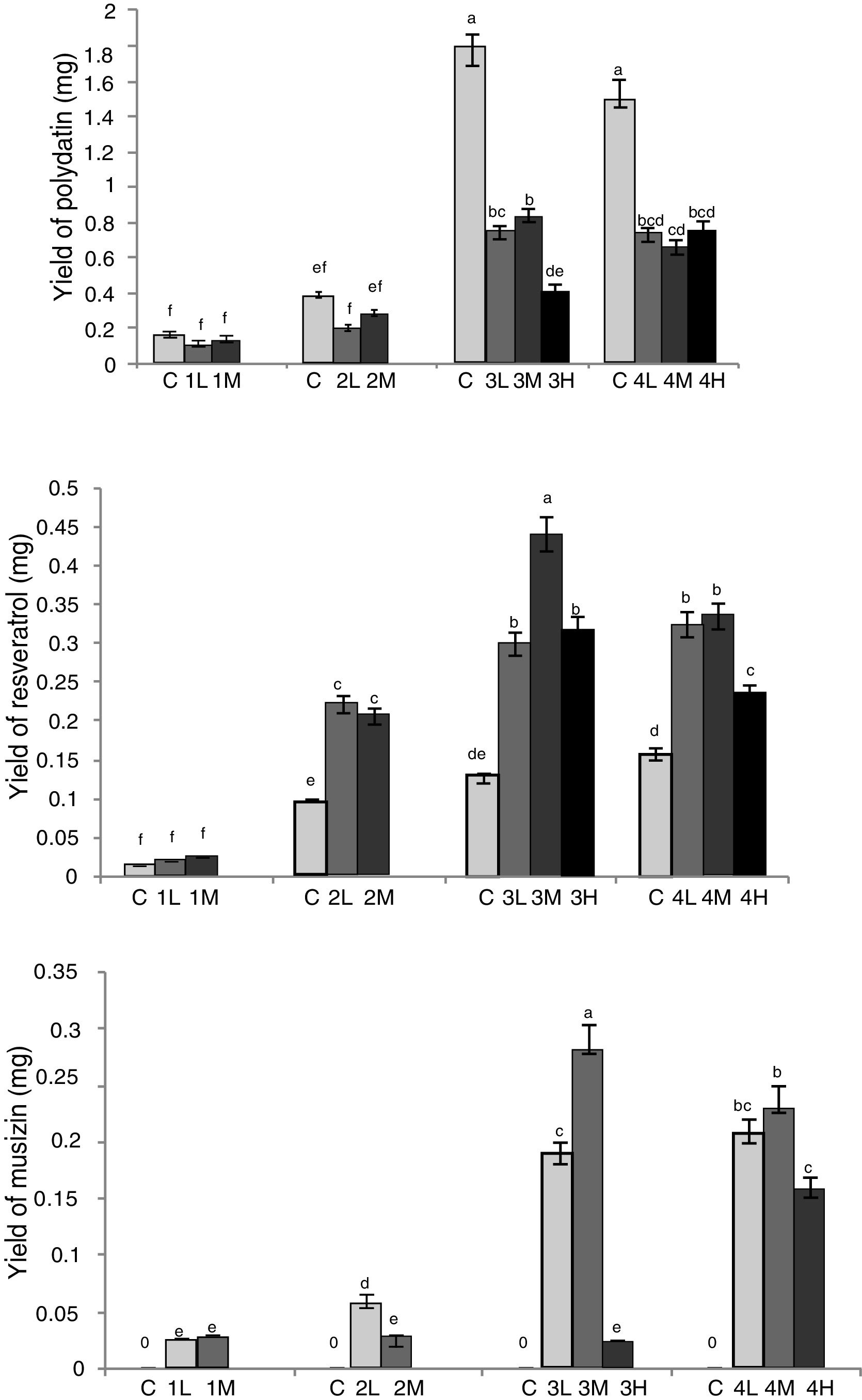
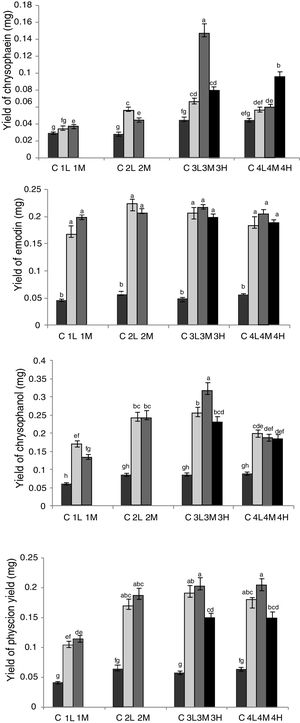
In view of dry weight of RGT seedlings, there were no significant differences between the group2 (L, M), 3 (L, M, H) and 4 (L, M, H), which showed that due to restriction of the culture environment, there was a certain plateau in the growth of RGT seedling. Through HPLC analysis (Fig. 4), the yield of effective components were changed between groups, the extreme value of musizin yield was 0.289mg in group 3M, which was not detected in control groups, the maximum yield of chrysophaein, resveratrol, chrysophanol and physcion were also appeared in group 3M, which were 3.52-, 3.70-, 3.60-, 3.85-fold of the control group, emodin yield in co-cultured groups had no significant difference, 3.37- to 4.43-fold of the control group. In addition, the polydatin yield of symbiotic group were lower than that of the control groups, group 3L, 3M, 4L and 4H were higher than the other. Therefore group 3M was the most suitable for the accumulation of bioactive components (inoculated Aspergillus sp. spores into media of RGT seedlings that had taken root for 20d, and made spore concentration in co-culture medium was 1×104mL–1). In this condition, fungi and RGT seedlings were in good growth condition and it was conducive to enhance the accumulation of bioactive components.
Optimization of co-culture conditions of RGT seedlings and Aspergillus sp. Different letters indicated significant differences among the treatments at p=0.05 level. C: control; 1: co-culture when RGT seedlings had taken root for 10d; 2: co-culture when RGT seedlings had taken root for 15d; 3: co-culture when RGT seedlings had taken root for 20d; 4: co-culture when RGT seedlings had taken root for 25d; L: final concentrations of Aspergillus sp. spores in media was 1×103mL–1;M: final concentrations of Aspergillus sp. spores in media was 1×104mL–1; H: final concentrations of Aspergillus sp. spores in media was 1×105mL−1.
In the establishment of co-culture system of endophytic fungi and RGT tissue culture seedlings, growth rate of the fungi were much faster than the host, in order to prevent fungi from excessively consuming medium nutrition, the medium should be always replaced to ensure the nutrients for seedlings. MS liquid medium for co-cultivation was easy to replace, could prevent excessive damage to the root which led to serious fungal infection. Meanwhile, fungi suspended in liquid medium could have more contact area with roots and rhizomes of RGT seedlings, which was conducive to interaction of fungi and plant.
In view of the effect of co-culture on the yield of bioactive secondary metabolites, it was implied that in the process of the symbiosis, Aspergillus sp. enhanced the synthesis and accumulation of some chemical substances in the seedlings. It had been reported that musizin had effects of antifungal and antioxidant.16,17 In normal conditions, musizin only exists in RGT roots and rhizomes when the plant grow bigger, however in co-cultured RGT seedlings musizin had been detected, maybe the plant need to resist exogenous fungus, resulting in the earlier synthesis of musizin. Polydatin had similar pharmacological effects to resveratrol, both of them could resist microbial attack and oxidative damage of adverse conditions.18,19 The yield of polydatin reduced, along with an increase of resveratrol yield. Former research showed that Aspergillus oryzae could transform polydatin to resveratrol with high yield and mild conditions.20 Therefore we speculated that the endophytic fungi of RGT might also have the ability of this biotransformation and more resveratrol was likely produced from polydatin hydrolysis by endophytic fungi. In co-cultured RGT seedlings, the contents of some kinds of anthraquinones, such as chrysophaein, chrysophanol, physcion and emodin were increased significantly. It may be due to symbiosis that promoted the synthesis of anthraquinone compounds, or inhibited the competitive pathway. Some studies showed that chrysophaein could be produced through microbial transformation of chrysophanol,21,22 but in this experiment the contents of chrysophaein and chrysophanol both increased significantly, therefore the biotransformation of endophytic fungi should happened in the process of formation of anthraquinone nucleus if it existed.
RGT seedlings co-cultured with Ramularia sp. had significantly lower yield of effective components than the control except emodin, which might be a self-protection mechanism when RGT seedlings were subjected to external microbial infection, plant produced emodin that had the effect of antifungal23 to resist the invasion of foreign fungus and prevent fungus further spread to other area. Emodin, polydatin and musizin all have antifungal effect, and they shared some common associated substrates in their biosynthesis, such as acetyl coenzyme A, phosphoric acid enol pyruvic acid and so on, since the contents of these substrate were limited in plant, competition existed in selection of synthetic directions. After co-culture of Ramularia sp. and RGT, there were no polydatin and musizin in seedlings, meanwhile emodin yield increased obviously. Hence the existence of certain strain might have synthetic limits on some components of RGT and such restrictions led to large-scale synthesis of other competitive components.
During optimization of co-culture conditions for RGT seedlings and Aspergillus sp., low and medium concentration of Aspergillus sp. spores were beneficial to build symbiotic state of fungus and RGT seedlings. After high concentration of Aspergillus sp. spores inoculated in medium, the environment was suitable for rapid growth of this strain, Aspergillus sp. massively infected RGT seedlings which led to death of plant. It was appropriate to inoculate fungus into RGT seedlings that had taken root for longer time, in that situation, RGT seedlings would become stronger, and the symbiosis between seedlings and fungus was easier to establish.
Those seven secondary metabolites that detected in this study were the main effective components of RGT. For human beings they had anti-cancer, anti-inflammatory, antiviral and other pharmacological effects. For RGT seedlings, these ingredients could help plant to resist the invasion of Aspergillus sp. and achieve peaceful coexistence by metabolic regulation.
The results indicated that co-cultured with its endophytic fungus Aspergillus sp. could significantly enhance the production of bioactive components in RGT seedlings, however, the specific regulation mechanism of Aspergillus sp. in accumulation of secondary metabolism of RGT seedlings needed further research. This co-culture system could be used to enhance the production of bioactive secondary metabolites in large scale.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the National Natural Science Foundation of China (Grant No. 30930700), Natural Science Foundation of Heilongjiang Province (C2016053), (QC2009C31).
Chang-hong Ding is in post doctoral mobile station in Chinese medicine direction of Heilongjiang University Of Chinese Medicine